1. Introduction
DDT, also known as DDT, chemically named dichlorophenyltrichloroethane, is an organochlorine insecticide with the chemical formula C14H9Cl5. It is a white crystal that is insoluble in water but soluble in kerosene, can be made into an emulsion, and is an effective insecticide. It significantly prevented agricultural pests and diseases in the first half of the 20th century and reduced the damage caused by mosquito and fly-borne diseases such as malaria and typhoid. However, many countries and regions have banned its use due to severe environmental pollution. Methoxyddt (MXC), as a structural analogue of DDT, has gradually become a substitute for DDT and is widely used in the control of vegetable and fruit tree pests because it tends to accumulate in body fat or be secreted into milk with little or no tendency. However, it was later found to cause more environmental pollution, and thus it was also regarded as a harmful substance [1]. Molecular docking is one of the common methods for studying the interaction patterns between small and large molecules. Compared with conventional test methods, molecular docking techniques have gradually been applied to study toxic mechanisms due to their advantages of rapidity and convenience. In this study, molecular ligation techniques were used to investigate the interaction mechanisms of DDT and metoxyddt and their metabolites with ERα, and to systematically compare and analyze the endocrine disruption effects of these molecules through key interaction parameters such as binding energy, thereby providing an essential theoretical basis and reference direction for further exploration of their mechanisms of action and risk assessment.
2. Literature review
The interaction of DDT and its substitutes and metabolites with estrogen receptor (ER) is the core for assessing their endocrine disruption effects. Numerous studies confirm that despite DDT bans, health risks from environmental residues persist [2]. Chinese scholars have made significant contributions in this field. For example, a team led by Professor Liu Weiping from Zhejiang University has delved deeply into the interaction mechanism between o,p'-DDT and ER. Molecular docking and kinetic simulations revealed the key binding sites and forces of o,p'-DDT and ERα ligand binding domains. The structural basis of its estrogenic activity was explained from the perspective of computational chemistry. In addition, a team led by Academician Jiang Guibin from the Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, has long focused on the environmental health effects of persistent organic pollutants. Their research confirmed the extensive residues of DDT compounds in environmental media and human samples in China. It systematically evaluated their potential to interact with nuclear receptors (including ER), providing key data for risk assessment. Specifically, o,p'-DDT isomers have been shown to bind directly to ER, miming the action of endogenous estrogen 17β -estradiol (E2), activating downstream gene transcription and stimulating estrogen-dependent cell proliferation [3]. Its main metabolite,p,p'-DDE, has less estrogenic activity. Still, studies have found its role as an androgen receptor antagonist, revealing the mechanism by which it harms health by interfering with other endocrine pathways [4]. Methoxyddt, which is widely used as an alternative, is less active on its own, but its metabolized products in the body, such as HPTE, show more substantial estrogenic effects [1]. Chinese CDC studies confirm DDT metabolite estrogen-like effects and reproductive toxicity in Chinese experimental models. Triclofenitol and its metabolites also interact with ER and induce estrogen-responsive genes [5]. The migration and transformation of these compounds in the environment and their ecological risks are also of great concern, indirectly supporting the issue of the sustainability of their biological activity. These interactions could eventually lead to health risks such as reproductive disorders, developmental abnormalities, and even cancer. For example, exposure to p,p'-DDE during pregnancy is associated with an increased risk of breast cancer in offspring [6]. Fudan's Chen Renjie et al. investigated environmental epidemiology, linking Chinese cohort exposure to persistent organic pollutants like DDT with various health outcomes, offering China-specific evidence on population health impacts.
Current research features mechanistic insights from receptor binding to molecular simulation and epigenetic regulation. Compound studies now include metabolites, environmental products, and mixture effects. Methodologies integrate computational toxicology, high-throughput screening, and in vivo validation. Health assessments focus on chronic and intergenerational effects of low-dose exposure.
