1. Introduction
Battery electrode materials are one of the hot research areas. The research on battery electrode materials is not only about energy storage and utilization, but also about environmental protection and sustainable development. During the last decades, research into battery electrode materials has made great strides, for example, lithium-ion batteries, sodium-ion batteries, and other new batteries have been introduced one after the other. With the continuous improvement of battery performance requirements, the research on electrode materials has become more and more urgent. Therefore, it is important to study the structure and performance of battery electrode materials and their applications. The research of battery electrode materials aims to solve many problems of batteries in this era. The improvement of electrode materials can increase the energy density of batteries, improve the cycle life of batteries, and thus extend the service time of batteries. In addition, improving the safety performance of batteries is also an important goal.
The research of battery electrode materials is a very active research direction in the field of battery technology, and a lot of research has been done on it. In recent years, electrode material research has become a hot topic in the field of battery technology due to the increasing demand for high-performance, long-life, safe and low-cost batteries in the expanding markets of electric vehicles, energy storage systems, and wearable devices. And compared with research papers that focus more on the development, innovation, and improvement of battery electrode materials, we can get a more comprehensive understanding of the performance and characteristics of different types of batteries by comparing several different types of current hot batteries for research, which has a wide range of guiding significance for the development of battery technology. And it can provide a more in-depth understanding of the performance and characteristics of batteries, which can help to design more optimized battery systems. This is of great significance for improving the energy density, cycle life, and safety of batteries, and is of higher practicality for the practical application of battery technology. By comparing electrode materials of different types of batteries, several subject areas can be involved, such as material science, chemistry, electrochemistry, etc. This can promote communication and cooperation among different disciplinary fields, thus promoting the interdisciplinary research and development of battery technology.
In this paper, three common types of batteries, lithium batteries, nickel-chromium batteries, and nickel-hydrogen batteries, are selected for comparative study. The study mainly focuses on the characteristics of the three types of batteries, the material preparation methods, and the advantages and disadvantages of the batteries as well as some of their current problems that still exist, detailing the differences in the electrode materials of various batteries, the impact on the performance and characteristics of the batteries. In this paper, we aim to provide a comprehensive analysis of the advantages, disadvantages, and scope of the application of various types of batteries. By comparing the charge and discharge mechanisms of different types of batteries, we can assess the impact on the battery’s cycle life, energy density, safety, and other performance factors. Through this analysis, we can gain a better understanding of the performance indexes of different batteries and how they vary across various applications. Ultimately, this research will help to inform the design of better battery systems that can meet the unique requirements of different applications.
2. Types of batteries
2.1. Lithium battery
Lithium-ion batteries utilize a non-aqueous electrolyte solution, which can be derived from either lithium metal or a lithium alloy, as either the negative or positive electrode material. Due to the highly reactive nature of lithium metal, the manufacture, storage, and use of this material require strict adherence to environmental regulations. However, thanks to advancements in science and technology, lithium batteries have become widely utilized throughout the world.
Lithium batteries are composed of a positive electrode, a negative electrode, an electrolyte (also known as electrolyte), a diaphragm, and a battery casing. The cathode material for lithium metal batteries is generally manganese dioxide. Lithium metal or its alloy y is generally the negative electrode material, in addition to the non-aqueous electrolyte solution. In contrast, a common configuration for lithium-ion batteries involves the utilization of a lithium alloy metal oxide as the positive electrode, the negative electrode in lithium-ion batteries is often composed of graphite, while a non-aqueous electrolyte solution is also utilized [1]. Unlike other types of batteries, non-polluting lithium-ion batteries do not contain harmful heavy metal substances. Instead, they offer several advantages, including small size, high storage capacity, and fast charging capabilities.
