1. Introduction
Nowadays, humans’ daily life has become more and more convenient as the technology is developing rapidly. However, the environment has deteriorated gradually since the progress occurs. The greenhouse gases such as carbon dioxide and methanol lead to the global warming, which makes the temperature increase slightly. Silicon dioxide and nitrogen oxides cause the formation of acid rain. Generally, the emissions from diesel or petrol vehicles are the main source of these gases. However, the emission of gas could be a practical chance to innovate the conventional factories, in order to enhance the energy efficiency. People need to find a new way to ensure sustainable development [1].
Fuel cell is a kind of equipment that provides energy by transferring the chemical energy into electrical energy. The major feature of the fuel cell is that it produces electricity through electrochemical reaction rather than combustion. Hydrogen and oxygen are combined together to form electricity, heat and water, which is extraordinary with conventional one by storing energy. Fuel cells are very advantageous in various aspects. They have no moving sections, which means that they are convenient to transport and carry. A lower maintenance requirement signifies that fuel cells have great durability and less manual work is taken on the fuel cell so that total costs can be reduced. What is more, a fuel cell can sustain a broad temperature range compared to the internal combustion engines [2]. The fuel cell also possesses high energy conversion efficiency which is greater than the internal combustion engines, so the energy dissipated is cut down. Usually, the hydrogen gas is regarded as the most frequently-used fuel. Other fuels, such as methanol, natural gas or nitrogen are available to be utilized as well. A fuel cell is comprised of two electrodes connected by the electrolyte. At the anode of the cell, hydrogen is placed while oxygen, acquired from the air, is existent at the cathode. They form into ions moving freely in the electrolyte, so as to produce a current. This is known as the basic operation and design of the fuel cell.
The classification of fuel cells is quite diverse, namely proton exchange membrane fuel cell (PEMFC), alkaline fuel cell (AFC), phosphoric acid fuel cell (PAFC), molten carbonate fuel cell (MCFC), solid oxide fuel cell (SOFC) and direct methanol fuel cell (DMFC). The categorization is based on the property of electrolyte applied. In addition, more kinds of fuel cells are known whose usage rate is relatively low, for instance, the air-depolarised cells, microbial fuel cell, enzymatic fuel cell, sodium amalgam fuel cell, etc [3].
Fuel cells still face some challenges and opportunities on the research and development of fuel cells. The development of fuel cells rockets through several years, on account of a series of causes. More environmental legislation is exerted in order to manipulate the exhaust emissions. The government remove the control of electric industry in aid of renewable energy production. Therefore, the fuel cell can display its own superiority into energy generation, due to high efficiency and energy density. It is significant that further research on the fuel cells is essential for promoting the quality and efficiency, contributing to the transportation, industrial engineering, mechanical manufacture, and other fields.
Hence, this article will focus on the research of fuel cells, aiming to summarize the working principle of fuel cells, and different kinds of fuel cells in respect of features, structures and applications. The article will review several types of common fuel cells, and provide some suggestions for the future development of fuel cells, laying the foundation for the research and development of fuel cells with higher efficiency and longer service life.
2. Fuel cells and their design and operation
A fuel cell is an electrochemical device that transforms chemical energy into electrical energy. Its general physical structure is comprised of the electrolyte and an anode and cathode with porous layers on each side. The basic reaction of the fuel cell is proceeded through reacting hydrogen gas with oxygen so that heat and water are formed, along with releasing electrical energy [4]. As shown in Figure 1, at the cathode, hydrogen gas releases electrons and protons. Electrons form a electric current through the external circuit. Protons then pass through the electrolyte membrane to reach the anode. Consequently, they can combine with the oxygen and electrons to produce water.
