1. Introduction
With the national vigorous promotion of the development of the new energy industry in recent years, many new energy materials have appeared on the market. As an industry that is still in a period of rapid development, there are many research areas to be developed. As a new material capable of overcoming many of today's significant challenges, lithium titanate holds great promise for future applications, and improvements based on its conditions are a significant development direction for the future new energy industry.
For lithium titanate materials, Xiong et al. [1] introduced the methods and successful cases of self-modification of lithium titanate materials in the article "Surface modification of lithium titanate", whose experiments are mainly based on changing the pH value of lithium titanate. In the article "Surface modification of lithium titanate", the experiments of the material itself are mainly based on changing the pH value of lithium titanate material to achieve the modification of lithium titanate material, The research in this paper is to apply nanotechnology to the doping of conventional lithium titanate material to improve the electrical conductivity of lithium titanate material for greater research progress in the energy conduction of batteries [2].
This paper focuses on conventional lithium titanate materials and nanotechnology. It later combines the application of both to improve the electrical conductivity of lithium titanate materials at the existing stage by combining the advantages of both with new means. Also, this paper presents a new outlook based on the improvement of lithium titanate anode materials
2. Nanotechnology
2.1. Classification of nanotechnology
In 1959, the famous physicist Feynman first heralded the emergence of nanotechnology when he proposed that humans could Sciencefreely manipulate individual atoms and molecules according to their will. Since then, research into nanosystems (1nm to 100 nm) has flourished. Nanotechnology was born and emerged in the 1980s. Nanotechnology is fully integrated and penetrated the research fields of modern science, and its classification is based on the classification of modern scientific fields, forming many new disciplines, which can be broadly classified into nanophysics, nanochemistry, nanomaterials, nanobiology, nanoelectronics, nanofabrication and so on. In the field of new energy batteries, the main applications of nanotechnology can be divided into nanofabrication, nanomaterials, and nanoelectronics.
2.2. Advantages and disadvantages of each nanotechnology
2.2.1. Nanofabrication technology. Nanofabrication is a high-precision, high-efficiency precision machining technology. It has a wide range of applications and can be used to manufacture a variety of nanostructures and devices, such as nanosensors, nanoelectronic components, nano-optical components, nano-memory devices, etc. The most critical aspect of nanofabrication technology is the ability to precisely control and process materials to enable the fabrication and improvement of nanostructures. Nanofabrication technology can also improve the properties of materials, such as strength, hardness, electrical conductivity, optical properties, etc. The improvement of these properties can greatly expand the field of application of materials.
The application of nanofabrication technology requires a high degree of expertise and sophisticated equipment. Unlike traditional processing techniques, nanofabrication technology requires the use of advanced material handling, imaging and control techniques such as scanning electron microscopes, atomic force microscopes and laser interferometers. These high-end equipment are not only expensive, but also require specialist technical personnel to maintain and operate. As a result, the cost of research and application of nanofabrication technology is very high and is currently a major barrier limiting its large-scale application.
However, the development and application of nanofabrication technology still holds great potential and promise. With the development and continuous innovation of nanotechnology, nanofabrication technology is also progressing and improving. Many research teams have already made important breakthroughs in the field of nanofabrication technology, for example, through self-assembly technology, nano-printing technology and electron-beam exposure technology, which enables precision processing at the nanoscale. It is expected that in the future, with the development of new materials and improvements in processing technology, nanofabrication technology will be more widely used and promoted.
2.2.2. Nanoelectronics. Nanoelectronics is an emerging discipline of electronics dedicated to the study and application of electronic components, circuits, integrated devices and information processing technologies at the nanoscale. The development of nanoelectronics has been made possible by the continuous advances in modern nanofabrication technology, which has enabled the fabrication of a wide range of tiny, high-precision electronic components and devices at the nanoscale. The challenges and opportunities facing nanoelectronics are unique compared to traditional electronics. The many new features of the morphology of electrons in nanomaterials and devices allow us to explore and exploit new quantum mechanical mechanisms, leading to more efficient, stable and reliable electronic devices and information processing systems.
Nanoelectronics has a wide range of applications and can be used in a variety of fields such as information technology, biomedicine, energy and more. For example, in the field of information technology, nanoelectronics can be used to create faster and more compact processors and memory devices, thus advancing computer technology. In biomedicine, nanoelectronics can be used to create highly sensitive, high-resolution biosensors and detectors, enabling more accurate disease diagnosis and treatment. In the energy sector, nanoelectronics can be used to make highly efficient solar cells, energy storage devices and energy saving devices, thereby promoting renewable energy.
