1. Introduction
In these days, silver nanoparticles (AgNCs) have become the representative of nanomaterials because of their special physical and chemical properties. The use of silver nanoparticles as fluorescent probes has been widely investigated due to their high fluorescence quantum yield, excellent photostability, subnanometre size, resistance to photobleaching, non-toxic and biocompatibility [1]. Silver nanoclusters are structures that are typically less than 3 nm in size and made of small clusters of a specific number of silver atoms with a well-defined structure and size. Silver nanoparticles exhibit SPR absorption in the visible range, however silver nanoclusters do not [2].
When stimulated by a light source, the AgNC can produce fluorescence. Silver nanoclusters' fluorescence characteristics can be exploited to create sensitive and targeted probes for a variety of analytes. To functionalize AgNCs as fluorescence probes, they are typically surface-modified with specific ligands or functional groups that can interact with the target analyte [3]. The function of AgNC can be amplified with different organic molecules or biomolecules by imparting a specific target signal, and their fluorescence varies according to their size, shape and surface chemistry [4].
While there are substantial reports on the application of AgNCs, the number of reports about factors that influenced AgNCs during fabrication is limited. So this article introduces recent breakthroughs about AgNCs for fluorescence probes from the four factors that influence the function mainly, which are size, shape, ligand, and surface chemistry. Furthermore, some applications of fluorescence AgNCs are also included, like sensing of metal ions, sensing of biomolecules, imaging of cells and tissues and even detection of environmental pollutants [5].
2. The effect of size of AgNC
2.1. Optical properties dependent on size
The size of metallic nanoclusters has a considerable impact on their optical characteristics. The sensitivity and dynamic range of a specific assay are determined by the optical properties most important for the design of fluorescence-based biosensors, especially for cancer diagnostics. These optical properties include fluorescence intensity, stability, and quenching efficiency in "off-on" probes [2].
The particle size of AgNCs is able to influence their absorption and emission wavelengths as biomarker, because the band gap of nanocluster is size-dependent. Smaller AgNCs with smaller band gap generally exhibit higher energy absorption and emission, resulting in a shift toward higher frequency (blue-shifted fluorescence) compared to larger AgNCs with a greater band gap. This tunability of optical properties allows for the optimization of AgNC size to match specific biomarker detection requirements [6].
2.2. Influence on stability due to surface energy changing by size
Although there is still no direct evidence of a relationship between the surface energy of AgNCs and their size, many studies agree with this conclusion. Theoretically, smaller AgNCs tend to have higher surface energy, which can lead to increased reactivity and potential aggregation. The contribution of the various surface sites, which are vertices and edges, which are predominate for small sizes, decreases with increasing size and decreases at the expense of the facets when there is an excess energy relative to a reference energy normalized to a surface area related to nanoclusters, which is defined as Gama. So larger AgNCs may possess enhanced stability due to a lower surface-to-volume ratio. It is crucial to balance the size to maintain the stability of AgNCs for long-term biomarker detection applications [7].
2.3. Effect on sensitivity of AgNC
AgNCs' sensitivity to the target biomarkers can be determined by the size of their particles. AgNC's huge surface area to volume ratio is an essential benefit in molecular detection when compared to bulk materials and other bigger nanoparticles. The surface of the nanocluster can be densely covered with components that can bind and recognize molecules thanks to this characteristic. Due to their higher surface area-to-volume ratio and higher density of biomarker binding sites, smaller AgNCs sometimes show higher sensitivity. When a smaller nanocluster is used to identify a cancer cell, for instance, numerous binding ligands are presented, which frequently enables multivalent effects that can increase an assay's sensitivity. However, depending on the specific application, the ideal particle size for achieving the maximum sensitivity may change [2].
2.4. Nanocluster size affects cellular uptake and biodistribution
The size of AgNCs can influence their cellular uptake and biodistribution in biological systems. Smaller AgNCs generally have better cellular penetration and distribution, enabling effective labeling and imaging of intracellular targets. However, larger AgNCs may offer advantages for specific applications, such as tumor targeting, where larger particles may exhibit enhanced retention and accumulation at the tumor site [2].