3. Materials and methods
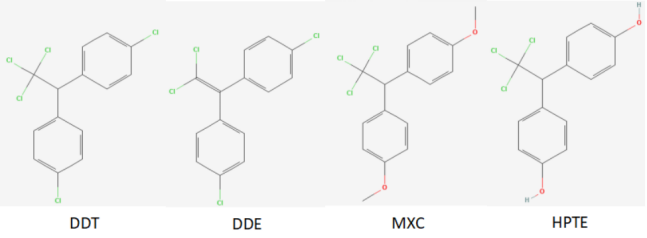
(1)Ligand molecule preparation The ligand molecules selected for docking in this study were DDT, DDE, MXC,2, 2-bis (4-hydroxybenzene) -1,1, 1-trichloroethane (HPTE), with chemical abstract numbers 50-29-3, 72-55-9, 72-43-5, 2971-36-0 respectively, and relative molecular masses They were 354.5, 318.0, 345.6, and 317.6 g/mol respectively, and the molecular structures are shown in Figure 1. Molecular conformations of ligand molecules were obtained from PubChem data and exported in SDF format. Further conformation conversion using Open Babel software to form the target conformation of the ligand molecule required for docking Convert it to MOL2 format and import it into AutoDockTools, a visualization tool in AutoDock 1.5.7 software (Olson Lab, Scripps Research, USA) for preprocessing to make the ligands rotatable during docking and form a crystal conformation that can be edited; Save the conformation file in PDBQT format for further molecular docking studies.
(2)Receptor molecule Preparation: Search the protein database for the ERα(6V8T) receptor's amino acid sequence, compare it with the protein structure database to obtain ERα's 3D conformation. Use PyMOL to display the 3D structure, removing redundant chains, water, and small molecules. Save the refined conformation in PDB format for molecular docking.
(3) Molecular Docking: Receptor molecules, prepared via AutoDock with hydrogenation and charge assignment, are saved as PDBQT files. Grid module analysis identifies potential docking sites, exported as GPF files post-selection. The autogrid module is then executed. Ligand-receptor docking, employing a genetic algorithm, yields probable ERα binding conformations, saved as DPF files. Docking results are ranked, data exported, and files saved in PDBQT format. The optimal docking conformation is chosen based on minimal energy and structural plausibility.
(4)Docking Result Analysis: Visualize docking results in PyMOL and LigPlus to identify optimal ERα binding sites, interaction forces, and key amino acids (Gly, Glu, Pro, Gln, Arg) for each ligand. Display 3D receptor-ligand interactions with red-green docking sites and blurred background. In 2D maps, represent ligands/protein side chains as balls, ligand bonds in purple, hydrogen bonds as green dashed lines, and non-bond contacts as eyelash bonds. Save all analyses.
4. Results and discussions
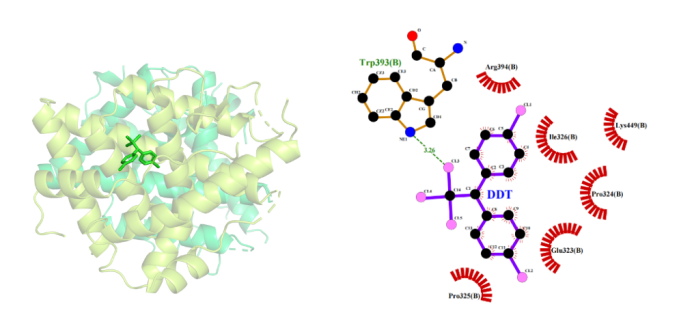
(1)Docking results of DDT to ERα: The optimal binding energy of DDT to Erα is -5.67kcal/mol. The hydrophobic amino acids that provide hydrophobic interaction are Arg394, Ile326, Lys449, Pro324, Glu323, and Pro325 (Figure 2), and the binding information is shown in Table 1.
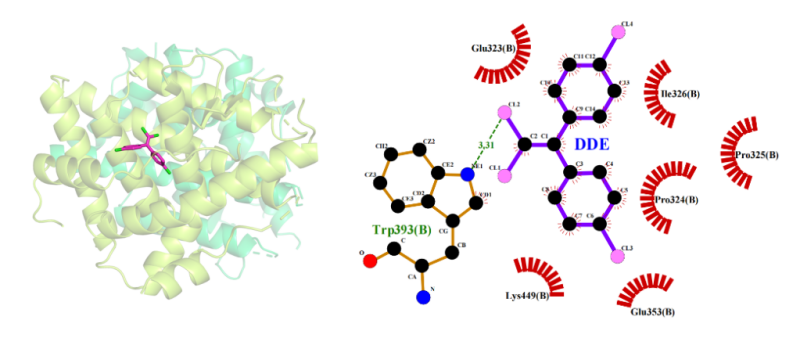
(2) Docking results of DDE to ERα: The optimal binding energy of DDE to Erα is -6.21kcal/mol, and the hydrophobic amino acids providing hydrophobic interaction are Glu323, Glu353, Pro324, Pro325, and Ile326 (Figure 3), and the docking information is shown in Table 1.
