1. Introduction
In recent years, with economic development and growth, the demand for energy has risen sharply, but humankind still can’t get rid of the dependence on disposable fossil energy that is gradually scarce. And the expand of carbon emissions making the greenhouse effect the world’s biggest environmental problem. All nations are dedicated to attaining carbon peaking and carbon neutrality in order to address the problems of energy scarcity and environmental damage. The usage of primary energy sources, such as coal, which produces significant amounts of carbon emissions, must be avoided if one wants to reduce carbon dioxide emissions and energy consumption. Meanwhile, renewable energy should be vigorously developed. And due to great dependent of renewable energy depends on climate, geography and other factors, the development of efficient, low-cost advanced energy storage technology is the key to the efficient use of renewable energy.
The energy storage technologies today can be divided into three main categories: thermal, electrical and hydrogen energy storage. A battery, which is a significant and portable form of energy storage at the moment, offers the benefits of adaptable power and energy configuration in response to various application needs, quick response, independence from external factors like geographic resources, and suitability for large-scale applications and mass production. Belonging to the electrochemical energy storage, LIBs with its high energy density, high operating voltage, high charging and discharging efficiency, long working life and other advantages, has gradually replaced the traditional secondary batteries, widely used in cell phones, laptops, cameras and other electronic products, and has been commonly selected as the battery for new energy vehicle. The cycle life of LIBs is hundreds or thousands of times, so a large number of retired LIBs will be produced one after another in the years after mass production and use. It makes how to deal with these spent LIBs a crucial problem. Containing a variety of valuable metals and organic substance, LIBs will cause heavy metal pollution, organic pollution of soil and groundwater, etc. if they are disposed of by general landfill. Moreover, LIBs are rich in metal resources, of which lithium, nickel and cobalt are mined in only a few countries, especially cobalt, which accounted for 6.2% of total global use in the electric vehicle industry in 2016, while 54% of all cobalt mined comes from DR Congo [1]. The resource industry is facing an increasingly tight situation of metal supply and demand. Effective recycling is needed to enable the sustainable development of electronic equipment, new energy vehicles and other industries. How to achieve green, efficient, economic and sustainable development of LIBs regeneration is the problem which industries needs long-term efforts to solve.
In this paper, we analyze the environmental hazards of LIBs from the composition of each part of their materials. Then the specific recycling processes of current LIBs are elaborated in terms of pretreatment, thermometallurgy, hydrometallurgy and direct regeneration. Different technologies are compared and evaluated to derive the challenges faced by current recycling technologies and to mark the future development direction of recycling technologies.
2. Environmental hazards of LIBs
Applied in new energy vehicles, electronic devices, medical devices and many other fields, LIBs are widely applicable. They are basically composed of positive electrode, negative electrode, separator, electrolyte and current collector [2]. Fig. 1 shows sketch structure diagram of common LIBs. The cathode accounts for the largest proportion in the battery (about 25%-30%), and the mainstream materials are LiCoO2, LiMn2O4, LiNiO2, LiNixCoyMn1-x-yO2, etc. If these materials containing Li, Co, Ni, Mn and other metals are disposed of in landfills, they will infiltrate into deep soil and groundwater and even into surface water, causing a large amount of heavy metal enrichment and raising the pH of the environment. The anode electrode materials are mainly graphite and other carbon materials, which are prone to dust pollution during the dismantling process. The electrolytes are mainly lithium salts such as LiPF6, LiBF4, LiClO4, etc., which will cause fluorine contamination, toxic gas and environmental pH change when exposed to air, water and heating. Electrolyte solvents such as EC, PC, DMC, etc., these organic solvents with aldehydes, ketones, alcohols, organic acids, etc. volatilized into the atmosphere or into the water will cause organic pollution and they are toxic and will cause health hazards to humans and other organisms. Separators such as polypropylene (PP), polyethylene (PE), and adhesives such as polyvinylidene fluoride (PVDF), can also cause organic contamination. The reaction between the electrolyte and the positive and negative electrodes will also produce polluting by-products, the former such as HF and LiF are not only toxic in themselves but also acidify the soil and water bodies thus aggravating the release of heavy metals [3].
