1. Introduction
The initial purpose of OLEDs is to utilize as display screens, and with recent innovations, OLEDs can now be use in many other aspects including the biomedical field, which includes the therapeutic treatment. Wearable OLEDs can be used as a light source for ensuring the outpatient care especially for people who are currently not available to go to hospital frequently. There are two main applications of OLEDs: PDT (photodynamic therapy) and PBM (photobiomodulation). PDT is one way to kill bacteria and cure cancer and has now been used in many aspects, e.g., in oncology, dermatology, ophthalmology, and dentistry [1]. It needs light at certain wavelength to send energy to a photosensitizer. Photosensitizer is kind of compound that can absorb energy within certain wavelengths in the ultraviolet region (25-420nm) or visible light region (400-800nm). After the excitation of photosensitizer, there will be interactions between oxygen molecules in the tissue, raising oxygen from the triplet ground state to the singlet state. In this case, the new created singlet oxygen molecules will be able to send radical attacks to kill the nearby bacteria [2]. The key principle of this transition is OLED, which help to develop new photosensitizers based on phosphorescent and TADF emitters. To have a better penetration through skin, red light is an optimal choice since there is high reliance on high power light sources to deliver the light to certain areas to achieve the target. One of the ramifications of OLED is NIR, which plays an essential role in the biological applications, due to the NIR has little overlap of the cell’s own autofluorescence (AFL). OLEDs are thin, light-weighted, and flexible solid-state devices that can be fabricated on large-area, conformable materials. This intrinsic flexibility allows them to conform to the curvilinear surfaces of human skin or organs, ensuring unparalleled uniformity of light emission across the entire treatment area.
2. The advantages of OLED’s application in medical treatment
OLEDs convert electrical energy into light energy through electroluminescence. OLEDs are flexible, lightweight and can be easily conformed to skin. These basic features make it outstanding to be an optimal choice for minimally invasive surgery. OELDs can be fabricated on flexible substrates (e.g., plastic films), which makes it possible to be integrated onto adhesive clothing or patches [3]. The whole working process is also explicit and efficient. The electrons are injected from the anode indium tin oxide and the cathode low work function metal respectively. After that, they recombine in the emissive layer to form excitons. The stack-like structure of anode, hole transport layer, emissive layer, electron transport layer, and cathode works on an energy-balanced carrier. Organic photodiodes (OPDs) are based on the reverse process. The photoactive layer absorbs photons to generate excitons. Although OLED’s function and OPD’s function work in an opposite way, they share the same exciton behavior control mechanism - OLEDs need to achieve an efficient radiative recombination of excitons, while OPDs need to maximize exciton dissociation efficiency. The integration of these technologies promotes the development of integrated display and sensing systems and photoelectric coupling systems. Compared with other conventional treatments, PDT combining OLEDs and OPDs has numerous advantages, such as fewer side-effects and enhanced cosmetic and clinical outcomes. It is typically used for treatments of basal cell carcinoma, Bowen disease, actinic keratosis.
3. An application of OLED in a clinical study
In a clinical study, Attili et al. used a wearable red OLED delivering 0.05 mW cm ⁻² at a peak wavelength of 620 nm to treat nonmelanoma skin cancer. In his study, patients contended lesser pain when the OLED was used, compared to a control group that was treated with an inorganic LED delivering a similar dose but at a higher fluence of 0.75 mW cm ⁻² [4]. Therefore, OLED is the key solution to alter the PDT treatment into a much more comfortable and convenient way for patients.
Due to the new technology is targeted to the elderly and the infants, therefore it is extremely important to minimize the pain, which is a key obstacle in PDT treatment. Combining The traditional PDT (photodynamic therapy) has one of its major flaws—the pain is sometimes significant to patients. That is mainly the consequence of its use in dermatology. Patients who take the conventional PDT described it as an extreme burning sense and is almost intolerant. Therefore, pain has been proved to be positively associated with the fluence rate and also been adduced by PpIx. And with innovations to reduce fluence rate is helpful to eliminate the painful feelings. When the fluence rate is < 60 mW cm ⁻², the pain score is consistently and significantly lower than with rates > 60 mW cm ⁻². Besides, pain intensity displays a direct proportion growth with fluence rates < 60 mW cm ⁻². Relatively, no significant correlation is observed when the fluence rate is > 60 mW cm ⁻². Figure1 represents a positive-related curve between PDT efficacy and fluence rate at fixed irradiation duration, and accurately depicts when the influence rate is larger than 60 mWcm ⁻², the curve is gradually switching into a horizontal line which has no significant change [5].
