1. Introduction
In the past decades, fossil fuels have been overexploited and abused to satisfy the rapid expansion of global production. This phenomenon has led to the depletion of fossil fuel, further casuing an energy crisis and severe environmental pollution. In such severe situation, society is compelled to accelerate the speed of energy transition. Consequently, in order to alleviate the energy crisis, people try to use electric energy instead of fossil fuels. Then, the lithium batteries are created by scientists. Lithium batteries are highly efficient energy storage devices and they play a crucial role across various sectors in our society [1]. In the electric devices and vehicles field, they are preferred choices for application because of their advantages of high energy density, long cycle life and low self-discharge rate. The advancement of lithium batteries accelerates the miniaturization and intelligent development of electronic equipment and provid great support for the widespread adoption. With demands for increased energy density and performance continue to rise, traditional graphite anodes are not available to bear these requirements.
For the purpose of enhancing the performance of lithium batteries, researchers continue to seek new anode materials. As it is commonly known that traditional graphite anodes (with a theoretical capacity of approximately 372 mAh/g) struggle to meet demands for higher energy density. In contrast, silicon-based anode materials can solve this problem due to their extremely high theoretical specific capacity (approximately 3600–4200 mAh/g, ten times that of graphite) [2]. Besides, silicon's abundant resources and low cost also increase its potential for application in next-generation lithium batteries. However, there are several problems need to be solved before silicon anodes realizing large-scale adoption and the most critical one is the substantial volume expansion during cycling. In the process of lithiation, silicon gets a volume expansion of up to 300–400% , and this may lead to bad consequences including material structural failure, electrode pulverization, and continuous cracking and rebuilding of the SEI film. Furthermore, the lithium battery will generate capacity decay and cycling performance degradation. Simultaneously, the repeated formation of the SEI film causes reduced initial coulombic efficiency and lithium loss [3].
Given that the morphology of silicon anodes is a critical factor in enhancing their performance, this paper focuses on the morphology of silicon anodes in lithium batteries. The paper aims to summarize the characteristics and properties of silicon anode materials with different morphologies and investigate their impact on energy storage performance. This paper reviews several common morphologies of silicon anodes and proposes recommendations for the future development of silicon anode materials, laying the foundation for the research and development of lithium batteries with high energy density and extended service life.
2. Advances and advantages and disadvantages of nano-silicon anode materials
The mechanism of lithium storage in silicon anode materials can be summarized as lithium intercalation and deintercalation onto silicon, with the specific process as follows:
In the processes of lithium batteries lithiation and delithiation, silicon undergoes a series of complex transformation. In the initial state, an amorphous lithium-silicon alloy (LixSi) is formed by crystalline silicon (Si) reacting with lithium ions (Li+) and electrons (e-) and it significantly enhances the battery’s specific capacity. Then the remaining crystalline silicon reacts with more lithium ions and electrons to form crystalline lithium-silicon alloy (Li15Si4), as the lithiation continues. Next, when reaction comes to the stage of delithiation, the crystalline Li₁₅Si₄ partly transforms into amorphous silicon and releases lithium ions and electrons. There may be some Li₁₅Si₁₄ left. The whole process of transition determines the battery's cycle stability and capacity retention rate, so researchers focus on the mechanism in silicon-based lithium battery researches [4]. The reason why silicon anodes are able to deliver a specific capacity far exceeding that of graphite is that silicon forms a series of non-stoichiometric lithium-silicon alloys (LiₓSi, 0 < x ≤ 4.4) in lithiation. The silicon undergoes a transformation from amorphous silicon to various lithiated silicon phases, but it also causes volume expansion reaching 300–400%. The expansion is significantly higher than graphite’s, which is less than 10%. Such drastic volume changes lead to electrode pulverization and failure of contact between active materials and current collectors. Simultaneously, the repeated formation and rupture of the solid electrolyte interphase film causes low initial coulombic efficiency and rapid capacity decay [5].