Despite the broad promise and potential of research and applications in nanoelectronics, there are a number of challenges and problems to be overcome. For example, there are material defects and inhomogeneities in the preparation of nanomaterials and devices, which can have an impact on the performance and reliability of the devices. In addition, quantum effects and noise issues in nanoelectronics need to be adequately addressed. Therefore, we need to continuously explore and innovate new nanomaterial and device preparation techniques to achieve more efficient, stable and reliable nanoelectronic components and information processing systems.
2.2.3. Nanomaterials science. Nanomaterials science is a discipline that studies the structure, property and preparation methods of materials at the nanoscale. Compared to conventional materials, nanomaterials have unique physical, chemical and mechanical properties due to their special size and surface effects. At the nanoscale, the properties of materials change significantly due to the self-organisation brought about by strong coherence, including thermal stability, electrical conductivity, magnetism, optics, etc. These changes make nanomaterials promising for a wide range of applications in many fields, such as energy, catalysis, biomedicine, sensors, etc.
Nanomaterials are prepared by physical, chemical and biological methods. Physical preparation methods include sputtering, ion beam and magnetron sputtering techniques, chemical preparation methods include sol-gel, precipitation and vapour phase deposition techniques, and biological preparation methods are the use of biological systems to synthesise nanomaterials. With the rapid development of nanotechnology, more and more new nanomaterials have been developed and applied to practical production and life, such as nanotubes, nanoparticles, nanocrystals, nanowires, etc.
3. Lithium titanate batteries
3.1. Conventional negative electrode materials
The negative electrode material is the carrier of lithium ions and electrons during the charging and discharging process of the battery and plays the role of energy storage and release. As the lithium ion embedding body negative electrode material to meet a number of requirements such as redox potential as close as possible to the lithium ion, can make the battery input voltage is high, the body of the negative electrode material in the reaction rarely change, have a good electron conductivity and ion conductivity and charge and discharge process smooth [3]. Currently there are several types of traditional negative electrode materials. The first is carbon materials including graphite, hard carbon, soft carbon etc. Graphite generally has relatively stable chemical properties as well as high temperature resistance and good electrical conductivity. This gives it a high commercial value. It also has the advantage of a low voltage plateau and high cycle efficiency. However, with long-term charging and discharging, graphite negative electrode batteries also have safety risks. During long-term charging and discharging, graphite cathode materials are prone to lithium dendrites, which can lead to short-circuiting of the battery, while the layer spacing becomes smaller and the graphite falls off. The second category is the transition technology oxide cathode material. The greatest characteristic of transition metal oxide as anode material is its high capacity. Compared to carbon materials, transition metal oxide anode materials are used in conversion reactions, so they have a very high energy density during the reaction but are not as efficient as carbon materials in terms of electrical conductivity, which destabilises the structure and causes it to collapse. The last type of anode material is alloyed anode materials, mainly tin alloys and silicon-based alloys. They have received a lot of attention because of their good electrical conductivity and stability [4].
3.2. Lithium titanate cells
lithium titanate battery is a lithium battery with lithium titanate as the anode material (figure 1) [5]. When the lithium titanate battery is charged, lithium positive ions from the lithium ternary material enter the electrolyte under the action of electric field forces, pass through the diaphragm, then migrate through the electrolyte to the surface of the negative lithium titanate crystal and then embed in the negative material [3].
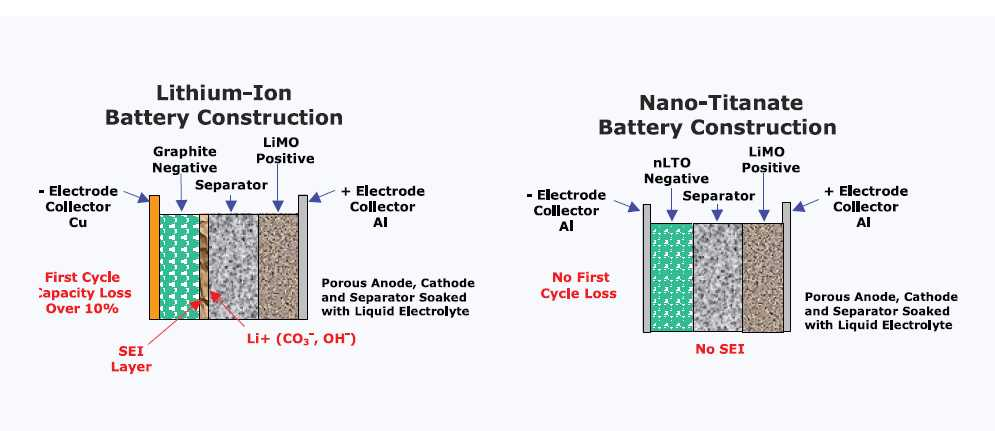
Figure 1. Lithium battery with lithium titanate as anode material [5].