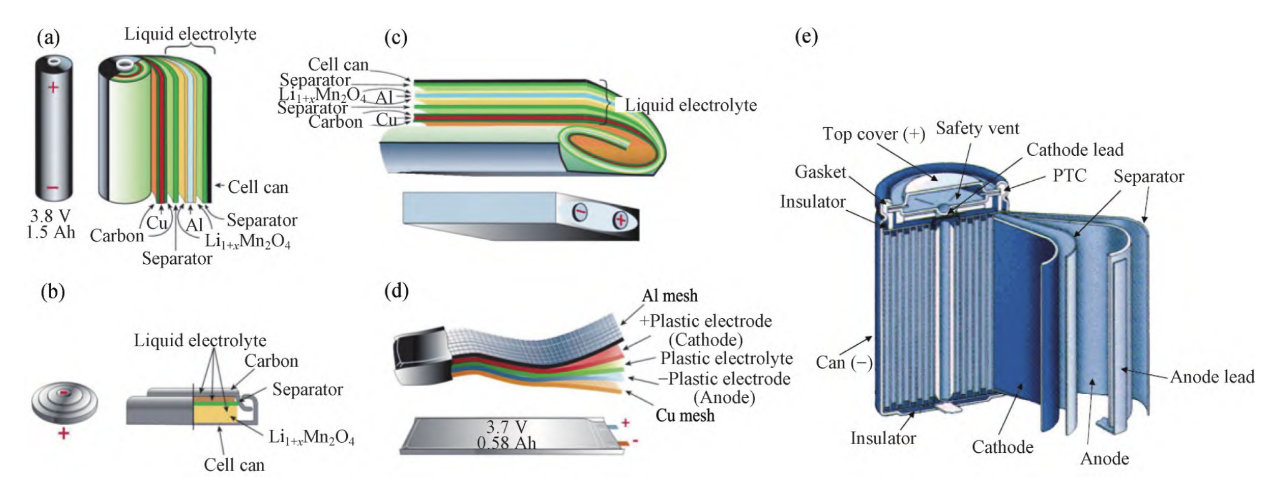
Figure 1. Sketch structure diagram of common LIBs [2].
If the spent LIBs are landfilled directly, it will bring a lot of pollution to the atmosphere, water and soil. Strengthening the recycling of LIBs can not only reduce environmental damage but also reuse the components in the battery, truly making LIBs a green, environmentally friendly new energy battery. However, it is worthy to note that the recycling process can also have pollution problems. For example, the current popular process of thermometallurgy, which requires high temperature calcination, emits greenhouse gases and toxic gases. Compared to the hydrometallurgy process, which consumes less energy and emits less greenhouse gases, but emits polluted wastewater, produces acid pollution, and may generate additional pollution when processing specific chemicals.
3. Recycling of LIBs
The existing recycling methods for spent LIBs are basically based on the idea of destroying the structure before extraction, and the general process is pretreatment, enrichment of valuable metals, and preparation of products. Pretreatment is the separation of each part of the battery individually. Then is the enrichment and recovery of valuable metals and the regeneration of materials, which is mainly divided into thermometallurgy and hydrometallurgy and other methods [4]. Finally, re-preparation of products through recycling. At present, the recovery process for valuable metals in cathode materials is relatively mature, but the recovery method for electrolyte and separator is still lacking. The following is a list of some of the mainstream recycling technologies for cathode materials.
3.1. Pre-treatment
The cathode material is stripped from the current collector via pretreatment. Pretreatment techniques are typically chosen for the cathode material to better separate it from the aluminum foil and to remove the binder from the cathode active material since it has the highest recovery benefit and the largest content share in LIBs. First of all, discharge of recyclable LIBs is an indispensable step, to prevent overheating or explosion and other hazards due to rapid release of residual power during dismantling, crushing and leaching process. Usually, salt solution immersion method and liquid nitrogen low temperature discharge method can be used to release the residual power in the battery [2]. The second step is to disassemble the different parts of the battery material, the most accurate method is manual dismantling, but the biggest problem this method faces is that it has great harm to the human body and requires too much manpower [5]. Therefore, there are mechanical treatment, solvent soaking, heat treatment [5-7]. Mechanical method is a separation method by different physical properties (specific gravity, etc.) of different components, such as mechanical grinding, centrifugal separation, electrostatic separation, ultrasonic assisted separation and other physical methods are used to separate the positive material from the aluminum foil, but the recovery rate and purity of the enrichment is relatively low [4, 6]. Solvent soaking is to separate the cathode material from the Al foil using organic solvents or alkali solutions. For example, NaOH is used to react with Al to form H2 and NaAlO2, thus stripping the active material from the aluminum foil. Or use organic solvents such as NMP and DMAC to dissolve the binder PVDF to achieve the same effect. In addition, high temperature calcination can also remove the binder [7]. Table 1. shows a comparison of the strength and shortage of the mentioned pretreatment methods.
Table 1. Comparison of different pretreatment methods.