2.2. NiMH batteries
NiMH batteries are alkaline batteries that utilize a nickel-based positive active substance and a negative active substance composed mainly of a hydrogen storage alloy. These batteries are an improved version of nickel-cadmium batteries that replace cadmium with a hydrogen-absorbing metal. In NiMH batteries, the “metal” component is typically in the form of metal intercalation. Various types of metal intercalation have been used in the production of NiMH batteries, which can be broadly divided into two main categories. The more common type used is AB5, which consists of a combination of rare earth elements and titanium to form A, and nickel, cobalt, manganese and aluminum to form B. Some high-capacity batteries with “multi-component” electrodes are primarily composed of AB2, where A is titanium or vanadium, and B consists of zirconium or nickel, along with some chromium, cobalt, iron, and manganese [2]. In contrast to NiMH batteries, alkaline batteries can leak slightly corrosive and harmful liquids after prolonged periods of non-use. These liquids can cause damage to the device using the battery and pose a potential risk to human health.
2.3. NiCd batteries
A rechargeable alkaline battery known as the nickel-cadmium battery utilizes cadmium for the negative electrode and nickel oxide hydroxide for the positive electrode. The positive electrode material is usually a mixture of nickel hydroxide and iron oxide, while the negative electrode material is typically made of cadmium hydroxide. The electrolyte is usually a solution of potassium hydroxide. The negative electrode is made of cadmium, the battery consists of a sponge mesh sieve filled with both cadmium powder and oxide powder. The electrolyte is a solution of either potassium hydroxide or sodium hydroxide.
NiCd batteries use different types of electrolytes depending on the ambient temperature. Sodium hydroxide solution is recommended for higher temperatures, while potassium hydroxide solution is recommended for lower temperatures. A more concentrated potassium hydroxide solution is recommended if the ambient temperature falls below -15 ℃. For sealed nickel-cadmium batteries, a potassium hydroxide solution with a density of 1.40 is used to balance low-temperature performance and charge retention. Lithium hydroxide is typically added to the electrolyte at a concentration of 15-20 grams per liter to improve battery capacity and cycle life [3].
The charging and discharging of NiCd batteries involved the conversion of active materials on the positive and negative plates. When a NiCd battery is charged, the active material on the positive plate is converted to nickel hydroxide, and the active material on the negative plate is transformed into cadmium metal. During discharging, the positive plate's active material becomes nickel suboxide hydroxide, while the negative plate's active material is converted to cadmium hydroxide. NiCd batteries are widely used in various small electronic devices due to their lightweight design, shock resistance, and long lifespan, making them a prevalent battery device in the current market.
3. Preparation method of battery
3.1. Preparation of lithium batteries
The lithium-ion battery is a complex system, which includes a cathode, cathode, cell separator, collector, binder, etc. Therefore, lithium-ion battery preparation is also a very complex process. Therefore, lithium-ion battery preparation is also a very complex process, which involves the electrochemical reaction of the cathode and cathode, the conduction of lithium ions and electron conduction reaction, and the diffusion of heat, among other things. The production process of lithium-ion is very long, and there are more than 50 processes involved before and after production.
3.1.1. Preparation of cathode materials for lithium batteries. The production goal of the first stage of the lithium battery manufacturing process is to complete the manufacture of the grade sheet, including the manufacturing of both the positive electrode materials and negative electrode materials. The cathode material accounts for a significant proportion of lithium batteries, with the mass ratio of positive to negative materials typically ranging from 3:1 to 4:1. However, because of its shortage of resources, high cost, low actual specific capacity, poor overcharge ability, and poor thermal stability, the cathode material has developed from a single lithium cobalt ate material in recent years to Lithium cobalt ate, lithium manganate, and lithium nickel cobalt ate have been developed in recent years from a single lithium cobalt ate material to a stage where lithium cobalt ate, lithium manganate, and lithium nickel cobalt ate are advancing together [4]. The preparation methods of lithium-ion battery cathode materials are generally solid-phase method, complex method, and sol-gel method. At present, in addition to the preparation of LiNixCoO1-x powder by the traditional solid phase method, people also use the water thermal method, co-precipitation method, sol-gel method, and other soft chemical methods, these soft chemical preparation methods have their characteristics, all of them can obtain LiNixCo1-xO(0<x<1) powder with fine particles and high purity [5].