Over the past decade, researchers have conducted extensive studies on the performance limitations of silicon anode materials. Latest research findings indicate that the development of silicon-based anodes primarily focuses on the following directions: First, reducing particle size through nanoscale processing to minimize fracture risks during cycling; second, utilizing porous or core-shell structures to buffer volume expansion and contraction [6]; third, enhancing electron conductivity and interface stability via carbon materials, conductive polymers, or metal coatings; fourth, mitigating low initial coulombic efficiency through methods such as pre-lithiation, electrolyte additives, and engineered SEI films [7]. Despite significant progress in research, the core issues of volume expansion and structural failure in silicon anodes remain prominent and challenging to resolve. These factors are also key contributors to the degradation of lithium battery cycle performance. Therefore, achieving a combination of high capacity and excellent stability has become a critical task in the development of silicon anode materials.
On this background, nanoscale silicon anode materials have emerged as a fundamental and crucial solution. The silicon nanomaterials primarily discussed in this paper include silicon nanoparticles, silicon nanowires/nanotubes, porous bulk silicon, and amorphous silicon films. Through nanoscale size effects, structural buffering, and composite integration with other materials, these structures significantly enhance the cycle life, interfacial stability, and rate performance of lithium-ion batteries [8].
2.1. Silicon nanoparticles
Silicon nanoparticles refer to silicon particles within a specific size range, typically measuring tens to hundreds of nanometers. Compared to conventional silicon particles, they exhibit a significantly increased specific surface area, substantially shortening lithium diffusion pathways within batteries. Additionally, the size effect of silicon nanoparticles helps mitigate stress concentration issues during lithiation, substantially reducing the risk of particle fracture and pulverization during charge-discharge cycles. As a result, the stability of lithium batteries will be improved and their cycle life will extend as well. As shown in Figure 1, multiple theoretical studies and experiments have demonstrated 150nm is the boundary diameter. When particle diameters are below approximately 150 nm, the fracture probability of silicon during lithium insertion decreases significantly.
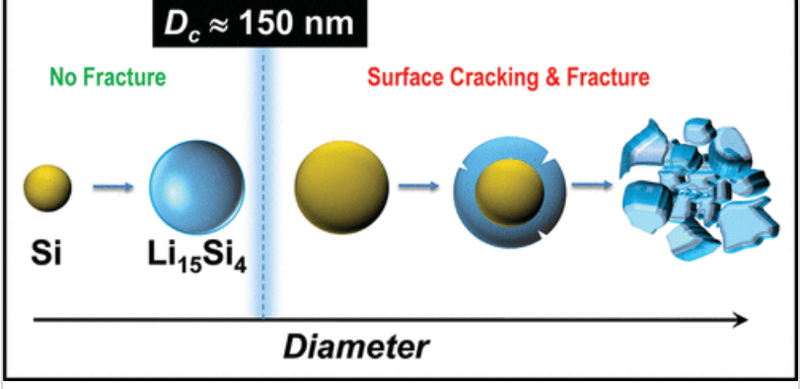
Moreover, nanoparticles can be tightly composite with conductive materials such as graphene, carbon nanotubes, or amorphous carbon. Under the action of elastic polymer binders, they form continuous electronic networks to enhance the mechanical toughness and electrical conductivity of electrodes [9]. However, silicon nanoparticles also present certain limitations. Their high specific surface area accelerates electrolyte decomposition reactions, leading to the continuous formation and rupture of the solid electrolyte interphase (SEI) film. This reduces the initial coulombic efficiency and increases the irreversible consumption of lithium during cycling. At the same time, the loose packing of nanoscale silicon particles results in low electrode packing density, diminishing the battery's volumetric energy density. Additionally, the complex and costly fabrication process for high-purity nanoscale silicon particles severely constrains its large-scale application [10].
