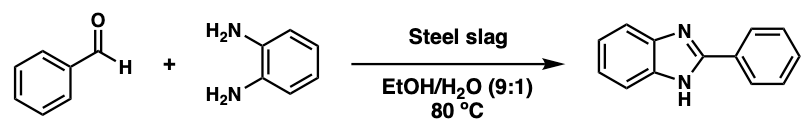
1. Introduction
Benzimidazole derivatives are a privileged class of heterocyclic compounds with broad applications in medicinal chemistry [1-4], agrochemicals [5-6], and materials science [7-8]. Among them, 1H-benzimidazole (1) has attracted particular attention from the organic chemistry community [9]. There are many marketed drugs that contain 1H-benzimidazole motif showing various medical activities (Figure 1).
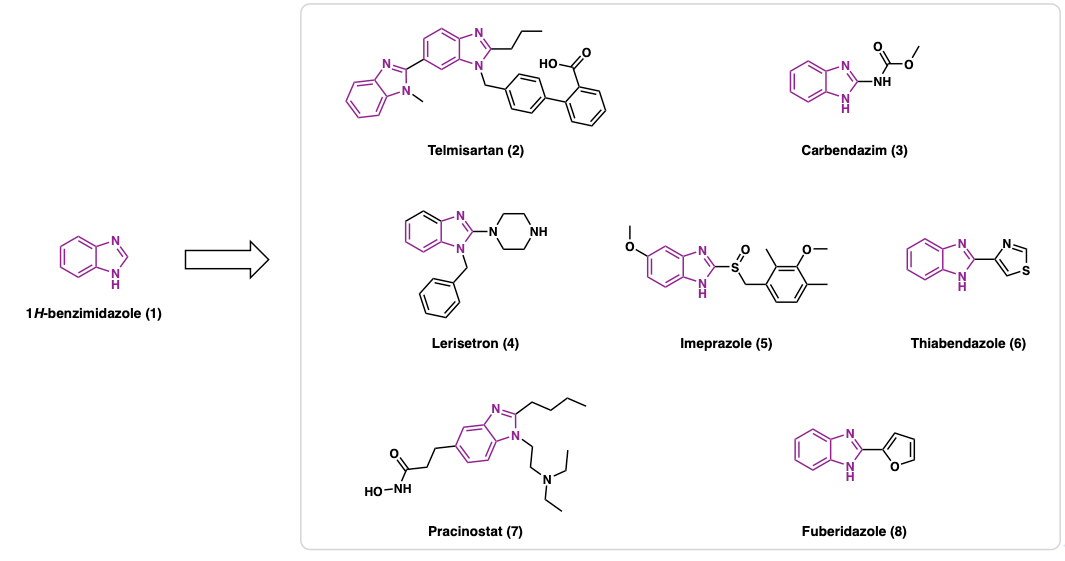
Among the marketed drugs, Telmisartan (2) is an angiotensin II receptor blocker used to treat hypertension and exhibits partial PPAR-γ agonism, and it is used to treat cardiovascular diseases [10].Carbendazim (3) and Fuberidazole (8) are widely used fungicide [11]. Lerisetron (4) is used for managing chemotherapy-induced nausea by blocking serotonin signaling [12]. Omeprazole (5) is a proton pump inhibitor that treats acid reflux by covalently inhibiting the H⁺/K⁺-ATPase in gastric parietal cells [13-14]. Thiabendazole (6) is an antifungal anthelmintic drug [15] and Pracinostat (7) is an histone deacetylase (HDAC) inhibitor under clinical development for breast cancer [16]. Overall, these benzimidazole-based drugs exhibit a wide range of different biological activities and highlight the benzimidazole scaffold’s versatility across therapeutic and agrochemical domains.
There have been many reports on synthetic methods for benzimidazole derivatives [9]. However, most conditions often involve harsh reaction conditions including strong acids [17] and oxidants [18], prolonged reaction times, elevated reaction temperatures or the use of toxic or expensive catalysts, such as Pd [19], In(OTf)3 [20], Zn(OTf)2 [21], Cu-Fe[22], or Pd-Cu[23]. These limitations underscore the need for more sustainable, environmentally friendly and green synthetic methodologies. In recent years, the development of green chemistry approaches has become an important area of research in organic synthesis [24-25], with a particular focus on discovering efficient, recyclable, and low-cost catalyst systems [26]. Steel slag, a byproduct of steel production, is typically considered industrial waste. However, it contains significant amounts of transition metals such as iron and copper, which are known to act as effective Lewis acid catalysts. The valorization of steel slag not only offers a sustainable pathway for waste utilization but also aligns with circular economy principles by converting waste into value-added materials.