3.1.2. Preparation of negative electrode materials for lithium batteries. The negative electrode material of a lithium battery typically consists of active material, binder, and additives, which are mixed to form a paste adhesive. The resulting mixture is then coated on both sides of a copper foil, dried, and rolled to create the electrode. The primary function of the negative electrode material is to store and release energy, which has a significant impact on the cycle performance and other key indicators of the lithium battery. The negative electrode material accounts for about 15% of the total mass of the battery. Li-ion battery cathode material generally has lithium metal cathode material, carbon-based cathode material, silicon-based cathode material, tin-based cathode material, germanium-based cathode material, and lithium titanate cathode material. Carbon and silicon anode are commonly used. The processing of artificial graphite anode materials requires a long process due to particle size and morphology design, which requires pre-treatment, pyrolysis, ball milling, outsourcing graphitization, sieving, and other steps. While in the design of different dimensions of silicon materials, carbon is most commonly used to composite with silicon to reduce the overall volume expansion and to enhance the electrical conductivity of the material [6]. Currently, silicon and carbon composites are prepared as electrode materials for lithium-ion batteries by hydrothermal, mechanical ball milling, spray drying, and chemical vapor deposition methods. High-capacity silicon/carbon composite anode materials can be synthesized by high-temperature pyrolysis [7].
3.2. Preparation of NiMH batteries
The primary equipment for the NiMH battery positive electrode is Ni(OH)2. The primary material for the negative electromagnet is the hydrogen storage alloy. The electrolyte is generally a 30% aqueous solution of KOH with a small quantity of LiOH and NaOH. The diaphragm is mostly made of polypropylene non-woven fabric. The commonly used NiMH batteries are cylindrical and square. The charging and discharging of the NiMH battery is realized by the function of hydrogen absorption and discharge of the hydrogen storage alloy when the potential changes, as shown in Figure 1.
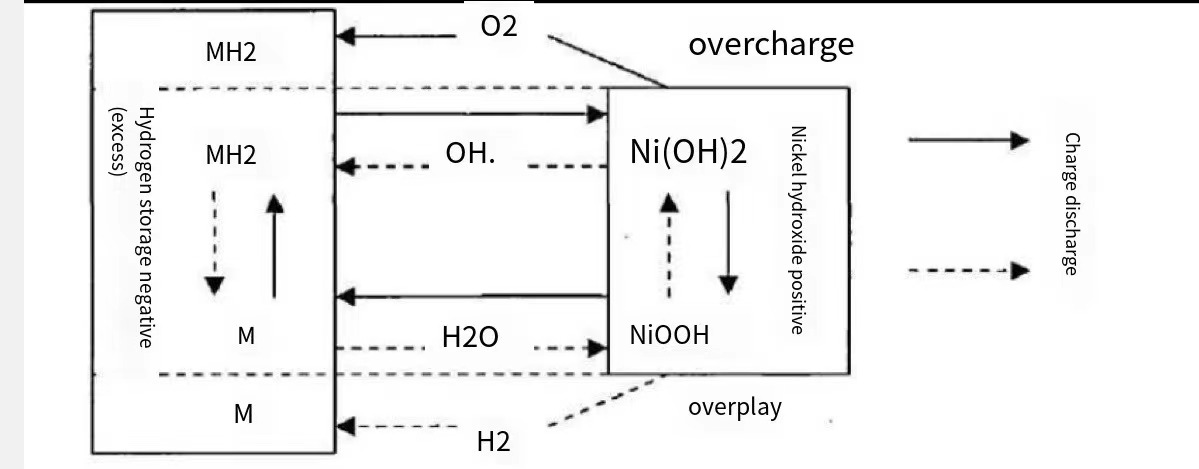
Figure 1. Illustration of the charging and discharging process of NiMH battery.
Nickel-metal hydride batteries commonly employ Ni(OH)2 as positive electrode material. The electrolyte used in these batteries consists of a 30% concentrated aqueous solution of KOH and LiOH.
Generally, NiMH batteries can be divided into three operating states: normal operating state, overcharge state, and over-discharge state.
Under normal conditions, the total chemical reaction equation of the NiMH battery is shown in equation (1). The reaction equation from left to right is the charging process, and the reaction equation from right to left is the discharging process. The positive and negative reaction equations during charging are shown in equations (2) and (3), respectively. The total reaction equation is shown in equation (4). The positive and negative reaction equations during discharge are shown in equations (5) and (6), respectively. The total reaction is shown in equation (7).