3. Effect from structure of probe
The conformation of the whole probe plays a critical role in the creation of the bright red emissive AgNCs and the stabilization of emissive species AgNCs. Pratik Shah developed AgNC-based DNA probes with bright red fluorescence emission for the detection of microRNAs (miRNAs). He showed that the propensity for mismatch self-dimer formation of the DNA probes are able to be a great indicator for the creation and stabilization of red emissive AgNCs. In his experiment, different DNA sequences were compared to show their ability to detect miRNA as we can see in Figure 1 [8]. In the result, unstructured 12nt, 15nt, and 18nt emissions revealed reduced, single-strand-like emission intensities. Whereas the 22nt and 23nt are able to develop mismatch self-dimers and emit more light at their maximal excitation (580 nm) than DNA-12nt-RED-160, and in the gel electrophoresis experiment, a self-dimer band was seen and bright red emissive AgNCs were generated in solution.
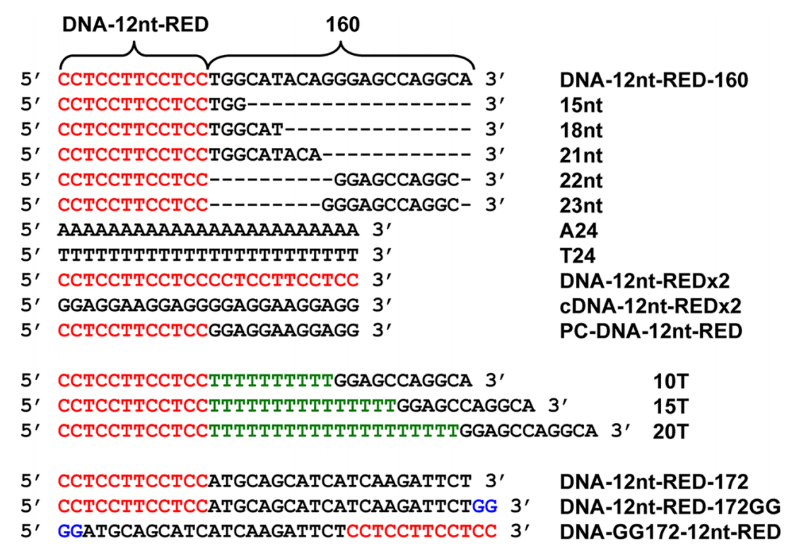
Figure 1. DNA sequences and their acronyms [8].
Because strong red emissive AgNC creation in the modified DNA-12nt-RED-160 probes may be related to the potential for building stable mismatch self-dimers and hairpins, DNA-12nt-RED-172 probe was less likely to notice red AgNC emission than the DNA-12nt-RED-160 probes. This event supports the conclusion that a high red emission can only be seen in the presence of an obvious mismatch self-dimer band [8].
4. The impact of ligands on AgNCs' photoluminescence
One of the optical characteristics of nanoclusters is photoluminescence (PL), which is extensively used in a wide range of scientific and technical applications, such as optical sensors, imaging, displays, and lighting. PL is the emission of light by a material following its absorption of photons, and it is a phenomenon commonly observed in various luminescent materials, like silver nanoclusters (AgNCs). In a comprehensive summary by Koustav Sahoo and Indranath Chakraborty, the impact of ligands on the optical properties, particularly PL features, of atomically accurate AgNCs has been thoroughly discussed. The authors highlight the significant impact of ligands on fine-tuning the emission characteristics of AgNCs, enabling their effective utilization in various applications [9].
The diversity of ligand types is uncommon for most nanoclusters, including AgNCs, in general because the ligand can greatly influence the atomicity of the ensuing NCs, and metal-ligand interactions during the NC formation are specific to distinct metals. In the article's example, Ag29 NCs, one of the silver family's most extensively investigated NCs at the moment, only contain the primary and secondary ligands, despite the fact that both NCs have the same crystal structure [9]. The overall characteristics of the NCs can be affected by the interaction between the primary and secondary ligands. The primary ligands provide stability and define the core structure of the NCs, while the secondary ligands offer flexibility for tailoring the surface properties and introducing desired functionalities.