When the battery is discharged, Li is de-embedded from the lithium titanate material and migrates to the surface of the lithium ternary material. The basic principle of lithium titanate batteries is that the lithium ions are de-embedded back and forth during the charging and discharging process.
3.3. Advantages and disadvantages of lithium titanate cells
The advantage of lithium titanate is that, on the one hand, lithium titanate batteries have an embedded lithium potential of up to 1.55V, which ensures that lithium titanate batteries will not have lithium dendrites and solid electrolyte interface film S E I film formation during operation, while the internal resistance of lithium titanate batteries increases sharply when short-circuiting, ensuring that the current will not be too large or produce a sudden temperature rise. The safety of lithium titanate batteries is guaranteed [4]. On the other hand, lithium titanate is known as a "zero strain" material due to the very small volume change during lithium embedding/de-embedding, which gives it excellent reversibility and a long cycle life [6]. Although lithium titanate has great potential as an anode material, there are still some issues that hinder the development of lithium titanate batteries [7]. 1.55V operating potential also leads to a lower energy density of lithium titanate batteries and is more pronounced in low temperature environments. The structural instability of lithium titanate at low temperatures prevents lithium-ion de-embedding and embedding, and changes in the diffusion path of lithium ions ultimately lead to reduced charge and discharge performance. Battery bulging is also a major problem in lithium titanate batteries today. The spinel structure of lithium titanate tends to absorb water and reacts to produce hydrogen gas during charging and discharging leading to battery bulging, while the unique (Ti-O) bonding of lithium titanate also catalyzes the production of gas from the electrolyte [8]. This is also the biggest safety concern of lithium titanate batteries.
4. Applications
4.1. Application of nanotechnology in the modification of lithium titanate
The current application of nanotechnology in lithium titanate materials is to modify lithium titanate cathode materials with nanotechnology to give them higher performance. Three examples of nanotechnology modification of lithium titanate cathode materials are described below. The first is nanosizing, by which lithium titanate materials can be nanosized to obtain some special electrical properties. Smaller lithium titanate particles shorten the diffusion distance of ions and electrons within the material, which improves the multiplicative properties of lithium titanate anode materials. With the development of technology, it is now easier to synthesise three-dimensional lithium titanate nanofibres from one-dimensional lithium titanate nanomaterials produced by electrostatic spinning, and the electrodes prepared by this method have a high stability. Kim et al, used tert-butanol as a solvent to synthesise lithium titanate nanofibres and formed a sea urchin-shaped structure by self-assembly, which has a high specific heat capacity for discharge [9]. Next is the surface wrapping technology. Because of the low conductivity of lithium titanate, the performance at high current densities does not excel the multiplicative performance of the electrodes affected. In order to improve this defect, lithium titanate materials covered with conductive materials on the surface of lithium titanate anode material conductivity optimization has become one of the mainstream strategies. This not only improves the conductivity but also isolates the electrolyte and reduces side reactions, which to some extent reduces the defect of bulging cells. Researchers are currently using an in-situ coating strategy to apply carbon coatings. The presence of carbon materials inhibits the growth of lithium titanate crystals and enables the synthesis of smaller lithium titanate particles, which helps to improve the multiplicative performance of the material. The material was coated with a 1-10 nm thick carbon layer [10]. The last type is doping modification, where the researchers replaced Ti, Li and O in lithium titanate with other doping elements. It was shown that a small amount of ion doping can significantly alter the chemical bond length and the local environment of the lattice of lithium titanate crystals, bringing about lattice defects and thus improving the lower electron and lithium ion transport properties of lithium titanate.