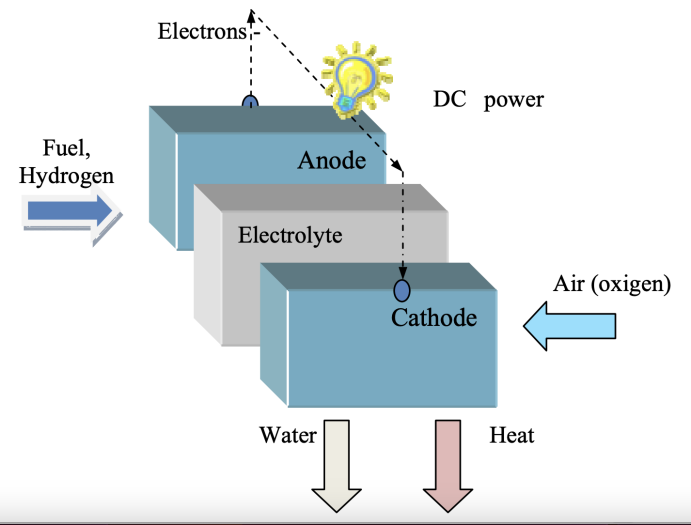
Electrolytes enable different types of particles, such as protons, hydroxide ions, hydrogen ions, carbonate ions, etc. to possess mobility and are also essential for completing the whole path of the fuel cell. Ideal Fuel cell electrolytes possess the properties of high ionic conductivity, thermal stability, chemical stability, great mechanical integrity and low gas permeability. Ion conduction is a process related to the temperature. Generally, the state of electrolytes is solid or liquid and this decides the temperature needed for the conduction to be started [5].
Some advantages appear obviously in the fuel cell. Fuel cells have high electrical energy conversion efficiency, arranging from 40% to 60%. If combined heat and power system is adopted, the comprehensive efficiency can reach to approximately 80%. No moving parts are placed in the fuel cell, so there is no need for maintenance. Owing to this, the operation has small oscillation and makes no noise. When hydrogen gas is reacted, the product is only water, which means that there is no emission of pollutants, such as carbon dioxide, nitrogen oxides and sulfur oxides. Besides this, natural gas, methanol and marsh gas can be utilized as hydrocarbon fuels in some fuel cells (SOFC, MCFC) [6].
However, there are some drawbacks that should be solved in the future. Catalysts used commonly employ noble metal lead and pricey high-temperature ceramics. Auxiliary systems such as air compression, cooling and humidification added increase the total cost. Hydrogen gas is hard for transportation because of great volume and high pressure. More importantly, hydrogen gas is explosive and flammable which is regarded as the biggest risk. In addition, electrolytes and catalysts are prone to aging, corrosion or poisoning which affect the life expectancy.
3. Categories of fuel cells
The categorization of fuel cells is generally based on the different types of electrolytes. The main types of fuel cells include proton exchange membrane fuel cell (PEMFC), phosphoric acid fuel cell (PAFC), molten carbonate fuel cell (MCFC), solid oxide fuel cell (SOFC), alkaline fuel cell (AFC) and direct methanol fuel cell (DMFC).
3.1. Proton exchange membrane fuel cell
In recent years, proton exchange membrane fuel cell has large potential. The core of PEMFC is the proton exchange membrane, which only allows protons to transit between electrodes. Hydrogen is oxidised at the anode and form protons. The protons pass through the proton exchange membrane which is fed into the channel between anode and cathode. When protons reach the cathode, they react with the oxygen to generate water and heat [7].
As shown in Figure 2, the structure of a proton exchange membrane fuel cell constitutes the anode and cathode, electrolytes, gas diffusion layers, catalyst layers and proton exchange membrane. These components are assembled to produce the membrane electrode assembly (MEA) [8].
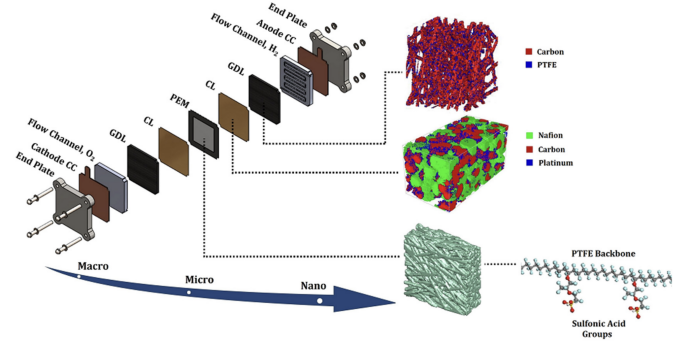
Catalyst layers are indispensable for promoting the reaction occurring in high efficiency and low temperature. At the anode, hydrogen molecules are attached on the catalyst layer. Hydrogen-hydrogen bond is broken so hydrogen atoms are produced. Protons are carried by ionomers and thus protons migrate to the cathode. However, electrons produced by the hydrogen atoms are prohibitive to pass through the membrane. They are transported by conductors such as carbon supports. These electrons are transferred to the external circuit and this is why the current is formed [9].