Primary ligands are the initial ligands that are typically attached to the surface of nanoclusters during their synthesis. These ligands play a crucial role in providing stability to the nanoclusters by forming strong bonds with the surface atoms. Due to their widespread distribution and close connection to the metal surface, they also have a substantial impact on the electrical, geometric, and optical properties of metal NCs. This view is supported by several phenomena. As illustrated in Figure 2 [10], the interactions between the transition dipole moment of individual nanoclusters and the induced dipole moments of nearby NCs are responsible for the small red shift in the absorption spectra of AgNCs in both crystal and solution forms. However, a more significant shift in emission maxima is observed in the crystalline form. This shift arises from electron-phonon connections and electronic linkage caused by non-radiative processes. Moreover, differences in the emission features between linear one-photon excitation fluorescence and two-photon excitation fluorescence spectra of Ag29 (DHLA)12 NCs are observed. These changes in symmetry laws governing metal-to-metal excitation within the central part of the NCs are the cause of these disparities. These results show that ligand-to-metal charge transmission or ligand-to-metal-to-metal charge transmission mechanisms are directly related to the generation of PL in nanoclusters. Understanding and modifying the optical characteristics of metal nanoclusters depends heavily on the individual ligands connected to the nanoclusters [9].
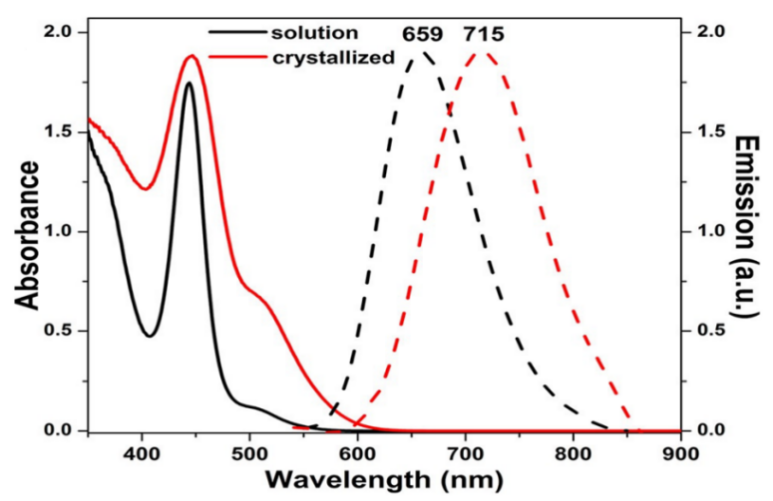
Figure 2. Solid curves are UV-Vis absorbance and dashed curves are emission of Ag29 (BDT) 12 (TPP) 4 NCs in acetonitrile (black) and dried (red) [9].
As opposed to primary ligands, secondary ligands are extra ligands that can be added to NC surfaces after the former. These ligands can establish coordinate bonds with the metal kernel, which results in much lower bond dissociation energies and straightforward dissociation in solution. The low PL seen in metal NCs is caused by the eventual emergence of non-radiative processes such thermal vibration relaxation. Additionally, secondary ligands act as surface capping ligands and are mostly present on the NCs' outer surface layers. These secondary ligands can alter the surface chemistry, charge, and characteristics of the NCs, which increases the stiffness and compactness of the metal NCs, improves stability, and intensifies photoluminescence. In Kang et al.'s experiment, the presence of excess TPP ligands in the solution led to an increase in the photoluminescence (PL) intensity. This increase was attributed to a decrease in the dissociation process, indicating a reversible dissociation-aggregation of TPP ligands. Ag29 (BDT) 12 (TPP) 4 nanoclusters showed a substantially greater PL in their solid-state form as compared to their solution-phase counterparts [9].
The influence of ligand chirality on the resulting PL properties presents an intriguing question. Researchers, such as Yoshida et al., have investigated this phenomenon by comparing Ag29NCs with racemic dihydrolipoic acid (DHLA) ligands to those protected by enantiopure DHLA ligands. The findings indicate that as compared to Ag29NCs shielded by optically pure chiral ligands with varying ratios of R and S enantiomers, racemic DHLA ligands had larger exposed surface areas on the core. This observation was revealed through the synthesis and characterization of Ag29 (DHLA) 12NCs. The difference in ligand chirality leads to distinct surface structures and arrangements, ultimately impacting the PL properties of the nanoclusters. Further investigations into the relationship between ligand chirality and PL behavior can provide valuable insights into the design and optimization of chiral ligand systems for tailoring the optical properties of metal NCs [11]. The Photoluminescence Quantum Yield (PLQY) data offers valuable insights into the efficiency of photoluminescence and provides information about the percentage of absorbed photons that are re-emitted as luminescence. By analyzing the PLQY data, we observe how the ligand orientation around the metal cores affects the photoluminescence characteristics, emphasizing the importance of the ligand's structure. An increase in PLQY indicates the influence of ligand arrangement and structure on enhancing the efficiency of photoluminescence. This emphasizes the crucial role played by ligands in determining the photoluminescence behavior of the nanoclusters [9].