In this study, we report a novel and efficient protocol for synthesizing 2-phenyl-1H-benzimidazole using recycled steel slag as a heterogeneous catalyst. The reaction proceeded under mild conditions in an ethanol–water mixture and afforded high yields of the target compound. The catalyst can be easily separated by filtration and reused. This work demonstrates the potential of industrial byproducts as practical catalysts in organic synthesis. The methodology offered an environmentally friendly alternative to conventional methods.
2. Methods
Steel slag was recycled and purchased from Ansteel Iron and Steel Group Corporation in Anshan, LiaoNing, China. Organic reagents and solvents were purchased from Sigma Aldrich. The reactions were monitored by thin-layer chromatography on Merck silica gel plates and by Agilent 6475 Triple Quadrupole LC/MS. 1H NMR was recorded on MR400 Agilent Technology NMR spectrometer using DMSO-d6 as solvent.
3. General procedure for the synthesis of 2-phenyl-1h-benzimidazole.
To a stirred solution of benzaldehyde (48.0 mg, 0.400 mmol) and recycled steel slug (14.4 mg, 30 wt%) in 9:1 EtOH:H2O (5.0 mL), was added o-phenylenediamine (55.9 mg, 0.516 mmol) in one portion. The reaction was stirred at 80 C for 30 minutes open to air. The reaction was cooled down to room temperature. The reaction was filtered over celite. The filtrate was evaporated in vacuo, and the residue was purified by silica chromatography eluting with 0-100% EtOAc in heptane.
4. Results
To optimize the reaction conditions for the synthesis of 2-phenyl-1H-benzimidazole, we investigated the effect of recycled steel slag catalyst loading. Reactions were carried out using benzaldehyde (1 equiv) and o-phenylenediamine (1.3 equiv) in a 9:1 ethanol–water mixture at 80 °C. The yields were determined after purification by silica chromatography (Table 1).
In the absence of any catalyst, the reaction proceeded slowly with moderate yields. The reaction reached 75% yield after 60 minutes (entry 1) and 56% after 30 minutes (entry 2). These two control experiments indicated a slow background reaction. When 10 wt% steel slag was added, the reaction rate improved significantly, affording 83% yield after 60 minutes (entry 3) and 70% after 30 minutes (entry 4). Increasing the catalyst loading to 30 wt% further improved the yield to 85% within 30 minutes (entry 5). The optimal result was obtained with 50 wt% steel slag, which delivered 89% yield in 30 minutes (entry 6). Product was confirmed by comparing with authentic sample (LCMS and 1H NMR).
|
Entry |
Steel Slag (weight %) |
Time (min) |
Yield (%)b |
|
1 |
0 |
60 |
75 |
|
2 |
0 |
30 |
56 |
|
3 |
10 |
60 |
83 |
|
4 |
10 |
30 |
70 |
|
5 |
30 |
30 |
85 |
|
6 |
50 |
30 |
89 |
aReaction condition: Benzaldehyde (1 equiv), o-phenylenediamine (1.3 equiv), 9:1 EtOH:H2O, 80 C. bIsolated yields.
5. Discussion
The results demonstrate the catalytic efficiency of recycled steel slag in promoting the synthesis of 2-phenyl-1H-benzimidazole. In the absence of catalyst, the condensation between benzaldehyde and o-phenylenediamine proceeded slowly, highlighting the need for a catalyst to enhance reaction efficiency. The introduction of steel slag significantly accelerated the reaction, with yields increasing in tandem with catalyst loading.
At 10 wt% loading (entry 4), steel slag provided notable improvement over the background reaction (entry 2). Higher loadings of 30 wt% (entry 5) and 50 wt% (entry 6) further enhanced the yield within a shorter reaction time, with the highest yield (89%) achieved at 50 wt% in just 30 minutes. This trend suggests that the active metals, such as iron and copper, in steel slag function effectively as catalysts, which can facilitate imine formation and subsequent cyclization under mild and aerobic conditions.