2.2. Silicon nanotubes and nanowires
Silicon nanotubes (nanowires) represent a typical one-dimensional hollow nanostructure, typically composed of a series of silicon atoms forming the outer wall, with the internal void space creating a cavity. Silicon nanotubes can be fabricated through methods such as template-assisted deposition or chemical vapor deposition. Their core advantage lies in the hollow cavity providing effective buffer space for volume expansion, significantly reducing structural fractures caused by volume expansion during lithiation. This results in a more stable surface-electrochemical interfacial (SEI) layer. Moreover, the axial continuity of silicon nanotubes (nanowires) provides a continuous pathway for the transport of electrons and lithium ions, enabling silicon nanotubes to exhibit outstanding rate performance. Kwon et al. [11] utilized a composite strategy to confine silicon particles within carbon nanotube channels, significantly improving cycle life and structural integrity while maintaining high specific capacity.
However, the one-dimensional hollow structure results in high porosity and insufficient compactness of silicon nanotubes (nanowires), limiting their ability to enhance volumetric energy density in batteries. Furthermore, their interfacial surfaces may still accumulate side reactions during long-term cycling, leading to increased polarization [12]. What is more, large-scale production applications cannot realize under current manufacturing processes because the synthesis ways for silicon nanotubes (and nanowires) are complex and heavily reliant on high-temperature or precision templating techniques. This results in high energy consumption and significant production costs so it is difficult to meet the demands of mass production. Therefore, silicon nanotubes or nanowires are more suitable for flexible devices, high-power batteries and fundamental mechanism research. The silicon nanotubes or nanowires are able to have the opportunity for widespread application in lithium batteries only if major breakthroughs come out in manufacturing.
2.3. Porous bulk silicon
The structural design principle of porous bulk silicon resembles that of silicon nanotubes (nanowires). By introducing porous structures or hierarchical pore channels into silicon particles at the micrometer to sub-micrometer scale, nanoscale buffer spaces can form within the particles. This structure enables enough space for volume expansion and enhances packing density so porous bulk silicon is a promising silicon nanomaterial for applications [13]. Additionally, cycle stability and volumetric energy density of bulk silicon are significantly improved by using hierarchical design.
Also, bulk silicon will get superior comprehensive properties through incorporating other materials to form composites in recent researches. For instance, silicon-carbon bulk composites prepared from biomass or polymer precursors exhibit high specific capacity and excellent cycle life. The composites show great potential in large-scale production and applications for their low cost. Additionally, porous bulk silicon can be combined with graphite or carbon-based frameworks to form composite systems. The composites are enhanced in mechanical toughness and electrical conductivity.
Nevertheless, this material still exhibits certain limitations. Similar to one-dimensional silicon nanotubes, the porous structure of multi-void bulk silicon inevitably increases its specific surface area, leading to higher reactivity to side reactions and lower first-cycle Coulombic efficiency [14]. What is more, due to the layered pores in bulk silicon, cracks may propagate along the pore direction toward the particle surface during cycling, ultimately causing structural failure. The performance characteristics is determined by the distribution of pores in bulk silicon structures and the consistency of their granular structure. Only when the consistency is high, the bulk silicon exhibit excellent compaction density and expansion buffering properties [15]. In laboratory settings, bulk silicon with consistent pore size distribution and granular structure can be prepared through deliberate intervention and control. However, achieving large-scale production applications requires breakthroughs in the stability of the fabrication process.
2.4. Silicon nano film
Silicon nano films are two-dimensional nanomaterials with thicknesses ranging from 1 to 100 nanometers which are usually fabricated by methods of physical vapor deposition (PVD), chemical vapor deposition (CVD), and sputtering. They have advantages in releasing the volume expansion and contraction of silicon materials during electrochemical cycling. Silicon nano films owns unique layered structure and the specific morphology enable them to effectively accommodate the stresses induced by volume expansion during lithium battery charging. As a result, the mechanical integrity of the electrode can be maintained for a long time. Li et al. [16] revealed the volumetric expansion characteristics of silicon thin-film electrodes during lithiation. The research found that in lithiation, silicon's volumetric expansion occurs perpendicular to the film surface, while in delithiation, lithium's volumetric contraction occurs both in the plane and perpendicular of the film.