5. Conclusion
This review highlights one nanomaterial, AgNCs, and researches some factors that may affect its fluorescence function as a biomarker. It can be influenced by particle size, surface energy, structure, ligand types and shapes, and so on.
The various particle sizes will cause different band gaps between AgNC particles, resulting in a red or blue shift in absorption and emission wavelengths. And the surface energy of AgNCs also depends on the surface-to-volume ratio, which is changed with particle size, which leads to tunable stability, sensitivity, and cellular uptake and biodistribution. In conclusion, the smaller AgNCs exhibit blue-shifted fluorescence, higher energy absorption and emission, lower stability, larger surface area-to-volume ratio, higher sensitivity and better cellular penetration and distribution compared to larger ones.
The structure of the fluorescence probe is another important aspect affecting how well AgNCs work. Comparing modified DNA-12nt-RED-160 probes with stable mismatch self-dimers and hairpins to DNA-12nt-RED-172 probes with less or unstable ones was done in the context of finding microRNAs. The results showed that solely when a clear mismatch self-dimer band exists can a noticeable red emission be seen.
This study also discusses the considerable impact of ligands on the photoluminescence (PL) characteristics of atomically exact silver nanoclusters, with an emphasis on Ag29 nanoclusters. The ligands were divided into the primary and secondary ligands, demonstrating their different effects on AgNCs and distinguishing them according to alterations to metal-ligand bonding and relative abundance. Given the current limited understanding of AgNC photo physics, there is a need for further exploration and comprehension of the energy transfer mechanisms involved in utilizing AgNCs and its various structures. By gaining a more precise knowledge, there will be increased interest in AgNCs for biosensing applications and it will unlock new opportunities and expand the potential applications of nanoclusters in the field of biosensing [12].
References
[1]. Díez, I., & Ras, R. H. (2011). Fluorescent silver nanoclusters. Nanoscale, 3(5), 1963-1970.
[2]. Chinen, A. B., Guan, C. M., Ferrer, J. R., Barnaby, S. N., Merkel, T. J., & Mirkin, C. A. (2015). Nanoparticle probes for the detection of cancer biomarkers, cells, and tissues by fluorescence. Chemical reviews, 115(19), 10530-10574.
[3]. Li, Q., Liu, L., Liu, J.-W., Jiang, J.-H., Yu, R.-Q., & Chu, X. (2014). Nanomaterial-based fluorescent probes for live-cell imaging. TrAC Trends in Analytical Chemistry, 58, 130-144.
[4]. Garg, S., Kamboj, S., Giri, S., Paul, B., Violina, D., Aggarwal, R., Tyagi, S., & Deepak, D. (2022). Small Silver Nanoparticles: A Multi-Functional Novel Delivery Vehicle For Therapeutics. Journal of Pharmaceutical Negative Results, 3504-3511.
[5]. Chatterjee, S., Lou, X.-Y., Liang, F., & Yang, Y.-W. (2022). Surface-functionalized gold and silver nanoparticles for colorimetric and fluorescent sensing of metal ions and biomolecules. Coordination Chemistry Reviews, 459, 214461.
[6]. Jiang, W., Kim, B. Y., Rutka, J. T., & Chan, W. C. (2008). Nanoparticle-mediated cellular response is size-dependent. Nature nanotechnology, 3(3), 145-150.
[7]. Amara, H., Nelayah, J., Creuze, J., Chmielewski, A., Alloyeau, D., Ricolleau, C., & Legrand, B. (2021). Is There Really a Size effect on the Surface Energy of Nanoparticles? https://hal.science/hal-03310351/
[8]. Shah, P., Rørvig-Lund, A., Chaabane, S. B., Thulstrup, P. W., Kjaergaard, H. G., Fron, E., Hofkens, J., Yang, S. W., & Vosch, T. (2012). Design aspects of bright red emissive silver nanoclusters/DNA probes for microRNA detection. Acs Nano, 6(10), 8803-8814.
[9]. Sahoo, K., & Chakraborty, I. (2023). Ligand effects on the photoluminescence of atomically precise silver nanoclusters. Nanoscale, 15(7), 3120-3129.