MH + NiOOH ↔ M + Ni(OH)2 (1)
Ni(OH)2 +OH— → NiOOH+H2O+e- (2)
M+H2O+ e-→ MH+ OH- (3)
Ni(OH)2 + M → MH + NiOOH (4)
NiOOH+ H2O + e-→ Ni(OH)2 + OH- (5)
MH + OH- → M + H2O + e- (6)
MH + NiOOH → M + Ni(OH)2 (7)
Where M is a hydrogen storage alloy and MH is a hydrogen storage alloy with adsorbed hydrogen atoms.
During the discharge of a NiCd battery, hydrogen atoms are formed through the transfer of protons from the positive electrode to the negative electrode in a reversible electrochemical reaction. Conversely, the charging process reverses this reaction, with protons moving from the negative electrode to the positive electrode to form hydrogen atoms. The electrolyte in this reaction does not take part in the chemical reaction itself but rather facilitates ion migration and charge transfer between the electrodes. However, during overcharge and over-discharge, water molecules are involved in the reaction.
When the NiMH battery is overcharged, the charging reaction on the positive electrode is transformed into the oxygen precipitation reaction of electrolytic water, and the concentration of electrolyte or the total amount of water in the battery will not change except for the hydrogen precipitation reaction of water generated on the negative electrode. The rate of oxygen compounding on the negative plate is very fast, and as long as the heat generated can be transferred out in time to avoid the thermal runaway phenomenon, the battery can fully withstand a certain rate of overcharging. The positive electrode reaction and negative electrode reaction equations are shown in equations (8) and (9), respectively.
4OH-→ 2H2O+O2 +4e- (8)
2H2O+O2 +4e- → 4OH- (9)
If a NiMH battery becomes over-discharged, hydrogen is generated at the positive electrode while hydrogen is consumed at an equivalent rate at the negative electrode. Therefore, the NiMH battery can be continuously discharged without hydrogen pressure accumulation or electrolyte concentration change. Its positive and negative electrode reaction equations are shown in equations (10) and (11), respectively. The NiMH batteries have good resistance to overcharge and over discharge as seen from the overcharge and over discharge reactions [8].
2H2O + 2e- → H2 + 2OH- (10)
H2 +2OH- → 2H2O+2e- (11)
3.3. Preparation of nickel-chromium cells
3.3.1. Preparation of NiCr cells. The NiCd battery is composed of a negative electrode made of cadmium (Cd), a positive electrode made of nickel oxyhydroxide (NiOOH), and an electrolyte that typically contains either sodium hydroxide (NaOH) or potassium hydroxide (KOH). The chemical reactions occurring at the positive and negative electrodes can be expressed by equations (12) and (13), respectively. The reaction process diagram is shown in Figure 2.
Cd+2OH-→ Cd(OH)2 +2e- (12)
2NiOOH +2H2O→ 2Ni(OH)2 +2OH- (13)
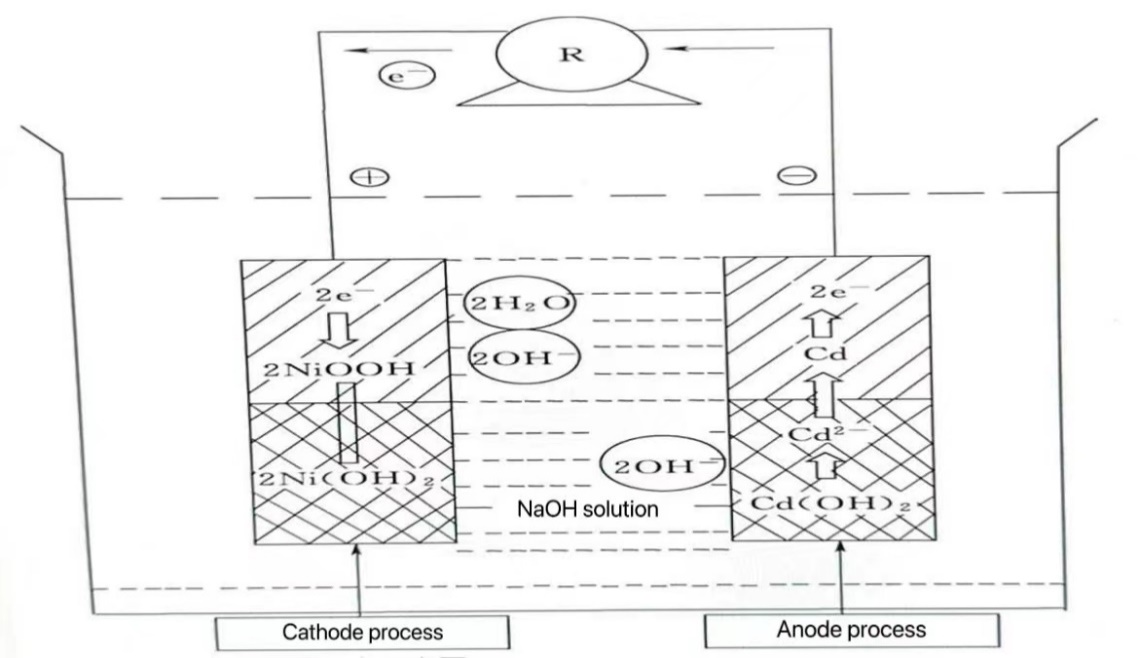
Figure 2. Illustration of electrode reaction of NiCr cell.