Among various forms of silicon, amorphous silicon (a-Si) is frequently used as a model material for studying lithiumation mechanisms due to its structural uniformity and isotropy. Amorphous silicon relatively enables stress distribution and maintains good structural integrity during cycling. Research indicates that through hydrogenation and doping control, a-Si:H films can achieve high specific capacity and outstanding cycling stability, which are outstanding performances in applications in small power sources and sensors [17]. As it is presented by the FESEM images in Figure 2, the aSi:H(i) films are strongly attached to the rough side of the Cu foil in order to improve the conductivity of Cu.
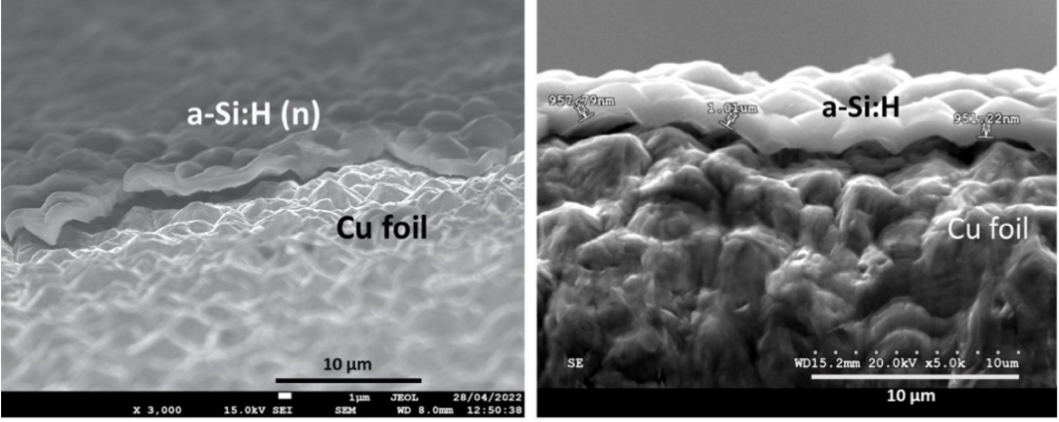
Furthermore, the tight integration between silicon thin films and current collectors provides a direct path for electrons, reducing interfacial contact resistance and making them suitable for high-rate charging and discharging applications. Research has introduced that diamond-like carbon layers onto the film surface to suppress cracking and powdering can enhance the mechanical properties.However, the limited areal capacity of thin-film materials fails to meet high-capacity demands in energy-intensive applications like batteries. Moreover, their deposition processes are costly, hindering large-scale implementation. Increasing film thickness to boost capacity often leads to cracking and delamination, further limiting their development [18]. Consequently, silicon nanolayers remain more suitable as candidate materials for fundamental research and miniature energy devices.
3. Prospects and recommendations for silicon anode materials
Silicon-based anodes are key materials for next-generation high-energy-density lithium batteries because their unique capacity advantages and abundant resources ensure promising development prospects. However, there are still several challenges such as volume expansion, low initial coulombic efficiency, and complex large-scale manufacturing processes waiting us to deal with. Future research and industrial applications of silicon anode materials should advance simultaneously through technological innovation and cost control. Silicon anode materials can combine the morphological advantages of nanoparticles, nanotubes, nano bulks, and nanolayers through multiscale design to construct composite structures. This approach effectively alleviates volume stress caused by lithium battery expansion and enhances structural integrity. Besides, researchers can find some new ways in binders and current collectors, artificial solid electrolyte interphase films, functionalized electrolyte additives, and pre-lithiation strategies. In this way, the properties in initial coulombic efficiency, cycle life, and cell stability of batteries will gain great improvement. In addition, finding low-cost silicon sources and environmentally sustainable preparation processes is also important. In the future, biomass precursor conversion, metallurgical silicon byproduct purification, and sol-gel methods may be the key directions. Through these methods, high-purity nano silicon can be highly optimized and complex deposition processes will be possible to meet industrial production demands. What is more, reducing costs is always the best way for silicon anode materials to achieve the large-scale production application.
As for future development trend, it is evident that silicon-based anodes will gradually transform from exploration of nanostructures to the development of macroscopically controllable layered structures and silicon-carbon composite materials, which will improve lithium battery’s energy density. Silicon anodes will enter the stage of large-scale application and emerge as one of the core materials propelling the advancement of electric vehicles and renewable energy storage as soon as manufacturing processes and industrial chain becomes mature.