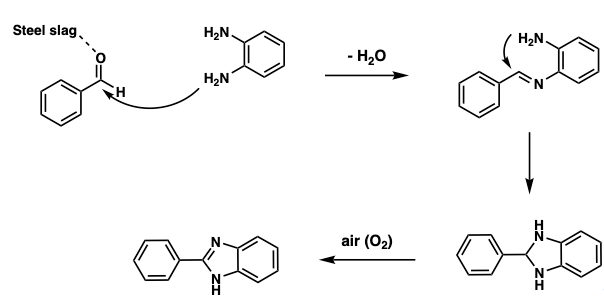
A proposed mechanism for the transformation is described in Scheme 1. The syntheses commenced with Steel slag coordinating with benzaldehyde as a Lewis acid. Then, the benzaldehyde was prone to react with o-phenylenediamine in a condensation reaction with the loss of 1 equivalence of water. As the imine formed, intramolecular attack from the diamine cyclized to form a dihydrobenzoimidazole, which can be easily oxidized in air to yield desired 2-phenyl-1H-benzimidazole.
In addition, the reaction is heterogeneous, and steel slag can be easily removed by filtration. The catalysts may be recycled for future use. These features of steel slag catalysts align with green chemistry principles. In summary, these results highlight steel slag as a low-cost, sustainable, and efficient catalyst for benzimidazole synthesis.
6. Conclusion
In summary, we have developed a green and efficient methodology to synthesize 2-phenyl-1H-benzimidazole using recycled steel slag as catalyst. Steel slag significantly shortened the reaction time and improved yield under mild conditions. Optimal results were achieved with 50 wt% catalyst loading of steel slag, affording 89% yield of 2-phenyl-1H-benzimidazole in 30 minutes. This work not only demonstrates the utility of steel slag in organic synthesis, but also offers a sustainable approach to recycle and reuse industrial waste. The ease of catalyst separation and its potential reusability further support its application in environmentally friendly chemical processes.
References
[1]. P. Barot, K.; Nikolova, S.; Ivanov, I.; D. Ghate, M. Novel Research Strategies of Benzimidazole Derivatives: A Review. Mini-Rev. Med. Chem. 2013, 13 (10), 1421–1447.
[2]. Brishty, S. R.; Hossain, M. J.; Khandaker, M. U.; Faruque, M. R. I.; Osman, H.; Rahman, S. M. A. A Comprehensive Account on Recent Progress in Pharmacological Activities of Benzimidazole Derivatives. Front. Pharmacol. 2021, 12. https: //doi.org/10.3389/fphar.2021.762807.
[3]. Pathare, B.; Bansode, T. Review- Biological Active Benzimidazole Derivatives. Results Chem. 2021, 3, 100200. https: //doi.org/10.1016/j.rechem.2021.100200.
[4]. Rashedy, A. A. E.; Aboul-Enein, H. Y. Benzimidazole Derivatives as Potential Anticancer Agents. Mini Rev. Med. Chem. 2013, 13 (3), 399–407. https: //doi.org/10.2174/138955713804999847.
[5]. Bai, S.; Zhang, M.; Tang, S.; Li, M.; Wu, R.; Wan, S.; Chen, L.; Wei, X.; Li, F. Research Progress on Benzimidazole Fungicides: A Review. Molecules 2024, 29 (6), 1218. https: //doi.org/10.3390/molecules29061218.
[6]. Quaranta, L. Benzimidazole Fungicides. In Bioactive Heterocyclic Compound Classes; John Wiley & Sons, Ltd, 2012; pp 103–118. https: //doi.org/10.1002/9783527664412.ch9.
[7]. Mamada, M.; Pérez-Bolívar, C.; Kumaki, D.; Esipenko, N. A.; Tokito, S.; Anzenbacher Jr., P. Benzimidazole Derivatives: Synthesis, Physical Properties, and n-Type Semiconducting Properties. Chem. – Eur. J. 2014, 20 (37), 11835–11846. https: //doi.org/10.1002/chem.201403058.
[8]. Marinescu, M. Chemistry and Applications of Benzimidazole and Its Derivatives; BoD – Books on Demand, 2019.
[9]. Alaqeel, S. I. Synthetic Approaches to Benzimidazoles from o-Phenylenediamine: A Literature Review. J. Saudi Chem. Soc. 2017, 21 (2), 229–237. https: //doi.org/10.1016/j.jscs.2016.08.001.
[10]. Benson, S. C.; Pershadsingh, H. A.; Ho, C. I.; Chittiboyina, A.; Desai, P.; Pravenec, M.; Qi, N.; Wang, J.; Avery, M. A.; Kurtz, T. W. Identification of Telmisartan as a Unique Angiotensin II Receptor Antagonist With Selective PPARγ–Modulating Activity. Hypertension 2004, 43 (5), 993–1002. https: //doi.org/10.1161/01.HYP.0000123072.34629.57.