3.3.2. Preparation of active substances. The anode active substance Ni(OH)2 can be divided into normal type Ni(OH)2 and spherical type Ni(OH)2. The reaction equations for the preparation of ordinary Ni(OH)2 and spherical Ni(OH)2 are shown in Eqs. (14) and (15), respectively. The preparation of negative electrode active material is generally done by preparing CdO first, then using CdO to prepare the Cd electrode, and converting CdO into Cd electrode when the battery is formed; it is also possible to prepare cadmium metal in sponge form directly. In addition, due to the actual use of the battery, the cadmium metal will be passivated, such as at higher overpotential, higher discharge current, or repeated charge and discharge of cadmium metal recrystallization. Therefore, a certain amount of surfactant or additive is added in the preparation of the negative electrode to inhibit the passivation of cadmium metal. In actual production, Sura oil or No.25 transformer oil is commonly used, and Fe3O4 is commonly used as an additive
NiSO4 +2NaOH → Ni(OH)2 +Na2SO4 (14)
Ni2 +2OH- →Ni(OH)2 (15)
3.3.3. Electrode preparation. The electrodes of NiCd battery can be divided into electrodes with plate cassette and electrodes without plate cassette according to the different requirements of battery performance by manufacturing process, among which electrode without plate cassette includes a sintered electrode, bonded electrode, nickel foam electrode, nickel fiber electrode, and electrodeposition electrode.
There is electrode plate box type electrode, also called bag type electrode, which has the advantages of strong structure, vibration resistance, long life, small self-discharge, and low manufacturing cost, but the specific energy is low. Sintered electrode, according to the thickness of the electrode plate is generally divided into plate sintered electrode and foil sintered electrode. Among them, the thickness of plate-sintered electrodes is 2~3 mm for the positive electrode and 1.3~1.8 mm for the negative electrode, which is characterized by. The electrode has a long life, small self-discharge, and low manufacturing cost, but the specific surface area is small, which limits its charging and discharging rate; the thickness of the electrode plate of foil electrode is 0.5~1.0 mm, the specific surface area is large, and the assembled battery has a small internal resistance, suitable for high current discharge and good resistance to overcharge, but the self-discharge is larger than that of plate electrode.
3.3.4. Preparation of NiCd battery. The construction of a typical cassette battery is as follows: firstly, the positive and negative electrodes are made into positive and negative electrode plates through powder coating, strip embossing, slicing, and ribbing, and finally pole plate forming; secondly, the positive and negative electrode plates are interspersed alternately, with one more negative electrode plate than positive electrode plate, and the plates are separated from each other by insulating isolators; then, the plates are put into iron or plastic shells, sealed, and the poles are led from the pole holes of the lid; finally, the electrodes are This step is to remove sulfate and carbonate, remove floating ash on the electrode surface, and activate the active material.