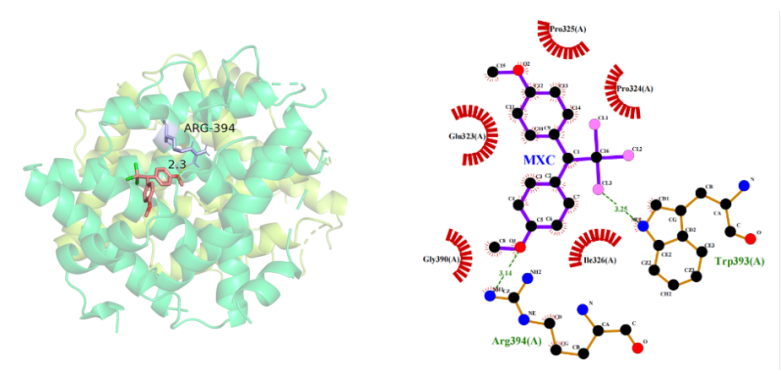
(3) Docking results of MXC with ERα: The optimal binding energy of MXC with Erα is -5.03kcal/mol, and MXC forms A hydrogen bond with the Arg394 residue of Erα at a distance of 3.14 A. The hydrophobic amino acids that provide hydrophobicity are Glu323, Gly390, Ile326, Pro324, and Pro325 (Figure 4), and the docking information is shown in Table 1.
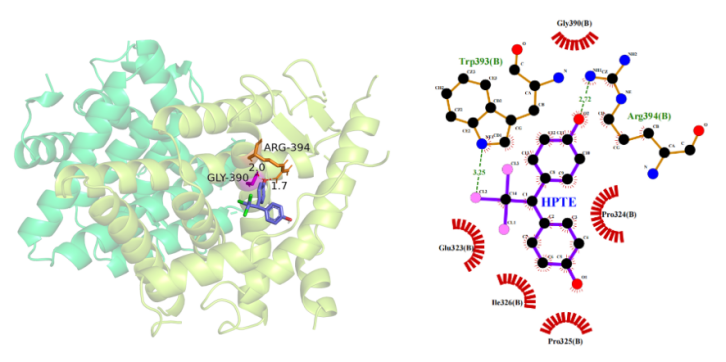
(4) Docking results of HPTE to ERα: The optimal binding energy of HPTE to Erα is -5.48kcal/mol, and HPTE forms A hydrogen bond with the Arg394 residue of Erα at a distance of 2.72 A. The hydrophobic amino acids that provide hydrophobic interaction are Glu323, Gly390, Ile326, Pro324, and Pro325 (Figure 5), and the docking information is shown in Table 1.
|
Optimal binding energy (kcal/mol) |
Hydrophobic amino acids |
Number of hydrogen bonds |
Hydrogen bond distance (A) |
|
|
DDT |
-5.67 |
Arg394, Ile326, Lys449, Pro324, Glu323, Pro325 |
- |
- |
|
DDE |
-6.21 |
Glu323, Glu353, Pro324, Pro325, Ile326, Lys449 |
- |
- |
|
MXC |
-5.03 |
Glu323, Gly390, Ile326, Pro324, Pro325 |
1 |
3.14 |
|
HPTE |
-5.48 |
Glu323, Gly390, Ile326, Pro324, Pro325 |
1 |
2.72 |
(5) Discussion: AutoDock as an efficient automated molecular docking tool, which demonstrates significant application advantages in the early screening of environmental pollutant health risks due to its outstanding computational efficiency and low resource cost [7]. It can rapidly predict the binding mode and affinity of compounds to biological macromolecules before experiments, significantly shortening the research cycle and reducing experimental costs. Unlike AutoDock Vina, AutoDock consists of two main programs: AutoDock performs ligand docking with a set of meshes describing the target protein; Autogrid pre-computes these grids. Ligplot functions similarly to PyMOL and is primarily used to automatically generate multiple two-dimensional interaction plots of ligand-protein interactions from three-dimensional coordinates. These plots depict hydrogen bond interaction patterns and hydrophobic contacts between ligands and proteins' principal or side chain elements. Using the tools above, this study investigated the interaction mechanisms of DDT, DDE, MXC, HPTE and ERα with molecular docking techniques. The results showed that the optimal binding energies of ERα to DDT, DDE, MXC, and HPTE were -5.67, -6.21, -5.03, and -5.48 kcal/mol, respectively, and ERα had sufficient affinity for all four molecules. The binding forces of ERα to the four molecules, from high to low, were DDE>DDT>HPTE>MXC. Among them, HPTE has a stronger affinity for ERα than MXC for ERα, which aligns with the phenomenon that HPTE has a more effective estrogenic effect than MXC. This study demonstrates that AutoDock-based molecular docking is broadly applicable in environmental science, drug development, and food safety. It enables rapid screening and assessment of interactions between emerging pollutants, industrial chemicals, drug candidates, and biological receptors. This facilitates early prediction and prioritization of endocrine-disrupting effects, ecotoxicity, and health risks, offering an efficient computational tool for large-scale compound library risk assessment [8]. This technique's key benefit is its efficient prediction of intermolecular binding and affinities with reduced computational cost and research time, alongside intuitive interaction