[11]. Singh, S.; Singh, N.; Kumar, V.; Datta, S.; Wani, A. B.; Singh, D.; Singh, K.; Singh, J. Toxicity, Monitoring and Biodegradation of the Fungicide Carbendazim. Environ. Chem. Lett. 2016, 14 (3), 317–329. https: //doi.org/10.1007/s10311-016-0566-2.
[12]. Jauregizar, N.; Calvo, R.; Suarez, E.; Quintana, A.; Raczka, E.; Lukas, J. C. Pharmacokinetics and Pharmacological Effect of Lerisetron, a New 5‐HT3 Antagonist, in Rats. J. Pharm. Sci. 2002, 91 (1), 41–52. https: //doi.org/10.1002/jps.1169.
[13]. Howden, C. W. Clinical Pharmacology of Omeprazole. Clin. Pharmacokinet. 1991, 20 (1), 38–49. https: //doi.org/10.2165/00003088-199120010-00003.
[14]. Maton, P. N. Omeprazole. N. Engl. J. Med. 1991, 324 (14), 965–975. https: //doi.org/10.1056/NEJM199104043241406.
[15]. Budetić, M.; Kopf, D.; Dandić, A.; Samardžić, M. Review of Characteristics and Analytical Methods for Determination of Thiabendazole. Molecules 2023, 28 (9), 3926. https: //doi.org/10.3390/molecules28093926.
[16]. Chen, J.; Li, N.; Liu, B.; Ling, J.; Yang, W.; Pang, X.; Li, T. Pracinostat (SB939), a Histone Deacetylase Inhibitor, Suppresses Breast Cancer Metastasis and Growth by Inactivating the IL-6/STAT3 Signalling Pathways. Life Sci. 2020, 248, 117469. https: //doi.org/10.1016/j.lfs.2020.117469.
[17]. Phillips, M. A. CCCXVII.—The Formation of 2-Substituted Benziminazoles. J. Chem. Soc. Resumed 1928, No. 0, 2393–2399. https: //doi.org/10.1039/JR9280002393.
[18]. Mann, J.; Baron, A.; Opoku-Boahen, Y.; Johansson, E.; Parkinson, G.; Kelland, L. R.; Neidle, S. A New Class of Symmetric Bisbenzimidazole-Based DNA Minor Groove-Binding Agents Showing Antitumor Activity. J. Med. Chem. 2001, 44 (2), 138–144. https: //doi.org/10.1021/jm000297b.
[19]. Ma, D.; Ji, X.; Wu, Z.; Cheng, C.; Zhou, B.; Zhang, Y. Synthesis of Benzimidazoles through Palladium-Catalyzed Amination of 2-Iodobenzimines with Diaziridinone. Adv. Synth. Catal. 2019, 361 (4), 739–746. https: //doi.org/10.1002/adsc.201801367.
[20]. Trivedi, R.; De, S. K.; Gibbs, R. A. A Convenient One-Pot Synthesis of 2-Substituted Benzimidazoles. J. Mol. Catal. Chem. 2006, 245 (1), 8–11. https: //doi.org/10.1016/j.molcata.2005.09.025.
[21]. Srinivasulu, R.; Kumar, K. R.; Satyanarayana, P. V. V. Facile and Efficient Method for Synthesis of Benzimidazole Derivatives Catalyzed by Zinc Triflate. Green Sustain. Chem. 2014, 2014. https: //doi.org/10.4236/gsc.2014.41006.
[22]. Wang, H.; Wang, Y.; Peng, C.; Zhang, J.; Zhu, Q. A Direct Intramolecular C−H Amination Reaction Cocatalyzed by Copper(II) and Iron(III) as Part of an Efficient Route for the Synthesis of Pyrido [1, 2-a]Benzimidazoles from N-Aryl-2-Aminopyridines. J. Am. Chem. Soc. 2010, 132 (38), 13217–13219. https: //doi.org/10.1021/ja1067993.
[23]. Li, J.; Gu, H.; Wu, C.; Du, L. The Mechanism of Transition-Metal (Cu or Pd)-Catalyzed Synthesis of Benzimidazoles from Amidines: Theoretical Investigation. Dalton Trans. 2014, 43 (44), 16769–16779. https: //doi.org/10.1039/C4DT01944J.
[24]. Kohansal, M. Advances in Green Chemistry: Sustainable Approaches in Organic Synthesis. Int. J. New Chem. 2025, 12 (4), 726–737. https: //doi.org/10.22034/ijnc.2025.719174.