4. Applications of battery
4.1. Lithium battery applications and problems
The battery industry has seen a significant increase in the utilization of lithium batteries in recent years, and they are now commonly used in energy storage systems. Additionally, lithium batteries have become increasingly popular in various electric-powered devices. Currently, lithium batteries are frequently utilized in electric bicycles and electric vehicles, among other applications.
In the field of electric vehicles in China, lithium batteries are lightweight, convenient as well as safe and other advantages, lithium batteries replace traditional lead-acid batteries as an inevitable trend in the development of electric vehicles [9]. Regarding the use of electric vehicles, the electric vehicle industry is actively developing the latest generation of lithium-ion batteries due to their pollution-free, low-pollution, and energy-diversifying features. Therefore, the application of lithium-ion batteries provides an additional viable solution to the current problem. Additionally, due to their exceptional performance characteristics, lithium batteries are increasingly being utilized in the aerospace industry to enhance launch and flight capabilities, as well as ground operating systems.
Lithium-ion batteries have become a prevalent energy storage option in recent years, thanks to their various advantages, such as high operating voltage, high specific energy. As a result, they are now an excellent substitute for conventional batteries. However, it also needs to improve its disadvantages. In contrast to other rechargeable batteries, the capacity of lithium-ion batteries decreases gradually with usage and is also influenced by temperature. The electrochemical reaction inside the lithium-ion battery generates heat. The composition of the cell collector and the microscopically inhomogeneous nature of the porous electrode make the electrostatic and chemical potentials non-uniformly distributed throughout the electrode; and the two, as the driving force of the electrochemical reaction, determine the non-uniform distribution of the reaction current in the electrode, so the heat generated by the electrochemical reaction will be high in the internal part of the electrode. Elevated temperatures in the vicinity of the graphite negative electrode can cause the solid electrolyte film on its surface to decompose and also result in the decomposition and evaporation of LiPF6 within the electrolyte [10]. The positive feedback mechanism of the decomposition is shown in.
Therefore, lithium-ion batteries have the problem of aging and fear of heat, so the high operating current of electronic products is more likely to reflect the phenomenon of heat decay, in addition, to avoiding the impact of external temperature. Replacing graphite with lithium titanate seems to extend its life. In addition, intolerance to overcharge, over-discharge is also one of the current problems of lithium-ion batteries. Therefore, lithium batteries must be used frequently, to avoid maintaining a full charge state and continuously plugged into the charger connector, to make the appropriate flow of electrons stored inside at regular intervals, to maintain the long-term health of the battery.
4.2. Development and problems of NiMH batteries
The development of NiMH batteries is facing competition with primary batteries, NiCd batteries, and Li-ion batteries at the same time, so it needs to show its relative competitiveness to develop a market segment that suits its performance characteristics and value. In the competition with primary batteries, it is necessary to satisfy customers so that they can use the batteries directly without recharging them after buying them. It is possible to keep the charge for a long time after charging and be available at any time.
The characteristics of different industries require very differentiated NiMH batteries. In the consumer market, high capacity and long life are important performance indicators for market segmentation; while power market demand such as vacuum cleaners and power tools have higher requirements for high discharge rate; fast charging, overcharge resistance, and over-discharge resistance are very demanding for high-end personal care products; high-temperature resistance is the most important indicator for the future emergency light market; high consistency is important for multi-series applications such as electric vehicles.
The future development of electric vehicles needs to be analyzed from three aspects: resource utilization, technology, and industrial policy, to obtain a suitable strategy for the development of China's electric vehicle industry. The rechargeable battery industry and related resources involve the main basic resources for battery manufacturing, various forms of energy, and resources required by the battery application market; the main basic resources required by rechargeable batteries include lead, lithium, cobalt, nickel, and rare earth. 74 million tons, and 100 million tons of rare earth. China's reserves are about 16.4% for lead, 10% for lithium, less than 0.1% for cobalt, 0.17% for nickel, and more than 50% for rare earth, respectively, and the future development of electric vehicles may be significantly limited by resource constraints [11].