Methods | Treatment condition | Advantages | Disadvantages |
Manual dismantling | Human power | Accurate | High cost, Great health harm; |
Mechanical treatment | Mechanical grinding | High efficiency, Low cost | Low yield, Low purity |
Alkaline soaking | NaOH soaking | Simple, High efficiency | Difficulty in aluminum recovery |
Organic solution soaking | NMP/DMF/TFA soaking | Simple, High efficiency | Environment pollution |
Heat treatment | 400~900 ℃ | Simple, High yield | High cost, Environment pollution |
3.2. Thermometallurgy
The thermometallurgy process involves roasting the spent LIBs at high temperatures and then applying other treatments to separate the valuable metals, depending on the needs. The most common methods of thermometallurgy today are carbon thermic reduction and salt-assisted roasting [8].
Carbon reduction roasting is to add reducing carbon, charcoal or coke during roasting, so that the valuable metals to be extracted in the cathode material are converted into low-valent oxidation or soluble states, and the valuable metals in the cathode materials can be extracted quickly. In this process, carbon as a reducing agent does not directly reduce lithium ions, but by first reducing other metal ions in the active cathode material, such as Co3+ and Mn4+ to a low-valence state, so that it can precipitate in an oxide or alloy state, thereby promoting the conversion of Li into soluble salt (Li2CO3) leaching and playing a separation effect. Carbonaceous materials are also more advantageous due to their low cost and abundant reserves. Since the vast majority of anode materials in LIBs are graphite, and the combustion of graphite at high temperature has a coupling relationship with the thermal decomposition of the positive electrode active material, the reaction can be more likely to occur [9]. This also led to the idea of in-situ recovery, without the need for additional reagents, the graphite anode in the battery is roasted with the collected active material to achieve leaching conditions [10]. However, the carbon-thermal reduction method still has hindrances of excessive energy use, large CO2 emissions, and low recovery rate.
Salt-assisted roasting is the addition of salts that reduce the roasting temperature as roasting agents to convert the metals to be recovered into different water-soluble products. This method is mainly divided into sulfated roasting, chlorination roasting and nitrification roasting according to the different salts added. Compared with chemical reduction roasting, this method effectively reduces secondary pollution during recycling and improves recovery efficiency [8].
3.3. Hydrometallurgy
Even if the thermometallurgy process is straightforward and has a sizable processing capacity, there are still issues like low recovered product purity, high energy usage, and a lot of waste gas. So at present, more research is on the wet process. The wet process to recover LIBs consists of three main steps, namely leaching, separation and regeneration.
3.3.1. Leaching. The leaching step is to transfer the cathode active material from the solid phase to the liquid phase by acid leaching, alkali leaching, biological leaching and other methods to obtain the leaching solution containing renewable metals, which is conducive to the subsequent precipitation and purification process.
Acid leaching typically includes inorganic acids, organic acids, and a mixture of organic and inorganic acids. Inorganic acids can achieve the leaching result effectively, but in the leaching process will produce Cl2, SO3, NOx and other harmful gases, SO42-, Cl-, PO43- and other ions in the solution will also bring acid waste liquid to form secondary pollution. Besides, hydrogen peroxide and other reducing agents are added in order to accelerate the leaching process, so as to increase the cost. The advantages of using organic acids such as oxalic acid are that they are less corrosive, do not react with used battery materials to produce toxic gases, are selective, and are environmentally friendly [10]. However, organic acids are more expensive and generally require high temperatures and reducing agents to enhance leaching, which further increases the cost. Therefore, in order to combine the advantages of both organic and inorganic acid systems, some researchers conducted experiments with a mixture of organic plus inorganic acids, which resulted in higher leaching rates and lower pollution. For example, some researchers created a mixed acid for leaching by using phosphoric acid and citric acid--the former acts as an leaching agent, and the latter as both leaching agent and reducing agent. In addition, some scholars have designed an electrochemically assisted cathodic reduction leaching process [11]. However, the selectivity of acid leaching for different metals is relatively poor, making the extraction and purification of metals from the leachate more difficult. For example, when NaOH is added to an acid leach solution containing Ni, Mn and Co, the pH change required for the precipitation of these ions is large [11]. Due to the overlapping pH range of each metal's precipitation, this process not only uses up a lot of alkaline solution and produces an excessive amount of wastewater discharge, but it also makes it difficult to precipitate each metal separately [12]. With the alkaline leaching approach, Co and Ni can be selectively leached, making the separation process easier. As an alkaline leaching agent, ammonia, ammonium carbonate, or other alkaline reagents may be utilized. Taking ammonia leaching as an example, the reaction equation is shown in Eq. (1)-Eq. (3) at the appropriate pH range.
Ni2+ + nNH3↔Ni(NH3)n2+(1)
Co2+ + nNH3↔Co(NH3)n2+ (2)
Cu2+ + 4NH3↔ Cu(NH3)42+(3)
Co, Ni and Cu have a high leaching efficiency because they can form soluble complex ions, while Mn cannot form soluble complex ions and enters the residue phase, thus achieving separation. However, compared with the acid leaching method, the generation of ammonia by alkaline leaching is more harmful to the environment, which is also a major problem in the industrial application of this method.