4.2. Application of lithium titanate batteries
Lithium titanate batteries can be used in many special applications because of their characteristics. For example, the auxiliary power storage of high-speed trains. The auxiliary power storage of high-speed rail trains is a backup power supply for the power supply system of the trains when it fails, and is a core component of the emergency system, mainly used for interior lighting and other purposes. Therefore, there must be special requirements for the auxiliary power storage of high-speed rail, such as light weight, high safety performance, and suitable for special temperatures. Compared to conventional lithium batteries, lithium titanate has a high safety level, a long service life and low temperature characteristics that make it perfectly suited to the auxiliary power storage system of high-speed trains. The low temperature resistance of lithium titanate is also suitable for many applications. Other anode materials have a reduced ability to embed and de-embed at low temperatures, which can lead to short circuits due to deformation per cell volume. Lithium titanate, however, has only 0.3% deformation per unit cell volume at low temperatures and does not produce lithium dendrites that can deteriorate the anode. Because of its low-temperature resistance, lithium titanate can also provide excellent power supply stability for 5G base station construction in cold northern regions [11]. There is also widespread use in public transport. For example, lithium titanate batteries are used in Changzhou's T1 tram line, which only requires four minutes of charging at the end of the station to ensure the normal operation of the whole station for 12 kilometres. Line 5 of the Kunming Metro also uses lithium titanate as an auxiliary battery. The electric capacity is able to meet the emergency lighting and emergency ventilation in the car. It also meets the requirements for the doors to open and close once as well as self-traction in the base. It is the safety of lithium titanate batteries and their long cycle life that make them an important emergency energy source.
4.3. Outlook
Lithium titanate has a promising future as an anode material in lithium-ion batteries. As a "zero-strain" material, lithium titanate has a high degree of stability and safety in the battery [12]. However, due to its own low electronic conductivity and high multiplicity performance capacity decay faster and other problems, hindered the widespread use of lithium titanate battery in the market. In order to solve these problems, later research can be carried out by the preparation process of lithium titanate with various schemes of doping modification, material nanosizing and surface covering to increase its electrical conductivity and improve the performance degradation problem and battery bulging problem.
Based on the advantages of lithium titanate such as high safety, stability and long cycle life, lithium titanate has a broad prospect of becoming the future mainstream anode material for power and energy storage lithium-ion batteries. Especially in the growing market of new energy vehicles and energy storage, lithium titanate will play an important role. In addition, lithium titanate battery is a green and environmentally friendly battery, which helps to reduce environmental pollution. It is believed that with the continuous technological progress and innovation, lithium titanate batteries will have a broader market prospect and play a significant industrial and commercial value.
5. Conclusion
With the booming development of the energy industry, lithium batteries have been widely studied for their high energy density and high charge/discharge efficiency. Lithium titanate, the anode material of lithium batteries, has been widely studied and applied for its higher performance and safety. This paper introduces the basic nanotechnology and the advantages and disadvantages of lithium titanate compared with conventional lithium batteries. To address the disadvantages of lithium titanate batteries, this paper discusses the surface modification of anode materials with nanotechnology to enhance the electrical conductivity of lithium titanate. These include making the material more stable by nanosizing, and the smaller distance between the particles after nanosizing greatly improves the efficiency of lithium ion and electron transport between the particles. The surface coverage makes the anode material more conductive while reducing the contact between the anode material and the electrolyte, thus reducing the generation of side reactions. Finally, doping modification improves the performance of lithium titanate by changing the chemical bond length and lattice layout. These methods of improving lithium titanate performance through nanotechnology have great promise for future applications. This paper also discusses the current profitability of lithium titanate batteries in various applications and analyzes the advantages and some problems that need to be improved. This paper provides an outlook for the future large-scale application of lithium titanate batteries.
References
[1]. Xiong Y.L., Jing L., Xu S.Z. et al. Study on surface modification of lithium titanate materials. Power Technology, 2015, 39(01): 52-53.
[2]. Wu Y.H., Lai C.B., Li X.X. et al. Design and preparation of capacitive lithium titanate batteries. Journal of Shanghai Jiaotong University, 2023, 1-9.
[3]. Wang G. Preparation of lithium titanate/carbon nanotube anode materials and their electrochemical properties. Hunan University, 2020.
[4]. Zhu H., Yao J., Jiang L. (2018). Lithium titanate anode materials for lithium-ion batteries: A review. Journal of Materials Science & Technology, 34(11), 1867-1882.