4. Conclusion
This article systematically summarizes the lithium storage mechanism, performance advantages, and key challenges of silicon anode materials in lithium batteries. Amounts of researches indicate that silicon can form lithium-silicon phases through alloying reactions with lithium ions and achieve a theoretical specific capacity far exceeding that of graphite. However, volume expansion and structural failure are the core problems limiting its cycling stability. Researchers have mentioned silicon nanomaterials with diverse morphologies and explored their advantages and limitations during the exploration.
Silicon nanoparticles reduce fracture risk through size effects, but their high specific surface area leads to decreased first-cycle coulombic efficiency. Silicon nanotubes and nanowires utilize their hollow structure to buffer volume changes and exhibit outstanding performance in cycling characteristics, but their preparation is complex and costly. Porous bulk silicon combines high compaction density with controlled volume expansion release capability, presenting promising prospects for industrialization. Nevertheless, challenges remain in achieving consistent pore size distribution and structural uniformity. Silicon nano films exhibit excellent mechanical integrity and strain tolerance due to their uniform structure and tight coupling with current collectors. They are primarily used for mechanism studies and miniature energy devices due to the constrain by low areal loading and high cost.
Overall, researches on silicon anodes has made significant progress in nanostructure design, material composites, and interface control. However, there remains a gap before achieving commercial-scale application. This paper reviews and summarizes silicon anode materials with different morphologies, proposing several promising research directions for silicon anode materials in future lithium batteries. Researchers can combine silicon nanomaterials with different morphologies in their designs, or improve other aspects of the battery, such as employing engineered SEI films, adding electrolyte additives, and performing electrode pre-lithiation. Also, exploring low-cost silicon sources and environmentally sustainable preparation processes is crucial for the industrial application of silicon anode materials. In the long run, with the optimization of production processes and the refinement of industrial chains, silicon anode materials are expected to gradually move beyond the laboratory setting and emerge as a core energy storage material driving the advancement of electric vehicles and renewable energy.
References
[1]. Li X.R., Cao K., Zhao X.Z. (2025). Progress in silicon/carbon based negative electrode materials by CVD method for Li-ion batteries. Materials Engineering, 53(07): 83-93. https: //doi.org/10.11868/j.issn.1001-4381.2024.000871
[2]. Reslan J, Saadaoui M, Djenizian T. (2024). Synthesis and Structural Design of Graphene, Silicon and Silicon‐Based Materials Including Incorporation of Graphene as Anode to Improve Electrochemical Performance in Lithium‐Ion Batteries. Advanced Materials Interfaces, 11(19): 2301062. https: //doi.org/10.1002/admi.202301062
[3]. Xia Y M, Liu Z, Chang Z H, (2023). Calendar aging mechanism of NCM811/graphite-SiOx pouch cells at different temperatures. Journal of Materials Engineering, 51 (9):148-157. https: //doi.org/10.11868/j.issn.1001-4381.2022.000351
[4]. Sun G.Q., Li H.B., Ding Z.Y. (2025) . Research Progress on Silicon-Based Anode Materials. Journal of Chemical Engineering of China, 25(34): 234532. https: //doi.org/ 10.11949/0438-1157.20241425
[5]. Liu X.H., Zhong L., Huang S. (2012). Size-dependent fracture of silicon nanoparticles during lithiation. ACS Nano, 6(2): 1522–1531. https: //doi.org/10.1021/nn204476h