visualization for mechanistic insights. However, accuracy is limited by force field parameters and scoring functions, static models cannot simulate complex dynamics, and biological relevance requires experimental validation. Thus, it remains an auxiliary tool. Future prospects involve integration with multi-scale methods like AI and molecular dynamics to enhance predictive accuracy and advance computational toxicology in risk assessment. Challenges include simulating complex biological systems, ensuring model repeatability, and promoting computational data acceptance in risk decisions.
5. Conclusion
ERα has sufficient affinity with DDT, DDE, MXC, HPTE, and the binding affinity of DDT and DDE to ERα is mainly provided by the hydrophobic interaction with the non-polar residue of the receptor. MXC and HPTE binding affinity to ERα is primarily supplied by hydrophobic interaction with non-polar receptor residues and hydrogen bond formation with key residues. MXC showed weaker endocrine-disrupting effects than DDT, while HPTE showed more substantial endocrine-disrupting effects than MXC. This study used molecular conjugation techniques to rapidly and cost-effectively reveal at the molecular level the potential binding patterns, interaction types, and relative affinity of DDT and its derivatives to estrogen receptor ERα, providing important theoretical hypotheses and preliminary mechanistic insights for understanding the possible endocrine disruption mechanisms of these environmental pollutants Especially in the assessment of metabolite activity, it demonstrated the unique advantages of computational methods. However, the reliance on computer simulations without experimental validation and uncertain biological relevance necessitates further confirmation of results. Molecular docking's binding energy, a preliminary estimate constrained by scoring function accuracy, does not fully reflect actual biological activity. The strongest binding energy DDE (-6.21 kcal/mol) contrasts with its reported weak estrogenic activity [9]. The author speculates that its binding conformation may not be conducive to recruiting coactivators, or that although it binds strongly, it may trigger antagonistic effects. This will need to be explored through more advanced simulations such as molecular dynamics.
References
[1]. Cummings, A. M. (1997). Methoxychlor as a model for environmental estrogens. Critical reviews in toxicology, 27(4), 367-379.
[2]. Turusov, V., Rakitsky, V., & Tomatis, L. (2002). Dichlorodiphenyltrichloroethane (DDT): ubiquity, persistence, and risks. Environmental health perspectives, 110(2), 125-128.
[3]. Amir, S., Shah, S. T. A., Mamoulakis, C., Docea, A. O., Kalantzi, O. I., Zachariou, A., ... & Tsatsakis, A. (2021). Endocrine disruptors acting on estrogen and androgen pathways cause reproductive disorders through multiple mechanisms: a review. International journal of environmental research and public health, 18(4), 1464.
[4]. Luccio-Camelo, D. C., & Prins, G. S. (2011). Disruption of androgen receptor signaling in males by environmental chemicals. The Journal of steroid biochemistry and molecular biology, 127(1-2), 74-82.
[5]. Zhang, C., Schiliro, T., Gea, M., Bianchi, S. Spinello, A., Magistrato, A., ... & Di Nardo, G. (2020). Molecular basis for endocrine disruption by pesticides targeting aromatase and estrogen receptor. International Journal of Environmental Research and Public Health, 17(16), 5664.