The economic value of recycling used NiMH batteries is very high, because the batteries contain a large amount of nickel, diamonds and rare earth, and other precious mineral resources. Increasing the recycling of used NiMH batteries is not only beneficial to reduce the cost of batteries, but also can effectively promote the improvement of social and economic benefits, which is of great practical significance to the sustainable development of society. However, from the current situation, China's recycling of used NiMH batteries is still in its initial stage, and there is still a gap between its recycling level and that of developed countries such as the West, and the reasons for this are mainly caused by the following three aspects. First, the production and sales of NiMH batteries are relatively small compared with those of developed countries, and the number of used batteries that fail is not large enough so the relevant technical personnel does not pay enough attention to the technical research on recycling of used batteries. Secondly, people's awareness of recycling is weak, and their awareness of environmental protection is not strong. They do not recognize the importance of recycling used NiMH batteries, and often discard and dispose of used NiMH batteries together with household garbage.
Third, the chemical composition of NiMH batteries is more complex, that is, NiMH batteries are not only more complicated in the internal metal structure, but also more complicated in the form of existence, which makes the recycling of used NiMH batteries more difficult [12].
4.3. Application and problems of NiCd battery
Nickel-cadmium battery is a commonly used rechargeable battery, which is widely used in various electronic devices and power tools. Nickel-cadmium batteries are mainly used in portable electronic products, such as digital cameras, video cameras, cell phones, stethoscopes, etc. In addition, NiCd batteries are also widely used in industrial, medical, and military fields, such as protective equipment, medical devices, wireless communication equipment, etc.
NiCd batteries have several unique features that make them one of the most commonly used types of rechargeable batteries. Some of these features include the fact that NiCd batteries have a high energy density and are therefore widely used in many fields. For example, in aviation and aerospace, NiCd batteries are used to store solar and wind energy to power flying vehicles. NiCd batteries have high reliability and can be used in a variety of harsh environments. Nickel-cadmium batteries can be recycled many times. Nickel-cadmium batteries can be charged and discharged for many cycles, so they have high value for long-term use. Nickel-cadmium batteries are more environmentally friendly and do not contain harmful substances, so they are friendly to the environment. However, NiCd batteries also have some shortcomings, such as lower charging efficiency and short cycle life. Therefore, more advanced battery technologies are now being sought to replace NiCd batteries.
Overall, NiCd batteries are an important battery technology with a wide range of applications in a variety of fields. With the continuous improvement of battery technology, NiCd batteries will continue to have a significant role in the future.
5. Conclusion
As the energy industry has continued to grow in recent years, new energy batteries have become increasingly critical, serving as the “heart” of the industry due to their pivotal role in power generation and storage. In particular, the popularity of new energy vehicles and the market's strong appeal have pushed new energy batteries to the forefront of technology. This paper mainly discusses the working principle, reaction mechanism, preparation method, advantages and disadvantages, application field, and application and problems of three types of batteries (Li-ion battery, NiMH battery, NiCd battery). Each of these three types of batteries has its advantages and disadvantages and plays an important role in various fields because of its unique characteristics. Lithium batteries are known for their extremely low auto-discharge rate and flat discharge voltage. The main characteristics of NiCd batteries are fast charging and discharging, no pollution as well a strong life. The biggest difference between NiCd batteries and the first two is their long storage period and high safety performance. As an emerging technology, the new energy battery has great potential and challenges in future development. Through continuous technological innovation and continuous application practice, new energy batteries will achieve a more efficient, environmentally friendly, and convenient energy supply, bringing more convenience and contribution to people's lives.
References
[1]. Gallard H., Laat J.D. (2000) Kinetic modelling of Fe(III)/H2O2 oxidation reactions in dilute aqueous solution using atrazine as a model organic compound. Water Research, 34(12): 3107-3116.
[2]. Sakai T., Uehara I., Ishikawa H. (1999) R&D on metal hydride materials and Ni-MH batteries in Japan[J]. Journal of Alloys & Compounds, 293-295:762-769.
[3]. Saotome Y., Nakazawa Y., Yamada Y. (1999) Disassembling and materials recovering process of alkaline manganese dry batteries by vacuum-aided recycling systems technology (VARS Tech). 53(1-2):101-104.
[4]. Takahashi M., Tobishima S.I., Takei K., et al. (2002) Reaction behavior of LiFePO4 as a cathode material for rechargeable lithium batteries. Solid State Ionics, 148(3-4):283-289.