Bio-leaching is also an innovative method of recycling. It uses certain specific microorganisms and metabolites of complexation, reduction, oxidation and leaching to achieve the purpose of valuable metal recovery and dissolution [5]. Compared to traditional methods, bio-leaching processes are subject to mild conditions, consume less energy, are friendly to the environment, and are technologies that have been worth developing in recent years. However, the difficulty and slowness of bacterial culture make it difficult to use it on a large scale in industry yet [13]. To improve the bioleaching efficiency, numerous researchers have attempted various techniques such as enhanced bacterial culture, adhesion of metal ions, and use of surfactants [13].
In order to achieve greener recycling, the use of deep eutectic solvents is an effective method. Deep eutectic solvents (DESs) are a class of low eutectic mixtures consisting of hydrogen bond donors (such as amides) and hydrogen bond acceptors (such as quaternary ammonium salts) in a certain stoichiometric ratio, whose freezing points are lower than the melting points of the pure substances of their constituents, and have benefits of being inexpensive, environmentally friendly, easy to prepare, and generally soluble in most metal oxides. In the leaching process, DESs act as both leaching and reducing agents, achieving a leaching rate of over 90% of cobalt and lithium from LiCoO2 waste without adding other chemical reagents [14]. Such a method is a good solution to the problem of high cost caused by the formation of acid waste solution and addition of additional reducing agent during acid leaching. Fig. 2 shows the general process of LIBs leaching using DESs: After the cathode of lithium-ion battery is stripped, it is inserted into low eutectic solvent for heating and stirring to extract cobalt ions and lithium ions by dissolving, and the aluminum foil, binder and conductive carbon can be separated in this step. Then the cobalt is recovered by deposition or electrodeposition method [15].
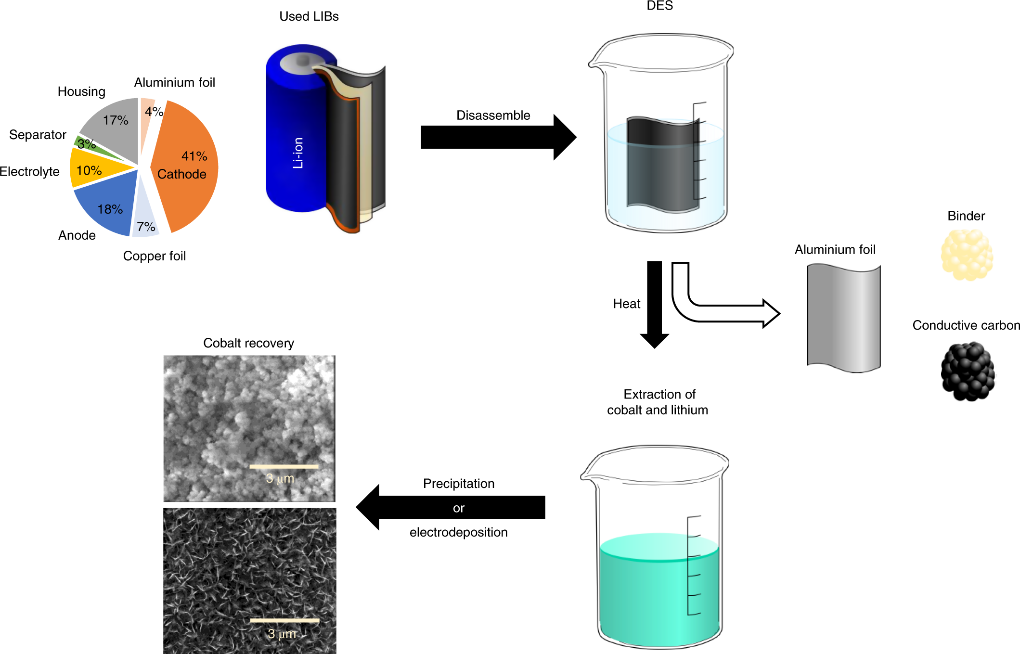
Figure 2. General process of LIBs leaching using DESs [15].
The reutilization of low eutectic solvents has also been studied. The presence of oxygen acceptors in the eutectic is theoretically essential for dissolving the oxide because it promotes the cleavage of the metal oxide bond, and dissolving the metal oxide implies a partial change in the chemical composition of the solvent. Even if this transition is not fully reversible upon recycling, partially oxidized compounds can still receive additional oxygen, while fully oxidized alcohols will provide protons that act as oxygen acceptors. In fact, researchers have also experimentally found that the leaching rate of the recovered low eutectic solvent reused as leaching agent is similar to the leaching rate of the first use [15]. This draws that low eutectic solvents can also be used in a truly green way for multiple uses.