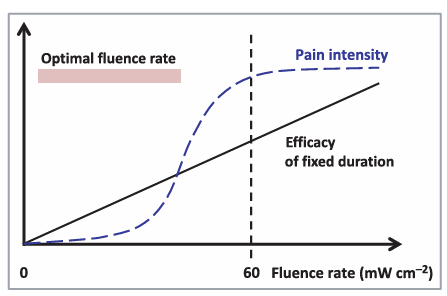
The newly-developed equipment consists of a low-irradiance, skin-fitting and light weight OLEDs, and emits light source for approximately 2 cm. Twelve patients with Bowen’s disease were recruited into this treatment utilizing the PDT method combining the OLEDs. Two treatments (45–60 J cm⁻² red light, 550–750 nm, peak 620 nm, irradiance 5 mW ⁻²) were administered 1 month apart following application of aminolaevulinic acid for 4 h. Results At the 12-month follow-up, seven of the 12 patients remained clear, with four of the non-responders exhibit margin failure. Patients were asked to rate their pain instantly after the treatment using the numerical rating scale (NRS)from 1-10. All 12 patients who take the treatment scored pain as < 2 using the NRS (median score 1).
4. Challenges
While OLEDs-based PDT has shown significant advantages in improving patient comfort and therapeutic efficacy for conditions like nonmelanoma skin cancer, its clinical application still faces notable challenges, with pain management remaining a core issue that requires in-depth analysis of contributing factors.
External factors that can cause pain, for example, battery is an external factor which can cause additional pain. According to studies, products consist of PpIX, ROS might trigger pain in PDT [6]. However, a plight is encountered when this situation is being balanced. It is not an ideal solution to remove the ROS although it release cytotoxicity and trigger pain. Since eliminating ROS would decrease the therapeutic effect of PDT, more researches need to be done in this field [7].
Internal factor that can cause pain, the characteristic features of skin lesions are significant contributors to pain variation during PDT. These factors primarily include the lesion's location, size, and type. PDT performed on the face and scalp generates greater pain than at other anatomical sites, a correlation likely explained by the highest sensory nerve innervation density in these areas. Furthermore, larger lesions are associated with increased pain. Among different conditions, psoriatic lesions tend to be the most painful, surpassing AK, BD, SCC, and acne. This is presumably due to their elevated metabolic rate and heightened local production of reactive oxygen species (ROS), which intensifies nerve stimulation [8].
5. Conclusion
PDT is an efficient method to treat specific kinds of skin diseases and due to nowadays technology, it takes much shorter time to treat patients and time to recover. During this process, OLEDs play an important role in the overall PDT process and present an innovative movement. By moving away from conventional rigid and bulky light sources towards a future of personalized, wearable medicine. Their fundamental mechanism of action—converting electrical energy into specific wavelengths of light with high efficiency—enables the precise activation of photosensitizers in PDT and the delivery of therapeutic photons for wound healing and neural stimulation. Since OLEDs take credits in significantly reducing the pain, the whole treatment is currently more comfortable. Future research on OLEDs in biomedical therapy will focus on addressing current limitations to enhance practicality and therapeutic efficacy. Efforts will prioritize optimizing wearable OLED device design—such as developing high-efficiency, low-power architectures to extend battery life and scaling device size while ensuring uniform light distribution—to better treat larger skin lesions. For the ROS-related pain dilemma, precision modulation strategies will be explored to balance cytotoxicity and pain reduction. Integrating real-time biosensors with OLEDs to enable dynamic adjustment of light parameters for personalized pain management is another key direction. These include overcoming material stability issues related to moisture and oxygen degradation, enhancing light output efficiency and penetration depth, especially for treating deeper tissues Additionally, expanding applications beyond nonmelanoma skin cancer using NIR OLEDs and unraveling anesthesia resistance mechanisms in PDT will further enhance OLEDs’ potential in transforming PDT into a more comfortable, accessible therapeutic option.