[5]. Bates J.B., Dudney N.J., Neudecker B., et al. (2000) Thin-film lithium and lithium-ion batteries[J]. Solid State Ionics, 135(1-4):33-45.
[6]. Zhang W.H., He W., Feng P., et al. (2013) Improved Electrochemical Properties of Al3+-doped 0.5Li2MnO3-0.5LiCo1/3Ni1/3Mn1/3O2 Cathode for Lithium Ion Batteries. Journal of Inorganic Materials, 28(11):1261-1264.
[7]. Huang W.L., Liang K.M., Gu S.R. (1999) Influence of Gelation Speed and Calcination on Surface Fractal Dimensions of Silica Xerogels. Journal of Inorganic Materials, 14(2):302-306.
[8]. Martin, Winter, Jürgen, et al. Insertion Electrode Materials for Rechargeable Lithium Batteries. Advanced Materials, 1998.
[9]. Master's e Journal Publication Information: Year: Issue 01, 2021.
[10]. Plett G.L. (2006) Sigma-point Kalman filtering for battery management systems of LiPB-based HEV battery packs. Journal of Power Sources, 161(2): 1369-1384.
[11]. Morgan D., Ven A., Ceder G. (2004) Li conductivity in LixMPO4 (M = Mn, Fe, Co, Ni) olivine materials. Electrochemical and Solid-State Letters, 7(2): A30.
[12]. Energy Storage Principles and Technology by C.G. Huang 5.1 NiCd Batteries, 2020-6.
Cite this article
Chen,S.;Chen,Z.;Liu,Z. (2023). Preparation and application of lithium batteries, nickel-hydrogen batteries and nickel-cadmium batteries. Applied and Computational Engineering,23,59-67.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2023 International Conference on Functional Materials and Civil Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Gallard H., Laat J.D. (2000) Kinetic modelling of Fe(III)/H2O2 oxidation reactions in dilute aqueous solution using atrazine as a model organic compound. Water Research, 34(12): 3107-3116.
[2]. Sakai T., Uehara I., Ishikawa H. (1999) R&D on metal hydride materials and Ni-MH batteries in Japan[J]. Journal of Alloys & Compounds, 293-295:762-769.
[3]. Saotome Y., Nakazawa Y., Yamada Y. (1999) Disassembling and materials recovering process of alkaline manganese dry batteries by vacuum-aided recycling systems technology (VARS Tech). 53(1-2):101-104.
[4]. Takahashi M., Tobishima S.I., Takei K., et al. (2002) Reaction behavior of LiFePO4 as a cathode material for rechargeable lithium batteries. Solid State Ionics, 148(3-4):283-289.
[5]. Bates J.B., Dudney N.J., Neudecker B., et al. (2000) Thin-film lithium and lithium-ion batteries[J]. Solid State Ionics, 135(1-4):33-45.
[6]. Zhang W.H., He W., Feng P., et al. (2013) Improved Electrochemical Properties of Al3+-doped 0.5Li2MnO3-0.5LiCo1/3Ni1/3Mn1/3O2 Cathode for Lithium Ion Batteries. Journal of Inorganic Materials, 28(11):1261-1264.
[7]. Huang W.L., Liang K.M., Gu S.R. (1999) Influence of Gelation Speed and Calcination on Surface Fractal Dimensions of Silica Xerogels. Journal of Inorganic Materials, 14(2):302-306.
[8]. Martin, Winter, Jürgen, et al. Insertion Electrode Materials for Rechargeable Lithium Batteries. Advanced Materials, 1998.
[9]. Master's e Journal Publication Information: Year: Issue 01, 2021.
[10]. Plett G.L. (2006) Sigma-point Kalman filtering for battery management systems of LiPB-based HEV battery packs. Journal of Power Sources, 161(2): 1369-1384.
[11]. Morgan D., Ven A., Ceder G. (2004) Li conductivity in LixMPO4 (M = Mn, Fe, Co, Ni) olivine materials. Electrochemical and Solid-State Letters, 7(2): A30.
[12]. Energy Storage Principles and Technology by C.G. Huang 5.1 NiCd Batteries, 2020-6.