[6]. Desaulniers, D., Leingartner, K., Russo, J., Perkins, G., Chittim, B. G., Archer, M. C., ... & Yang, J. (2001). Modulatory effects of neonatal exposure to TCDD, or a mixture of PCBs, p, p'-DDT, and pp'-DDE, on methylnitrosourea-induced mammary tumor development in the rat. Environmental Health Perspectives, 109(7), 739-747.
[7]. Ishfaq, M., & Shah, S. W. A. (2025). An overview of computational tools and approaches for green molecular design to minimize toxicological risk in chemical compounds. Computational Methods in Medicinal Chemistry, Pharmacology, and Toxicology, 223-238.
[8]. Enni, M. A., & Maraj, M. A. A. (2022). IN SILICO DRUG REPURPOSING FOR INFLAMMATORY DISEASES: A SYSTEMATIC REVIEW OF MOLECULAR DOCKING AND VIRTUAL SCREENING STUDIES. American Journal of Advanced Technology and Engineering Solutions, 2(04), 35-64.
[9]. Donohoe, R. M., & Curtis, L. R. (1996). Estrogenic activity of chlordecone, o, p′-DDT and o, p′-DDE in juvenile rainbow trout: induction of vitellogenesis and interaction with hepatic estrogen binding sites. Aquatic toxicology, 36(1-2), 31-52.
Cite this article
Huang,T. (2025). The Interaction Between DDT and Its Substitutes and Metabolites and Estrogen Receptors was Studied Based on Molecular Docking Technology. Applied and Computational Engineering,200,69-75.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of CONF-MCEE 2026 Symposium: Advances in Sustainable Aviation and Aerospace Vehicle Automation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Cummings, A. M. (1997). Methoxychlor as a model for environmental estrogens. Critical reviews in toxicology, 27(4), 367-379.
[2]. Turusov, V., Rakitsky, V., & Tomatis, L. (2002). Dichlorodiphenyltrichloroethane (DDT): ubiquity, persistence, and risks. Environmental health perspectives, 110(2), 125-128.
[3]. Amir, S., Shah, S. T. A., Mamoulakis, C., Docea, A. O., Kalantzi, O. I., Zachariou, A., ... & Tsatsakis, A. (2021). Endocrine disruptors acting on estrogen and androgen pathways cause reproductive disorders through multiple mechanisms: a review. International journal of environmental research and public health, 18(4), 1464.
[4]. Luccio-Camelo, D. C., & Prins, G. S. (2011). Disruption of androgen receptor signaling in males by environmental chemicals. The Journal of steroid biochemistry and molecular biology, 127(1-2), 74-82.
[5]. Zhang, C., Schiliro, T., Gea, M., Bianchi, S. Spinello, A., Magistrato, A., ... & Di Nardo, G. (2020). Molecular basis for endocrine disruption by pesticides targeting aromatase and estrogen receptor. International Journal of Environmental Research and Public Health, 17(16), 5664.
[6]. Desaulniers, D., Leingartner, K., Russo, J., Perkins, G., Chittim, B. G., Archer, M. C., ... & Yang, J. (2001). Modulatory effects of neonatal exposure to TCDD, or a mixture of PCBs, p, p'-DDT, and pp'-DDE, on methylnitrosourea-induced mammary tumor development in the rat. Environmental Health Perspectives, 109(7), 739-747.
[7]. Ishfaq, M., & Shah, S. W. A. (2025). An overview of computational tools and approaches for green molecular design to minimize toxicological risk in chemical compounds. Computational Methods in Medicinal Chemistry, Pharmacology, and Toxicology, 223-238.
[8]. Enni, M. A., & Maraj, M. A. A. (2022). IN SILICO DRUG REPURPOSING FOR INFLAMMATORY DISEASES: A SYSTEMATIC REVIEW OF MOLECULAR DOCKING AND VIRTUAL SCREENING STUDIES. American Journal of Advanced Technology and Engineering Solutions, 2(04), 35-64.
[9]. Donohoe, R. M., & Curtis, L. R. (1996). Estrogenic activity of chlordecone, o, p′-DDT and o, p′-DDE in juvenile rainbow trout: induction of vitellogenesis and interaction with hepatic estrogen binding sites. Aquatic toxicology, 36(1-2), 31-52.