[25]. Simon, M.-O.; Li, C.-J. Green Chemistry Oriented Organic Synthesis in Water. Chem. Soc. Rev. 2012, 41 (4), 1415–1427. https: //doi.org/10.1039/C1CS15222J.
[26]. Shaikh, I. R. Organocatalysis: Key Trends in Green Synthetic Chemistry, Challenges, Scope towards Heterogenization, and Importance from Research and Industrial Point of View. J. Catal. 2014, 2014 (1), 402860. https: //doi.org/10.1155/2014/402860.
Cite this article
Lu,Y. (2025). Green and Efficient Synthesis of 2-phenyl-1H-benzimidazole Using Recycled Steel Slag as a Novel and Readily Removable Catalyst. Applied and Computational Engineering,200,63-68.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of CONF-MCEE 2026 Symposium: Advances in Sustainable Aviation and Aerospace Vehicle Automation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. P. Barot, K.; Nikolova, S.; Ivanov, I.; D. Ghate, M. Novel Research Strategies of Benzimidazole Derivatives: A Review. Mini-Rev. Med. Chem. 2013, 13 (10), 1421–1447.
[2]. Brishty, S. R.; Hossain, M. J.; Khandaker, M. U.; Faruque, M. R. I.; Osman, H.; Rahman, S. M. A. A Comprehensive Account on Recent Progress in Pharmacological Activities of Benzimidazole Derivatives. Front. Pharmacol. 2021, 12. https: //doi.org/10.3389/fphar.2021.762807.
[3]. Pathare, B.; Bansode, T. Review- Biological Active Benzimidazole Derivatives. Results Chem. 2021, 3, 100200. https: //doi.org/10.1016/j.rechem.2021.100200.
[4]. Rashedy, A. A. E.; Aboul-Enein, H. Y. Benzimidazole Derivatives as Potential Anticancer Agents. Mini Rev. Med. Chem. 2013, 13 (3), 399–407. https: //doi.org/10.2174/138955713804999847.
[5]. Bai, S.; Zhang, M.; Tang, S.; Li, M.; Wu, R.; Wan, S.; Chen, L.; Wei, X.; Li, F. Research Progress on Benzimidazole Fungicides: A Review. Molecules 2024, 29 (6), 1218. https: //doi.org/10.3390/molecules29061218.
[6]. Quaranta, L. Benzimidazole Fungicides. In Bioactive Heterocyclic Compound Classes; John Wiley & Sons, Ltd, 2012; pp 103–118. https: //doi.org/10.1002/9783527664412.ch9.
[7]. Mamada, M.; Pérez-Bolívar, C.; Kumaki, D.; Esipenko, N. A.; Tokito, S.; Anzenbacher Jr., P. Benzimidazole Derivatives: Synthesis, Physical Properties, and n-Type Semiconducting Properties. Chem. – Eur. J. 2014, 20 (37), 11835–11846. https: //doi.org/10.1002/chem.201403058.
[8]. Marinescu, M. Chemistry and Applications of Benzimidazole and Its Derivatives; BoD – Books on Demand, 2019.
[9]. Alaqeel, S. I. Synthetic Approaches to Benzimidazoles from o-Phenylenediamine: A Literature Review. J. Saudi Chem. Soc. 2017, 21 (2), 229–237. https: //doi.org/10.1016/j.jscs.2016.08.001.
[10]. Benson, S. C.; Pershadsingh, H. A.; Ho, C. I.; Chittiboyina, A.; Desai, P.; Pravenec, M.; Qi, N.; Wang, J.; Avery, M. A.; Kurtz, T. W. Identification of Telmisartan as a Unique Angiotensin II Receptor Antagonist With Selective PPARγ–Modulating Activity. Hypertension 2004, 43 (5), 993–1002. https: //doi.org/10.1161/01.HYP.0000123072.34629.57.
[11]. Singh, S.; Singh, N.; Kumar, V.; Datta, S.; Wani, A. B.; Singh, D.; Singh, K.; Singh, J. Toxicity, Monitoring and Biodegradation of the Fungicide Carbendazim. Environ. Chem. Lett. 2016, 14 (3), 317–329. https: //doi.org/10.1007/s10311-016-0566-2.