3.3.2. Separation. Due to the different cathode materials, the leachate composition is also different, so the objectives and methods of separation and extraction are also different. At present, the main separation methods are precipitation separation method, extraction method, etc.
Precipitation is the most common method for industrial synthesis of LiNixCoyMn1-x-yO2 cathode materials, using precipitating agents to selectively precipitate Li and Co ions in the leaching solution, and the product is later separated by filtration. The precipitation method focuses on preventing the co-precipitation of impurity ions and the sequential precipitation of valuable metal ions to obtain higher purity products. Commonly used precipitation agents are sulfuric acid, sodium sulfide, oxalic acid, sodium carbonate, etc. [2]. Chemical precipitation method has the preponderance of high recovery rate, low equipment requirements, low cost and short process, but this method requires high pH control and easily affects product purity due to co-precipitation of multiple metal ions.
Extraction method is to selectively separate valuable metals such as Co, Li, Ni, etc. by adding some organic solvent to the leaching solution. This method has high recovery rate, mild conditions, low energy consumption, high product purity, and can better recover the metal ions in the leaching solution of waste batteries, in which the extractant is a more important influencing factor, and the commonly used extractants are P507, Cyanex272, Acorga M5640, etc. [10]. Extraction method commonly used solvents are mostly organic solvents, which are widely used and more mature technology, but the pollution of the environment by organic solutions cannot be ignored, contrary to the development of green and friendly process, for this reason, the solution two-phase system extraction can be proposed and studied, the solution two-phase system is mainly composed of five toxic, non-flammable or even biodegradable or recycled solution as the solvent, compared to traditional organic solvents, with green features, it is worth studying for replacing the traditional extraction process.
3.4. Direct regeneration
The recovery methods for cathode materials currently established are mainly based on hydrometallurgy, where the acid is dissolved followed by chemical precipitation. Using a lot of acid and alkali solutions creates more waste and makes the recovery procedure more challenging. Moreover, the available energy in the cathode material particles is lost during such a damaging recovery process. Therefore, the direct recovery of cathode materials is the focus of many scholars' research.
Lithium ions are taken out of the cathode and embedded into the anode during charging to achieve energy storage, and then taken out of the anode and embedded back into the cathode during discharging. The cathode material's crystal structure expands and shrinks as the charging and discharging process goes on, and the material's structural stability gradually declines as a result. This causes the battery capacity to continuously degrade. In response to composition loss and structural damage, various lithiation methods, supplemented by heat treatment, are usually used for direct repair of failed cathode materials in retired LIBs. Compared with traditional thermal and wet processes, the main advantages of direct recovery are simple process, low energy consumption and low pollution, which can slash the cost of LIBs recovery [15].
The key to direct regeneration of cathode materials lies in ensuring the stoichiometric ratio of each metal element, repairing the broken or collapsed crystal structure and ensuring the smooth passage of lithium ions. For example, it is possible to directly repair the recycled waste LiCoO2 material by high temperature sintering method. The LiCoO2 cathode material is obtained by sintering under different temperature conditions by adding the corresponding stoichiometric amount of Li2CO3 directly without a series of processes such as "acid leaching - precipitation - precursor preparation - resynthesis of material" in the traditional recycling method.
Another idea is to use LiOH as a lithium replenishing agent. As in Fig. 3, the lithium lost during the cycle is replenished by hydrothermal method, and then the repaired active material is obtained by annealing, which is used in lithium cobaltate, ternary materials and their blends, and also shows good electrochemical performance. However, the reaction pressure of hydrothermal method is much higher than the ambient pressure, and there are certain safety hazards. Therefore, many researchers have devoted themselves to finding methods to achieve re-lithiation of failed cathodes under atmospheric pressure, in order to further realize green, energy-saving and efficient recycling of LIBs cathode materials [16].