[10]. AbdulHalim, L. G., Bootharaju, M. S., Tang, Q., Del Gobbo, S., AbdulHalim, R. G., Eddaoudi, M., Jiang, D.-e., & Bakr, O. M. (2015). Ag29 (BDT) 12 (TPP) 4: a tetravalent nanocluster. Journal of the American Chemical Society, 137(37), 11970-11975.
[11]. Yoshida, H., Kumar, J., Ehara, M., Okajima, Y., Asanoma, F., Kawai, T., & Nakashima, T. (2020). Impact of Enantiomeric Ligand Composition on the Photophysical Properties of Chiral Ag29 Nanoclusters. Bulletin of the Chemical Society of Japan, 93(7), 834-840.
[12]. Yourston, L. E., & Krasnoslobodtsev, A. V. (2020). Micro RNA Sensing with Green Emitting Silver Nanoclusters. Molecules, 25(13), 3026.
Cite this article
Zhang,X. (2023). Research on the influence of various factors on the fluorescence function of silver nanoclusters as biomarkers. Applied and Computational Engineering,25,16-21.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2023 International Conference on Functional Materials and Civil Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Díez, I., & Ras, R. H. (2011). Fluorescent silver nanoclusters. Nanoscale, 3(5), 1963-1970.
[2]. Chinen, A. B., Guan, C. M., Ferrer, J. R., Barnaby, S. N., Merkel, T. J., & Mirkin, C. A. (2015). Nanoparticle probes for the detection of cancer biomarkers, cells, and tissues by fluorescence. Chemical reviews, 115(19), 10530-10574.
[3]. Li, Q., Liu, L., Liu, J.-W., Jiang, J.-H., Yu, R.-Q., & Chu, X. (2014). Nanomaterial-based fluorescent probes for live-cell imaging. TrAC Trends in Analytical Chemistry, 58, 130-144.
[4]. Garg, S., Kamboj, S., Giri, S., Paul, B., Violina, D., Aggarwal, R., Tyagi, S., & Deepak, D. (2022). Small Silver Nanoparticles: A Multi-Functional Novel Delivery Vehicle For Therapeutics. Journal of Pharmaceutical Negative Results, 3504-3511.
[5]. Chatterjee, S., Lou, X.-Y., Liang, F., & Yang, Y.-W. (2022). Surface-functionalized gold and silver nanoparticles for colorimetric and fluorescent sensing of metal ions and biomolecules. Coordination Chemistry Reviews, 459, 214461.
[6]. Jiang, W., Kim, B. Y., Rutka, J. T., & Chan, W. C. (2008). Nanoparticle-mediated cellular response is size-dependent. Nature nanotechnology, 3(3), 145-150.
[7]. Amara, H., Nelayah, J., Creuze, J., Chmielewski, A., Alloyeau, D., Ricolleau, C., & Legrand, B. (2021). Is There Really a Size effect on the Surface Energy of Nanoparticles? https://hal.science/hal-03310351/
[8]. Shah, P., Rørvig-Lund, A., Chaabane, S. B., Thulstrup, P. W., Kjaergaard, H. G., Fron, E., Hofkens, J., Yang, S. W., & Vosch, T. (2012). Design aspects of bright red emissive silver nanoclusters/DNA probes for microRNA detection. Acs Nano, 6(10), 8803-8814.
[9]. Sahoo, K., & Chakraborty, I. (2023). Ligand effects on the photoluminescence of atomically precise silver nanoclusters. Nanoscale, 15(7), 3120-3129.
[10]. AbdulHalim, L. G., Bootharaju, M. S., Tang, Q., Del Gobbo, S., AbdulHalim, R. G., Eddaoudi, M., Jiang, D.-e., & Bakr, O. M. (2015). Ag29 (BDT) 12 (TPP) 4: a tetravalent nanocluster. Journal of the American Chemical Society, 137(37), 11970-11975.
[11]. Yoshida, H., Kumar, J., Ehara, M., Okajima, Y., Asanoma, F., Kawai, T., & Nakashima, T. (2020). Impact of Enantiomeric Ligand Composition on the Photophysical Properties of Chiral Ag29 Nanoclusters. Bulletin of the Chemical Society of Japan, 93(7), 834-840.
[12]. Yourston, L. E., & Krasnoslobodtsev, A. V. (2020). Micro RNA Sensing with Green Emitting Silver Nanoclusters. Molecules, 25(13), 3026.