[12]. Jauregizar, N.; Calvo, R.; Suarez, E.; Quintana, A.; Raczka, E.; Lukas, J. C. Pharmacokinetics and Pharmacological Effect of Lerisetron, a New 5‐HT3 Antagonist, in Rats. J. Pharm. Sci. 2002, 91 (1), 41–52. https: //doi.org/10.1002/jps.1169.
[13]. Howden, C. W. Clinical Pharmacology of Omeprazole. Clin. Pharmacokinet. 1991, 20 (1), 38–49. https: //doi.org/10.2165/00003088-199120010-00003.
[14]. Maton, P. N. Omeprazole. N. Engl. J. Med. 1991, 324 (14), 965–975. https: //doi.org/10.1056/NEJM199104043241406.
[15]. Budetić, M.; Kopf, D.; Dandić, A.; Samardžić, M. Review of Characteristics and Analytical Methods for Determination of Thiabendazole. Molecules 2023, 28 (9), 3926. https: //doi.org/10.3390/molecules28093926.
[16]. Chen, J.; Li, N.; Liu, B.; Ling, J.; Yang, W.; Pang, X.; Li, T. Pracinostat (SB939), a Histone Deacetylase Inhibitor, Suppresses Breast Cancer Metastasis and Growth by Inactivating the IL-6/STAT3 Signalling Pathways. Life Sci. 2020, 248, 117469. https: //doi.org/10.1016/j.lfs.2020.117469.
[17]. Phillips, M. A. CCCXVII.—The Formation of 2-Substituted Benziminazoles. J. Chem. Soc. Resumed 1928, No. 0, 2393–2399. https: //doi.org/10.1039/JR9280002393.
[18]. Mann, J.; Baron, A.; Opoku-Boahen, Y.; Johansson, E.; Parkinson, G.; Kelland, L. R.; Neidle, S. A New Class of Symmetric Bisbenzimidazole-Based DNA Minor Groove-Binding Agents Showing Antitumor Activity. J. Med. Chem. 2001, 44 (2), 138–144. https: //doi.org/10.1021/jm000297b.
[19]. Ma, D.; Ji, X.; Wu, Z.; Cheng, C.; Zhou, B.; Zhang, Y. Synthesis of Benzimidazoles through Palladium-Catalyzed Amination of 2-Iodobenzimines with Diaziridinone. Adv. Synth. Catal. 2019, 361 (4), 739–746. https: //doi.org/10.1002/adsc.201801367.
[20]. Trivedi, R.; De, S. K.; Gibbs, R. A. A Convenient One-Pot Synthesis of 2-Substituted Benzimidazoles. J. Mol. Catal. Chem. 2006, 245 (1), 8–11. https: //doi.org/10.1016/j.molcata.2005.09.025.
[21]. Srinivasulu, R.; Kumar, K. R.; Satyanarayana, P. V. V. Facile and Efficient Method for Synthesis of Benzimidazole Derivatives Catalyzed by Zinc Triflate. Green Sustain. Chem. 2014, 2014. https: //doi.org/10.4236/gsc.2014.41006.
[22]. Wang, H.; Wang, Y.; Peng, C.; Zhang, J.; Zhu, Q. A Direct Intramolecular C−H Amination Reaction Cocatalyzed by Copper(II) and Iron(III) as Part of an Efficient Route for the Synthesis of Pyrido [1, 2-a]Benzimidazoles from N-Aryl-2-Aminopyridines. J. Am. Chem. Soc. 2010, 132 (38), 13217–13219. https: //doi.org/10.1021/ja1067993.
[23]. Li, J.; Gu, H.; Wu, C.; Du, L. The Mechanism of Transition-Metal (Cu or Pd)-Catalyzed Synthesis of Benzimidazoles from Amidines: Theoretical Investigation. Dalton Trans. 2014, 43 (44), 16769–16779. https: //doi.org/10.1039/C4DT01944J.
[24]. Kohansal, M. Advances in Green Chemistry: Sustainable Approaches in Organic Synthesis. Int. J. New Chem. 2025, 12 (4), 726–737. https: //doi.org/10.22034/ijnc.2025.719174.
[25]. Simon, M.-O.; Li, C.-J. Green Chemistry Oriented Organic Synthesis in Water. Chem. Soc. Rev. 2012, 41 (4), 1415–1427. https: //doi.org/10.1039/C1CS15222J.
[26]. Shaikh, I. R. Organocatalysis: Key Trends in Green Synthetic Chemistry, Challenges, Scope towards Heterogenization, and Importance from Research and Industrial Point of View. J. Catal. 2014, 2014 (1), 402860. https: //doi.org/10.1155/2014/402860.