Proton exchange membrane is most vital for constructing the PEMFC. Nafion is a very generally used type of proton exchange membrane, which consists of fluorocarbon skeleton and sulfonic acid functional group. It possesses excellent chemical and thermal stability, and offers mechanical strength and high corrosion resistance. Although the structure is quite loose, it displays a nature of hydrophobicity [10].
Gas diffusion layer is one of the most significant sections in membrane exchange assembly, which is located between the catalyst layer and flow field. It has the same function as the proton exchange membrane, enhancing the mechanical strength. Besides, the gas diffusion layer makes the control of reactants, water and affects the effectiveness of PEMFC. The gas diffusion layer possesses a range of capabilities, transporting the electrons, and eliminating the water produced from the proton exchange membrane to the flow channels. Ordinarily, the gas diffusion layer consists of two parts, namely macroporous substrate and microporous layer. The macroporous substrate is composed of carbon paper or carbon cloth, which forms the backbone of the gas diffusion layer. The function of this part is offering gas passage and basic mechanical support. The microporous layer is coated between the base layer and catalyst layer, constituting carbon black and hydrophobic agent, generally PTFE, aiming to improving gas distribution and aqueous management.
PEMFCs exist several benefits such as remaining clean and environmentally friendly on account of generating water only, high energy conversion efficiency due to low operating temperature and high current density, quick launch, peaceful operation and the probability of costing less. On the contrary, some drawbacks are presented which means that a large amount of space is existent on PEMFCs. The marketization of PEMFCs is proceeding yet not enough information and technical data are noticed to the public. Many enterprises marketing the PEMFCs are not likely to share their own important information for the sake of their own profit [11].
Because of having the property of high efficiency and green use, PEMFCs are applied in a wide range of fields. PEMFC becomes as a core power source in the transportation, mainly used in the fuel cell electric vehicles, such as Toyota Mirai, Honda Clarity, etc. Buses and cars utilize PEMFCs so that exhaust gas emissions can be reduced. Portable digital gadgets and laptops employ PEMFCs as the power source since they have longer battery life. Additionally, PEMFCs also have some potential on the field of military research.
3.2. Direct methanol fuel cell
The direct methanol fuel cell is a special type of PEMFC. The primary distinction between DMFC and PEMFC is that the fuel for DMFC is methanol directly. DMFC is regarded as a better choice for the power source. Hydrogen is in gaseous state while methanol is in liquid state which facilitates the disposal and increases the contact area of the reaction. Comparatively, it has higher energy density and plays a role as green fuel as well. Due to the state of liquid, the stockpile of methanol can be very simple and safe [12]. The direct methanol fuel cell is comprised of several components--anode, cathode, proton exchange membrane, catalyst, fuel supply system and oxygen supply system. The basic principle of DMFC is reacting methanol and water electrochemically and catalytically at the anode to generate carbon dioxide, protons and electrons. The equation can be shown as CH3OH+H2O→CO2+6H++6e-. At the cathode, the oxygen is oxidised reacting with protons to form water. The electrons released at the anode are transported into the external circuit and thus electric current is produced. Electrons also combine with protons and oxygen to form water. The equation can be shown as 3/2O2+6H++6e-→3H2O. The DMFC must use an acidic electrolyte to assist the carbon dioxide elimination, because insoluble carbonates are produced in the electrolytes. The overall reaction can be shown as CH3OH+3/2O2→CO2+2H2O [13].
Mostly, platinum or platinum-based alloys are used as the catalyst for the DMFC, since it has extremely superior catalytic performance. It is suitable for catalyzing the reaction between methanol and oxygen under a low temperature. Platinum is a good metal conductor, in favour of the burst transmission of electrons. This enhances the cell input efficiency. In addition, under acidic condition (Nafion film), platinum is not prone to be subject to corrosion and has favourable chemical inertness and durability. However, during the oxidation of methanol, the intermediate product carbon monoxide will adsorb on the surface of platinum, causing CO poisoning. Also, platinum metal belongs to noble metal since the resource is scarce. This would lead to an increase in the cost of fuel cell system.