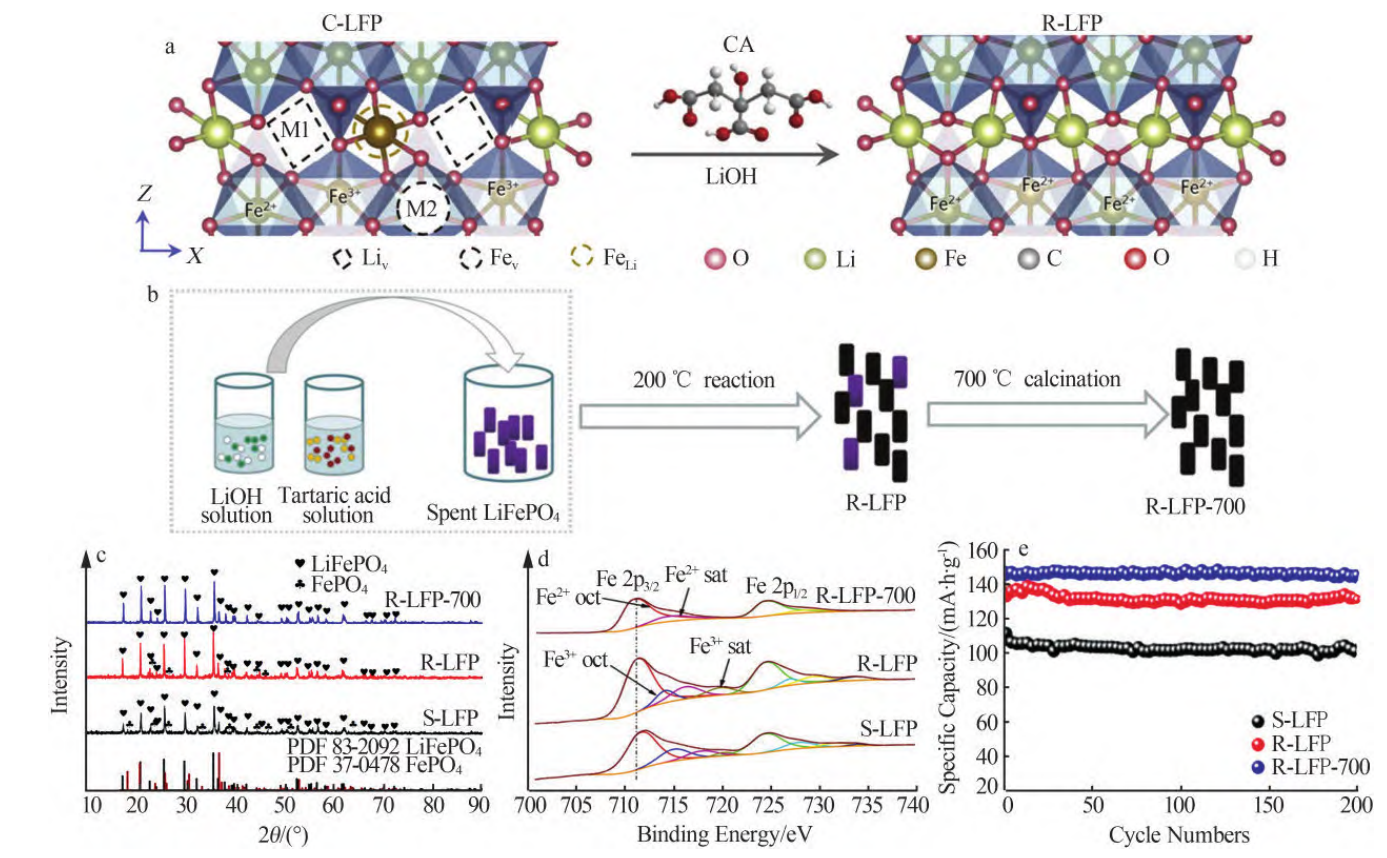
Figure 3. Direct regeneration of spent LPF via hydrothermal technique. (a) Mechanism of direct generation of LFP cathode; (b) Schematic diagram of lithiation of solution [16].
4. Prospects
At present, the world produces a lot of spent LIBs every year, and these batteries are generally still recycled through the landfill method. This is because the way of commercial utilization of recycling technology is not yet consummate, the value of some recovered products in the recycling process is low, and some valuable components cannot be fully recovered, which cannot bring the expected revenue for most companies; and the positive electrode material is constantly updated, while the regeneration technology of used lithium batteries is relatively backward, which makes it difficult for the existing regeneration technology to keep pace with it.
The existing recycling technology recovers precious metal elements like C, Ni, and Co based on a thorough analysis of the economic and environmental advantages. As the application areas and fields of LIBs have changed and large LIBs are increasingly used in a wide range of fields, there are not only great environmental problems but also great resource issues if the existing treatment method is still followed. Therefore, the future regeneration of used LIBs needs to be focused on: There is not much research on the recycling of cathode graphite materials and electrolytes in used LIBs. Due to the widespread use of electric power systems, large capacity and large volume batteries produce a large amount of electrolyte, and the electrolyte of LIBs is itself a volatile substance, and when the batteries are recycled and disassembled, lithium hexafluorophosphate will produce HF, PF5 and other toxic gases [1]. In the long run, the environmentally sound disposal of electrolytes is also a pressing issue.
The recycling technologies developed so far are for the liquid LIBs which accounts for vast majority on the market. While the all-solid-state LIBs are a new generation of batteries with simpler structure, higher value of lithium metal cathode and solid electrolyte, which have been applied to a certain extent, but there is still less research on its recycling technologies.