[6]. Zhang Z, Wu Y, Mo Z. (2025). Research progress of silicon-based anode materials for lithium-ion batteries. RSC advances, 15(14): 10731-10753.https: //doi.org/ 10.1039/D5RA01268F
[7]. Cheng L, Wang Z, Wang T. (2024). Understanding and research progress on the initial coulombic efficiency of silicon-based anodes in lithium-ion batteries. Journal of Electroanalytical Chemistry, 973: 118670. https: //doi.org/10.1016/j.jelechem.2024.118670
[8]. Chen X, Cheng W, Liu H. (2025). Research Progresses on Nano-Structured Silicon-Based Materials as Anode for Lithium-Ion Batteries. Materials, 18(4): 830. https: //doi.org/10.3390/ma18040830
[9]. Fereydooni A, Yue C, Chao Y. (2024). A Brief Overview of Silicon Nanoparticles as Anode Material: A Transition from Lithium‐Ion to Sodium‐Ion Batteries. Small, 20(17): 2307275. https: //doi.org/10.1002/smll.202307275
[10]. Toki G F I, Hossain M K, Rehman W U, (2024). Recent progress and challenges in silicon-based anode materials for lithium-ion batteries. Industrial Chemistry & Materials, 2(2): 226-269.https: //doi.org/ 10.1039/D3IM00115F
[11]. Kwon H J, Hwang J Y, Shin H J, (2019). Nano/microstructured silicon–carbon hybrid composite particles fabricated with corn starch biowaste as anode materials for Li-ion batteries. Nano letters, 20(1): 625-635. https: //doi.org/10.1021/acs.nanolett.9b04395
[12]. Feyzi E, MR A K, Li X, (2024). A comprehensive review of silicon anodes for high-energy lithium-ion batteries: Challenges, latest developments, and perspectives. Next Energy, 5(23): 100176. https: //doi.org/10.1016/j.nxener.2024.100176
[13]. Liang X.D., Ding Y.F. (2024). Research Progress on Silicon-Based Materials for Lithium-Ion Batteries. Ship and Electrical Technology, 44(12): 87–91. https: //doi.org/10.13632/j.meee.2024.12.021
[14]. Feng Z, Peng W, Wang Z. (2021). Review of silicon-based alloys for lithium-ion battery anodes. International Journal of Minerals, Metallurgy and Materials, 28(10): 1549-1564. https: //doi.org/10.1007/s12613-021-2335-x
[15]. Khan M, Yan S, Ali M. (2024). Innovative solutions for high-performance silicon anodes in lithium-ion batteries: overcoming challenges and real-world applications. Nano-Micro Letters, 16(1): 179. https: //doi.org/10.1007/s40820-024-01388-3
[16]. Li J, Dozier A K, Li Y, (2011). Crack pattern formation in thin film lithium-ion battery electrodes. Journal of The Electrochemical Society, 158(6): 689. https: //doi.org/ 10.1149/1.3574027
[17]. González N, García T, Morant C, (2024). Fine-tuning intrinsic and doped hydrogenated amorphous silicon thin-film anodes deposited by PECVD to enhance capacity and stability in lithium-ion batteries. Nanomaterials, 14(2): 204. https: //doi.org/10.3390/nano14020204
[18]. Yu C, Li X, Ma T, (2012). Silicon thin films as anodes for high-performance lithium-ion batteries with effective stress relaxation. Advanced Energy Materials, 2(1): 68-73. https: //doi.org/10.1002/aenm.201100634
Cite this article
Liu,H. (2025). The Influence of Silicon Anode Morphology on Energy Storage Performance in Lithium Batteries. Applied and Computational Engineering,188,303-310.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of CONF-MCEE 2026 Symposium: Advances in Sustainable Aviation and Aerospace Vehicle Automation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Li X.R., Cao K., Zhao X.Z. (2025). Progress in silicon/carbon based negative electrode materials by CVD method for Li-ion batteries. Materials Engineering, 53(07): 83-93. https: //doi.org/10.11868/j.issn.1001-4381.2024.000871