References
[1]. Hauswald, S., Duque-Afonso, J., Wagner, M. M., Schertl, F. M., Lübbert, M., Peschel, C., et al. (2009). Histone deacetylase inhibitors induce a very broad, pleiotropic anticancer drug resistance phenotype in acute myeloid leukemia cells by modulation of multiple ABC transporter genes. Clinical Cancer Research, 15(11), 3705-3715.
[2]. Hagendoorn, J., Padera, T. P., Kashiwagi, S., Isaka, N., Noda, F., Lin, M. I., ... & Jain, R. K. (2004). Endothelial nitric oxide synthase regulates microlymphatic flow via collecting lymphatics. Circulation research, 95(2), 204-209.
[3]. Robertson, C. A., Evans, D. H., & Abrahamse, H. (2009). Photodynamic therapy (PDT): a short review on cellular mechanisms and cancer research applications for PDT. Journal of Photochemistry and Photobiology B: Biology, 96(1), 1-8.
[4]. Wang, B., Shi, L., Zhang, Y. F., Zhou, Q., Zheng, J., Szeimies, R. M., & Wang, X. L. (2017). Gain with no pain? Pain management in dermatological photodynamic therapy. British Journal of Dermatology, 177(3), 656-665.
[5]. Eljamel, M. S., Goodman, C., & Moseley, H. (2008). ALA and Photofrin® Fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: A single centre Phase III randomised controlled trial. Lasers in medical science, 23(4), 361-367.
[6]. DeNicola, G. M., Karreth, F. A., Humpton, T. J., Gopinathan, A., Wei, C., Frese, K., ... & Tuveson, D. A. (2011). Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature, 475(7354), 106-109.
[7]. Bedard, K., & Krause, K. H. (2007). The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiological reviews, 87(1), 245-313.
[8]. Rai, P., Kumar, P. S., & Varadan, V. K. (2010, March). Integration of OLEDs in biomedical sensor systems: design and feasibility analysis. In Nanosensors, Biosensors, and Info-Tech Sensors and Systems 2010 (Vol. 7646, pp. 206-212). SPIE.
Cite this article
Sun,S. (2025). Organic Light-Emitting Diodes Applications in Biomedical Therapy Mechanisms Clinical Studies and Challenges. Applied and Computational Engineering,187,31-34.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of CONF-FMCE 2025 Symposium: Semantic Communication for Media Compression and Transmission
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Hauswald, S., Duque-Afonso, J., Wagner, M. M., Schertl, F. M., Lübbert, M., Peschel, C., et al. (2009). Histone deacetylase inhibitors induce a very broad, pleiotropic anticancer drug resistance phenotype in acute myeloid leukemia cells by modulation of multiple ABC transporter genes. Clinical Cancer Research, 15(11), 3705-3715.
[2]. Hagendoorn, J., Padera, T. P., Kashiwagi, S., Isaka, N., Noda, F., Lin, M. I., ... & Jain, R. K. (2004). Endothelial nitric oxide synthase regulates microlymphatic flow via collecting lymphatics. Circulation research, 95(2), 204-209.
[3]. Robertson, C. A., Evans, D. H., & Abrahamse, H. (2009). Photodynamic therapy (PDT): a short review on cellular mechanisms and cancer research applications for PDT. Journal of Photochemistry and Photobiology B: Biology, 96(1), 1-8.
[4]. Wang, B., Shi, L., Zhang, Y. F., Zhou, Q., Zheng, J., Szeimies, R. M., & Wang, X. L. (2017). Gain with no pain? Pain management in dermatological photodynamic therapy. British Journal of Dermatology, 177(3), 656-665.
[5]. Eljamel, M. S., Goodman, C., & Moseley, H. (2008). ALA and Photofrin® Fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: A single centre Phase III randomised controlled trial. Lasers in medical science, 23(4), 361-367.
[6]. DeNicola, G. M., Karreth, F. A., Humpton, T. J., Gopinathan, A., Wei, C., Frese, K., ... & Tuveson, D. A. (2011). Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature, 475(7354), 106-109.
[7]. Bedard, K., & Krause, K. H. (2007). The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiological reviews, 87(1), 245-313.
[8]. Rai, P., Kumar, P. S., & Varadan, V. K. (2010, March). Integration of OLEDs in biomedical sensor systems: design and feasibility analysis. In Nanosensors, Biosensors, and Info-Tech Sensors and Systems 2010 (Vol. 7646, pp. 206-212). SPIE.