[5]. Lithium Ion Batteries (LI-ION) | Energy Storage Association. (2021, April 7). Energy Storage Association. https://energystorage.org/why-energy-storage/technologies/lithium-ion-li-ion-batteries/
[6]. Luo., Chu., Huang., Sun., Li. (2014). Fundamental scientific issues in lithium-ion batteries (VIII)- anode materials. Energy Storage Science and Technology, 3(2), 146-163.
[7]. Xie H., Zhu X., Zhang J., Wang L., Liu Y. (2021). Review on synthesis, modification and application of lithium titanate as anode material for lithium-ion batteries. Journal of Energy Storage, 42, 103959.
[8]. Xiao M., Chen J., Liu Y., Zhu K., Zhang Q. (2020). Lithium titanate as anode material for lithium-ion batteries: A review. Journal of Energy Chemistry, 49, 225-240.
[9]. Yin Y.H., Li S.Y., Fan Z.J., et al. Synthesis of novel anode Li4Ti5O12/C with PAN as carbon source and its electrochemical performance. Materials Chemistry and Physics, 2011, 130(1-2): 186-1900.
[10]. Zhu K., Gao H., Hu G., et al. Scalable synthesis of hierarchical hollow Li4Ti5O12 microspheres assembled by zigzag-like nanosheets for high rate lithium-ion batteries. Journal of Power Sources, 2017.
[11]. Zhang Y., Wang Y., Liu Z., et al. A battery for minimalist 5G base stations in low-temperature environment regions. Post & Telecom Design Technology, 2022, 560(10): 23-27.
[12]. Qin S.K., Liu D., Deng J.J. Research progress of lithium titanate for lithium-ion batteries. Jiangxi Chemical Industry, 2022, 38(03): 58-61.
Cite this article
Sheng,Z.;Yu,R. (2023). Application of nanotechnology in lithium titanate batteries. Applied and Computational Engineering,23,68-74.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2023 International Conference on Functional Materials and Civil Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Xiong Y.L., Jing L., Xu S.Z. et al. Study on surface modification of lithium titanate materials. Power Technology, 2015, 39(01): 52-53.
[2]. Wu Y.H., Lai C.B., Li X.X. et al. Design and preparation of capacitive lithium titanate batteries. Journal of Shanghai Jiaotong University, 2023, 1-9.
[3]. Wang G. Preparation of lithium titanate/carbon nanotube anode materials and their electrochemical properties. Hunan University, 2020.
[4]. Zhu H., Yao J., Jiang L. (2018). Lithium titanate anode materials for lithium-ion batteries: A review. Journal of Materials Science & Technology, 34(11), 1867-1882.
[5]. Lithium Ion Batteries (LI-ION) | Energy Storage Association. (2021, April 7). Energy Storage Association. https://energystorage.org/why-energy-storage/technologies/lithium-ion-li-ion-batteries/
[6]. Luo., Chu., Huang., Sun., Li. (2014). Fundamental scientific issues in lithium-ion batteries (VIII)- anode materials. Energy Storage Science and Technology, 3(2), 146-163.
[7]. Xie H., Zhu X., Zhang J., Wang L., Liu Y. (2021). Review on synthesis, modification and application of lithium titanate as anode material for lithium-ion batteries. Journal of Energy Storage, 42, 103959.
[8]. Xiao M., Chen J., Liu Y., Zhu K., Zhang Q. (2020). Lithium titanate as anode material for lithium-ion batteries: A review. Journal of Energy Chemistry, 49, 225-240.
[9]. Yin Y.H., Li S.Y., Fan Z.J., et al. Synthesis of novel anode Li4Ti5O12/C with PAN as carbon source and its electrochemical performance. Materials Chemistry and Physics, 2011, 130(1-2): 186-1900.
[10]. Zhu K., Gao H., Hu G., et al. Scalable synthesis of hierarchical hollow Li4Ti5O12 microspheres assembled by zigzag-like nanosheets for high rate lithium-ion batteries. Journal of Power Sources, 2017.
[11]. Zhang Y., Wang Y., Liu Z., et al. A battery for minimalist 5G base stations in low-temperature environment regions. Post & Telecom Design Technology, 2022, 560(10): 23-27.
[12]. Qin S.K., Liu D., Deng J.J. Research progress of lithium titanate for lithium-ion batteries. Jiangxi Chemical Industry, 2022, 38(03): 58-61.