There are still some challenges faced by DMFCs. Methanol crossover takes place when methanol molecules spread through the membrane and are oxidised by oxygen right away. This is a severe issue since the voltage is cut down. The current density and fuel are also affected and therefore the DMFC performance is undermined. Moreover, the commercialization of DMFC is suffered by great pressure. Lithium-based cell ranks in a dominating position in terms of portable devices and energy storage. PEMFC makes a good performance through high efficiency [14].
3.3. Solid oxide fuel cell
Solid oxide fuel cell, just as it names implies, employ inorganic oxide as the electrolyte, mostly yttria-stabilized zirconia, instead of liquid electrolyte. It has the ability to utilize various types of fuel, including hydrogen gas, natural gas, methanol and even coal gas, to generate electricity. The core principle of SOFC is carrying out electrochemical reactions through the conduction of oxygen ions in solid electrolytes under upper temperature, ranging from 600 to 1000 degrees approximately. At the cathode, oxygen molecules in air adsorb onto the surface of the cathode so as to form oxygen ions. The equation can be demonstrated by 1/2O2+2e-→O2-. The electrolytes will block electrons and only permit the conduction of oxygen ions. At the anode, oxygen ions combine with fuel, such as hydrogen gas, to make the oxidation occur. Water is formed with the emission of electrons. The equation can be displayed as H2+O2-→H2O+2e-. The overall reactions can be shown as H2+1/2O2→H2O+electrical energy + thermal energy. The electrons released from the anode cannot pass through the electrolyte so they can only flow into the cathode through the external circuit to produce electricity [15].
Solid oxide fuel cells involve a wide range of advantages compared with conventional fuel cells. The electricity-to-heat conversion efficiency is high, roughly 50% to 60% individually. Some SOFCs are triumphantly applied as combustors in gas turbines. This type of SOFCs could acquire the efficiencies of 70%. Low material cost is acceptable since SOFCs commonly utilize ceramics or normal metals such as nickel, LSM, etc. as the electrode materials. There are very few emissions of pollutants, namely sulfur oxides and nitrogen oxides, which is environmentally friendly. The operation is noiseless because the oscillation is slight [16].
In contrast, a number of research and papers have referred to the issue in respect of the challenges and shortage presented in SOFCs. The operation of SOFCs require an elevated temperature, generally from 600 to 1000 degrees. As a result, the demand for the raw material becomes relatively high, which needs material with high temperature resistance. The coefficient of difficulty for manufacturing will be greatly leveled up. Recently, review papers show that the gross challenges for the marketization of SOFCs are lowering the cost and improving the effectiveness of fuel cell system. More research should be carried out to balance the operation temperature with the efficiency and total cost [17].
Several applications are available through employing SOFCs. They are suitable for combined heat and power generation. Many infrastructures, such as communities, hospitals, schools and industrial estates, are likely to use distribution power and heat by SOFCs. The utilization of gas turbines is also renowned in only theories, otherwise some has been used in pressurized running. Hence, large potential has been present in SOFCs and it is feasible to be commercialized widely in the near future [18].
4. The development and prospect of fuel cells
Fuel cells have been regarded as environmentally friendly and efficient technologies. It is occupying a larger proportion of energy system gradually. The research has demonstrated that hydrogen energy and hydrogen fuel cells technique can be expected to apply in vehicles, portable electronic devices and fixed power station and other fields. They are also prepared to become the major advanced technology in aerospace technology and ship power system. However, some problems are still faced, cost, structural compactness, stack durability, reliability and power stability. The general strategies for promoting the development of fuel cell would be very essential. Significantly, the orientation of the development is to remain clean and highly efficient. What is more, the adaptability to multiple fuels helps apply in various places and industries. In addition, coupling with renewable energy can achieve more environmentally friendly closed cycle system.