Therefore, both now and in the future, we are seeking a way that is economically viable and has a low environmental impact to regenerate LIBs to the maximum extent. The development of a complete recycling program that can balance the environmentally sound and efficient use of organic components such as electrolyte, the effective recycling of batteries of different materials, and the maximum use of the recovered products.
5. Conclusion
Spent LIBs are important secondary resources with high recycling value. Currently, the recycling process of spent LIBs mainly includes coarse separation of battery parts, leaching and extraction of reusable metals and product preparation process. Among them, the extraction step is the most critical one in the whole recovery process, the process mainly uses thermometallurgy, hydrometallurgy, etc. Thermometallurgy has low-cost and simple processes but greater energy consumption, lower purity, more serious secondary pollution; general acid leaching and alkali leaching method of hydrometallurgy leaching efficiency, a certain degree of selectivity, but higher costs, resulting in a large amount of waste liquid and harsher conditions control. Deep eutectic solvent leaching, bioleaching and direct regeneration methods are potential directions for development. DESs leaching and bioleaching methods have mild conditions, low energy consumption and are extremely environmentally friendly, but the subsequent regenerative synthesis process still requires significant energy consumption and other processes, and the microbial leaching method needs to be commercially improved in terms of microbial culture; direct regeneration of cathode materials can greatly simplify the recycling process of lithium-ion batteries and reduce energy consumption, but relevant research is currently limited and the process is not mature enough. In addition, the recycling of electrolyte and cathode has not yet received enough attention and development, which is a significant step to achieve full recycling of LIBs and minimize environmental pollution. How to achieve maximum green recycling and economic viability is a long-term issue to be explored in the future.
References
[1]. Mayyas, A., Steward, D., Mann, M. (2019) The case for recycling: Overview and challenges in the material supply chain for automotive li-ion batteries. Sustainable Materials and Technologies., 19: e00087.
[2]. Li M., Chen L., Yang Y., Niu F., Zhang X., Liu D. (2020) Research progress of lithium-ion battery recycling technology. Chinese Journal of Rare Metals., 46(3): 349-366.
[3]. Mrozik, W., Rajaeifar, M.A., Heidrich, O., Christensen, P. (2021) Environmental impacts, pollution sources and pathways of spent lithium-ion batteries. Energy Environ. Sci., 14: 6099-6121.
[4]. Xu, Z., Liu, Z., Wang, S., Lu, Z., Zhang, Z., Wang, H., Jiang, F. (2022) Review on hydrometallurical recovery of valuable metals from spent lithium-ion batteries. Journal of China University of Mining & Technology., 51(3):454-465.
[5]. Xiao, JF., Li, J., Xu, ZM. (2020) Challenges to future development of spent lithium-ion batteries recovery from environmental and technological perspectives. Environmental Science & Technology., 54(1):9-25.
[6]. Zheng, X., Zhu, Z., Lin, X., Zhang, Y., He, Y., Cao, H., Sun, Z.(2018) A mini-review on metal recycling from spent lithium ion batteries. Engineering.,4(3):361-370.
[7]. Ferreira, D.A., Prados, L.M.Z., Majuste, D., Mansur, M.B.(2009) Hydrometallurgical separation of aluminium, cobalt, copper and lithium from spent Li-ion batteries. Journal of Power Sources., 187(1):238–46.
[8]. Makuza, B., Tian, Q.H., Guo, X.Y., Chattopadhyay, K., Yu, D. (2021) Pyrometallurgical options for recycling spent lithium-ion batteries: A comprehensive review. Journal of Power Sources., 491: 229622.
[9]. Mao, J.K., Li, J., Xu, Z.M. (2018) Coupling reactions and collapsing model in the roasting process of recycling metals from LiCoO2 batteries. Journal of Cleaner Production., 205:923-929.
[10]. Sattar, R., Ilyas, S., Bhatti, H.N., Ghaffar, A. (2019) Resource recovery of critically-rare metals by hydrometallurgical recycling of spent lithium ion batteries. Separation and Purification Technology., 209:725-733.
[11]. Wang, R.C., Lin, Y.C., Wu, S.H. (2009) A novel recovery process of metal values from the cathode active materials of the lithium-ion secondary batteries. Hydrometallurgy., 99(3-4):194-201.
[12]. Ferreira, D.A., Prados, L.M.Z., Majuste, D., Mansur, M.B. (2009) Hydrometallurgical separation of aluminium, cobalt, copper and lithium from spent Li-ion batteries. Journal of Power Sources., 187(1):238-246.
[13]. Roy, J.J., Cao, B., Madhavi, S. (2021) A review on the recycling of spent lithium-ion batteries (LIBs) by the bioleaching approach. Chemosphere., 282(0):0-13.