[2]. Reslan J, Saadaoui M, Djenizian T. (2024). Synthesis and Structural Design of Graphene, Silicon and Silicon‐Based Materials Including Incorporation of Graphene as Anode to Improve Electrochemical Performance in Lithium‐Ion Batteries. Advanced Materials Interfaces, 11(19): 2301062. https: //doi.org/10.1002/admi.202301062
[3]. Xia Y M, Liu Z, Chang Z H, (2023). Calendar aging mechanism of NCM811/graphite-SiOx pouch cells at different temperatures. Journal of Materials Engineering, 51 (9):148-157. https: //doi.org/10.11868/j.issn.1001-4381.2022.000351
[4]. Sun G.Q., Li H.B., Ding Z.Y. (2025) . Research Progress on Silicon-Based Anode Materials. Journal of Chemical Engineering of China, 25(34): 234532. https: //doi.org/ 10.11949/0438-1157.20241425
[5]. Liu X.H., Zhong L., Huang S. (2012). Size-dependent fracture of silicon nanoparticles during lithiation. ACS Nano, 6(2): 1522–1531. https: //doi.org/10.1021/nn204476h
[6]. Zhang Z, Wu Y, Mo Z. (2025). Research progress of silicon-based anode materials for lithium-ion batteries. RSC advances, 15(14): 10731-10753.https: //doi.org/ 10.1039/D5RA01268F
[7]. Cheng L, Wang Z, Wang T. (2024). Understanding and research progress on the initial coulombic efficiency of silicon-based anodes in lithium-ion batteries. Journal of Electroanalytical Chemistry, 973: 118670. https: //doi.org/10.1016/j.jelechem.2024.118670
[8]. Chen X, Cheng W, Liu H. (2025). Research Progresses on Nano-Structured Silicon-Based Materials as Anode for Lithium-Ion Batteries. Materials, 18(4): 830. https: //doi.org/10.3390/ma18040830
[9]. Fereydooni A, Yue C, Chao Y. (2024). A Brief Overview of Silicon Nanoparticles as Anode Material: A Transition from Lithium‐Ion to Sodium‐Ion Batteries. Small, 20(17): 2307275. https: //doi.org/10.1002/smll.202307275
[10]. Toki G F I, Hossain M K, Rehman W U, (2024). Recent progress and challenges in silicon-based anode materials for lithium-ion batteries. Industrial Chemistry & Materials, 2(2): 226-269.https: //doi.org/ 10.1039/D3IM00115F
[11]. Kwon H J, Hwang J Y, Shin H J, (2019). Nano/microstructured silicon–carbon hybrid composite particles fabricated with corn starch biowaste as anode materials for Li-ion batteries. Nano letters, 20(1): 625-635. https: //doi.org/10.1021/acs.nanolett.9b04395
[12]. Feyzi E, MR A K, Li X, (2024). A comprehensive review of silicon anodes for high-energy lithium-ion batteries: Challenges, latest developments, and perspectives. Next Energy, 5(23): 100176. https: //doi.org/10.1016/j.nxener.2024.100176
[13]. Liang X.D., Ding Y.F. (2024). Research Progress on Silicon-Based Materials for Lithium-Ion Batteries. Ship and Electrical Technology, 44(12): 87–91. https: //doi.org/10.13632/j.meee.2024.12.021
[14]. Feng Z, Peng W, Wang Z. (2021). Review of silicon-based alloys for lithium-ion battery anodes. International Journal of Minerals, Metallurgy and Materials, 28(10): 1549-1564. https: //doi.org/10.1007/s12613-021-2335-x
[15]. Khan M, Yan S, Ali M. (2024). Innovative solutions for high-performance silicon anodes in lithium-ion batteries: overcoming challenges and real-world applications. Nano-Micro Letters, 16(1): 179. https: //doi.org/10.1007/s40820-024-01388-3
[16]. Li J, Dozier A K, Li Y, (2011). Crack pattern formation in thin film lithium-ion battery electrodes. Journal of The Electrochemical Society, 158(6): 689. https: //doi.org/ 10.1149/1.3574027
[17]. González N, García T, Morant C, (2024). Fine-tuning intrinsic and doped hydrogenated amorphous silicon thin-film anodes deposited by PECVD to enhance capacity and stability in lithium-ion batteries. Nanomaterials, 14(2): 204. https: //doi.org/10.3390/nano14020204
[18]. Yu C, Li X, Ma T, (2012). Silicon thin films as anodes for high-performance lithium-ion batteries with effective stress relaxation. Advanced Energy Materials, 2(1): 68-73. https: //doi.org/10.1002/aenm.201100634