5. Conclusion
The article’s research on different types of fuel cell systems shows that current fuel cell technologies have formed the pattern dominated by the hydrogen fuel cells (PEMFC), supplemented by other types of fuel cells, such as SOFC, DMFC, etc. Fuel cells exhibit the own superiority of high efficiency and environmental protection performance. PEMFC takes up the majority of the fuel cell market, but it is still faced by some challenges. The commercialization of PEMFC confronts some risks, such as low publicity rate. Direct methanol fuel cells possess some advantages on the aspect of the operation temperature and high energy density. However, it is necessary to overcome the efficiency bottleneck in order to expand its market. Solid oxide fuel cells and other types of fuel cells demonstrate enormous potential, yet the industrialization and commercialization are still affected by some companies and costs in any aspect.
Hence, in the future, the industry of fuel cells must enhance its basic research and development so that the relationship between structure performance and cost consideration can be deepened for people to comprehend. Emphasis should be placed on cooperation among industry, academia and research, since this can accelerate the transformation from experimental research results to the practical applications. Meanwhile, the promotion for developing fuel cells is conducive to the green environment and sustainable development.
The essay has concluded the three main kinds of fuel cells, which can be useful for the future research. The content shows the basis of fuel cell principles and different structures and their properties. Fuel cells provide an alternative route for energy distribution, particularly contributing to the environment. Furthermore, the use of fuel cells can reduce the degree of dependency on the fossil fuels. Fuel cells can be regarded as one of the most significant components of new generation of energy. This essay can be used for reference to other researchers.
References
[1]. Lucia, U. (2014). Overview on fuel cells. Renewable and Sustainable Energy Reviews, 30, 164-169.
[2]. Qasem, N. A., & Abdulrahman, G. A. (2024). A recent comprehensive review of fuel cells: history, types, and applications. International Journal of Energy Research, 2024(1), 7271748.
[3]. Stambouli, A. B., & Traversa, E. (2002). Fuel cells, an alternative to standard sources of energy. Renewable and sustainable energy reviews, 6(3), 295-304.
[4]. Giorgi, L., & Leccese, F. (2013). Fuel cells: Technologies and applications. The Open Fuel Cells Journal, 6(1), 1-20.
[5]. Haile, S. M. (2003). Materials for fuel cells. Materials today, 6(3), 24-29.
[6]. Ali, A. B., Nemah, A. K., & Al Bahadli, Y. A. (2024). Principles and performance and types, advantages and disadvantages of fuel cells: A review. Case jStudies in Chemical and Environmental Engineering, 10, 100920.
[7]. Bose, S., Kuila, T., Nguyen, T. X. H., Kim, N. H., Lau, K. T., & Lee, J. H. (2011). Polymer membranes for high temperature proton exchange membrane fuel cell: Recent advances and challenges. Progress in Polymer Science, 36(6), 813-843.
[8]. Ye, D. H., & Zhan, Z. G. (2013). A review on the sealing structures of membrane electrode assembly of proton exchange membrane fuel cells. Journal of power sources, 231, 285-292.
[9]. Zhao, J., Liu, H., & Li, X. (2023). Structure, property, and performance of catalyst layers in proton exchange membrane fuel cells. Electrochemical Energy Reviews, 6(1), 13.
[10]. Dafalla, A. M., Wei, L., Habte, B. T., Guo, J., & Jiang, F. (2022). Membrane electrode assembly degradation modeling of proton exchange membrane fuel cells: A review. Energies, 15(23), 9247.
[11]. Wee, J. H. (2007). Applications of proton exchange membrane fuel cell systems. Renewable and sustainable energy reviews, 11(8), 1720-1738.
[12]. Alias, M. S., Kamarudin, S. K., Zainoodin, A. M., & Masdar, M. S. (2020). Active direct methanol fuel cell: An overview. International Journal of Hydrogen Energy, 45(38), 19620-19641.
[13]. Hogarth, M. P., & Hards, G. A. (1996). Direct methanol fuel cells. Platinum Metals Review, 40(4), 150-159.
[14]. Kamarudin, S. K., Achmad, F., & Daud, W. R. W. (2009). Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices. International Journal of hydrogen energy, 34(16), 6902-6916.
[15]. Ormerod, R. M. (2003). Solid oxide fuel cells. Chemical Society Reviews, 32(1), 17-28.
[16]. Singhal, S. C. (2000). Advances in solid oxide fuel cell technology. Solid state ionics, 135(1-4), 305-313.