[14]. Wang, S.B., Zhang, Z.T., Lu, Z.G., Xu, Z.G. (2020) A novel method for screening deep eutectic solvent to recycle the cathode of Li-ion batteries. Green Chemistry., 22(14): 4473-4482.
[15]. Tran, M.K., Rodrigues, M.T.F., Kato, K., Babu, G., Ajayan, P.M. (2019) Deep eutectic solvents for cathode recycling of Li-ion batteries. Nature Energy., 4:339-345.
[16]. Tang D., Wang J.X., Chen W., Ji G.J., Ma J.,Zhou G.M. (2023) Research status and prospect on direct regeneration of cathode materials from retired lithium⁃ion batteries. Inorganic Chemicals Industry., 55(1):15-25.
Cite this article
Peng,J. (2023). Environment impacts and recycling methods of spent lithium-ion batteries. Applied and Computational Engineering,23,16-24.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2023 International Conference on Functional Materials and Civil Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Mayyas, A., Steward, D., Mann, M. (2019) The case for recycling: Overview and challenges in the material supply chain for automotive li-ion batteries. Sustainable Materials and Technologies., 19: e00087.
[2]. Li M., Chen L., Yang Y., Niu F., Zhang X., Liu D. (2020) Research progress of lithium-ion battery recycling technology. Chinese Journal of Rare Metals., 46(3): 349-366.
[3]. Mrozik, W., Rajaeifar, M.A., Heidrich, O., Christensen, P. (2021) Environmental impacts, pollution sources and pathways of spent lithium-ion batteries. Energy Environ. Sci., 14: 6099-6121.
[4]. Xu, Z., Liu, Z., Wang, S., Lu, Z., Zhang, Z., Wang, H., Jiang, F. (2022) Review on hydrometallurical recovery of valuable metals from spent lithium-ion batteries. Journal of China University of Mining & Technology., 51(3):454-465.
[5]. Xiao, JF., Li, J., Xu, ZM. (2020) Challenges to future development of spent lithium-ion batteries recovery from environmental and technological perspectives. Environmental Science & Technology., 54(1):9-25.
[6]. Zheng, X., Zhu, Z., Lin, X., Zhang, Y., He, Y., Cao, H., Sun, Z.(2018) A mini-review on metal recycling from spent lithium ion batteries. Engineering.,4(3):361-370.
[7]. Ferreira, D.A., Prados, L.M.Z., Majuste, D., Mansur, M.B.(2009) Hydrometallurgical separation of aluminium, cobalt, copper and lithium from spent Li-ion batteries. Journal of Power Sources., 187(1):238–46.
[8]. Makuza, B., Tian, Q.H., Guo, X.Y., Chattopadhyay, K., Yu, D. (2021) Pyrometallurgical options for recycling spent lithium-ion batteries: A comprehensive review. Journal of Power Sources., 491: 229622.
[9]. Mao, J.K., Li, J., Xu, Z.M. (2018) Coupling reactions and collapsing model in the roasting process of recycling metals from LiCoO2 batteries. Journal of Cleaner Production., 205:923-929.
[10]. Sattar, R., Ilyas, S., Bhatti, H.N., Ghaffar, A. (2019) Resource recovery of critically-rare metals by hydrometallurgical recycling of spent lithium ion batteries. Separation and Purification Technology., 209:725-733.
[11]. Wang, R.C., Lin, Y.C., Wu, S.H. (2009) A novel recovery process of metal values from the cathode active materials of the lithium-ion secondary batteries. Hydrometallurgy., 99(3-4):194-201.
[12]. Ferreira, D.A., Prados, L.M.Z., Majuste, D., Mansur, M.B. (2009) Hydrometallurgical separation of aluminium, cobalt, copper and lithium from spent Li-ion batteries. Journal of Power Sources., 187(1):238-246.
[13]. Roy, J.J., Cao, B., Madhavi, S. (2021) A review on the recycling of spent lithium-ion batteries (LIBs) by the bioleaching approach. Chemosphere., 282(0):0-13.
[14]. Wang, S.B., Zhang, Z.T., Lu, Z.G., Xu, Z.G. (2020) A novel method for screening deep eutectic solvent to recycle the cathode of Li-ion batteries. Green Chemistry., 22(14): 4473-4482.
[15]. Tran, M.K., Rodrigues, M.T.F., Kato, K., Babu, G., Ajayan, P.M. (2019) Deep eutectic solvents for cathode recycling of Li-ion batteries. Nature Energy., 4:339-345.
[16]. Tang D., Wang J.X., Chen W., Ji G.J., Ma J.,Zhou G.M. (2023) Research status and prospect on direct regeneration of cathode materials from retired lithium⁃ion batteries. Inorganic Chemicals Industry., 55(1):15-25.