[17]. Choudhury, A., Chandra, H., & Arora, A. (2013). Application of solid oxide fuel cell technology for power generation—A review. Renewable and Sustainable Energy Reviews, 20, 430-442.
[18]. Laosiripojana, N., Wiyaratn, W., Kiatkittipong, W., Arpornwichanop, A., Soottitantawat, A., & Assabumrungrat, S. (2009). Reviews on solid oxide fuel cell technology. Engineering Journal, 13(1), 65-84.
Cite this article
Liu,Y. (2025). Research Progress of Various Types of Fuel Cells. Applied and Computational Engineering,188,230-237.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of CONF-MCEE 2026 Symposium: Advances in Sustainable Aviation and Aerospace Vehicle Automation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Lucia, U. (2014). Overview on fuel cells. Renewable and Sustainable Energy Reviews, 30, 164-169.
[2]. Qasem, N. A., & Abdulrahman, G. A. (2024). A recent comprehensive review of fuel cells: history, types, and applications. International Journal of Energy Research, 2024(1), 7271748.
[3]. Stambouli, A. B., & Traversa, E. (2002). Fuel cells, an alternative to standard sources of energy. Renewable and sustainable energy reviews, 6(3), 295-304.
[4]. Giorgi, L., & Leccese, F. (2013). Fuel cells: Technologies and applications. The Open Fuel Cells Journal, 6(1), 1-20.
[5]. Haile, S. M. (2003). Materials for fuel cells. Materials today, 6(3), 24-29.
[6]. Ali, A. B., Nemah, A. K., & Al Bahadli, Y. A. (2024). Principles and performance and types, advantages and disadvantages of fuel cells: A review. Case jStudies in Chemical and Environmental Engineering, 10, 100920.
[7]. Bose, S., Kuila, T., Nguyen, T. X. H., Kim, N. H., Lau, K. T., & Lee, J. H. (2011). Polymer membranes for high temperature proton exchange membrane fuel cell: Recent advances and challenges. Progress in Polymer Science, 36(6), 813-843.
[8]. Ye, D. H., & Zhan, Z. G. (2013). A review on the sealing structures of membrane electrode assembly of proton exchange membrane fuel cells. Journal of power sources, 231, 285-292.
[9]. Zhao, J., Liu, H., & Li, X. (2023). Structure, property, and performance of catalyst layers in proton exchange membrane fuel cells. Electrochemical Energy Reviews, 6(1), 13.
[10]. Dafalla, A. M., Wei, L., Habte, B. T., Guo, J., & Jiang, F. (2022). Membrane electrode assembly degradation modeling of proton exchange membrane fuel cells: A review. Energies, 15(23), 9247.
[11]. Wee, J. H. (2007). Applications of proton exchange membrane fuel cell systems. Renewable and sustainable energy reviews, 11(8), 1720-1738.
[12]. Alias, M. S., Kamarudin, S. K., Zainoodin, A. M., & Masdar, M. S. (2020). Active direct methanol fuel cell: An overview. International Journal of Hydrogen Energy, 45(38), 19620-19641.
[13]. Hogarth, M. P., & Hards, G. A. (1996). Direct methanol fuel cells. Platinum Metals Review, 40(4), 150-159.
[14]. Kamarudin, S. K., Achmad, F., & Daud, W. R. W. (2009). Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices. International Journal of hydrogen energy, 34(16), 6902-6916.
[15]. Ormerod, R. M. (2003). Solid oxide fuel cells. Chemical Society Reviews, 32(1), 17-28.
[16]. Singhal, S. C. (2000). Advances in solid oxide fuel cell technology. Solid state ionics, 135(1-4), 305-313.
[17]. Choudhury, A., Chandra, H., & Arora, A. (2013). Application of solid oxide fuel cell technology for power generation—A review. Renewable and Sustainable Energy Reviews, 20, 430-442.
[18]. Laosiripojana, N., Wiyaratn, W., Kiatkittipong, W., Arpornwichanop, A., Soottitantawat, A., & Assabumrungrat, S. (2009). Reviews on solid oxide fuel cell technology. Engineering Journal, 13(1), 65-84.









