1. Introduction
Fossil fuels have become a significant energy source due to a growing part of human industrial activity [1]. However, the conversion of chemical energy to other forms of energy by fuels is relatively low efficiency and generates greenhouse gases which reach their limitations and pose environmental threats. In order to gain green energy, new energy sources must be found, and various options are available, including wind, solar, and even nuclear power. The majority of these sources required intermediate forms of energy for electricity to be transferred between generators and consumer devices, thereby increasing the importance of batteries as a means of storing electricity. There have been several decades since the invention of lithium-ion batteries, and their outstanding storage and rechargeability have made them the most commonly chosen battery for portable devices [2]. In spite of the limited cycle life and energy density of existing lithium-ion batteries, long-range electric vehicles such as trucks or fully electric aircraft require further improvement in these aspects [3]. In order to overcome the current backward performance of advanced lithium-ion batteries, progression improvements such as adding transition metals, surface treatment, microstructure control, and nanostructure fabrication are increasing in popularity [4]. It is becoming increasingly popular to develop alternative battery chemistries to enhance these properties, and to improve the energy density of lithium-ion batteries, which can be accomplished by adding nanomaterials or nanostructures to these anodes or cathodes, which can significantly overcome some limitations imposed by bulk materials. The mechanisms underlying those phenomena should therefore be explored and the potential of nanomaterials to be applied further in these fields summarized. As part of this report, partial modifications of anodes or cathodes, such as surface treatments, as well as the full synthesis of nanostructure materials will be discussed, along with their mechanisms and potential improvements for lithium-ion batteries. The report discusses graphene, carbon nanotubes, silicon-based anodes, and other metal-based anodes, followed by lithium cobalt oxide, lithium iron phosphate, and nickel-based cathodes.
2. Anode
1. Graphene
Graphene is a two-dimensional signal layer of carbon arranged in hexagonal order, that is a conductive substrate being widely researched with its potential to make high-performance Lithium-ion batteries. While large-scale graphene synthesis is under development, the practical experimental scale of graphene-modified electrodes can be made by classical coating or vacuum filtration [5]. However, those production methods can only produce dense multilayered graphene anodes, which may cause lithium-ions to experience steric hindrance effects during diffusion and limit the Li-ion storage capacity of anode under high charging or discharging rates. Consequently, multilayered graphene is not an ideal anode for lithium-ion batteries if the space between layers is hard to control.
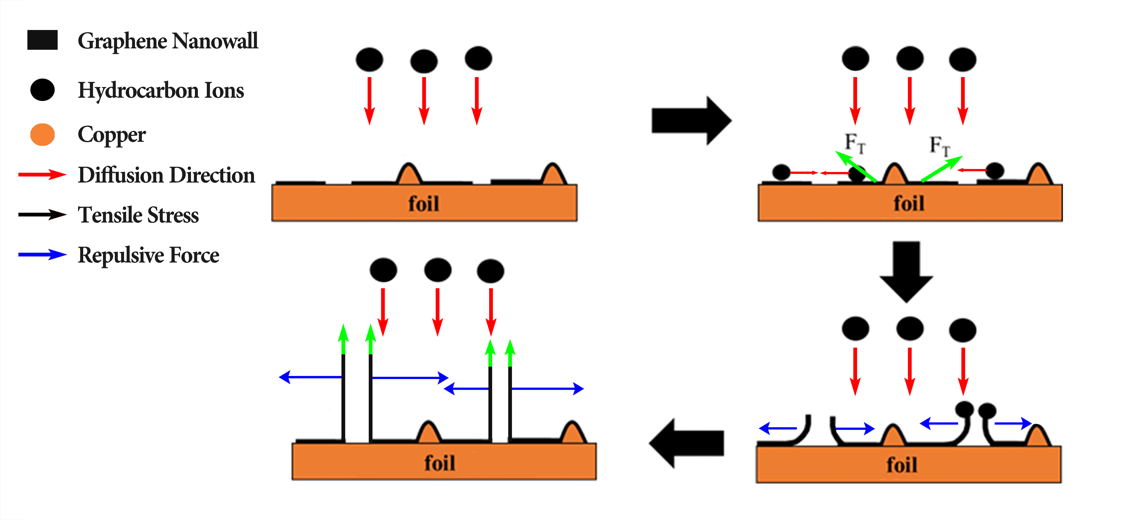
As part of improving graphene-based anode performance under high charging and discharging rates, addictive materials, such as metal oxides, are being used to increase Li-ion diffusion. As shown in Figure 1, the vertically oriented graphene nanowall structure based on copper foil was prepared under a methane environment [6]. The three-dimensional structure of graphene is expected to achieve approximately a Coulombic efficiency of 98% after 100 cycles with a specific capacity of around 80 mAhg-1 by reducing the transport resistance of Li-ions. Despite this, the plasma-enhanced tube furnace deposition system severely limits growth parameters and morphology, which results in the need to develop a new fabrication process in order to achieve these goals.
Figure 1. Schematics of nanowall structure synthesis [6].
Another improvement of the graphene-based anode was based on the titanium oxide-graphene nanostructure, which used surfactants to disperse metal oxide into the high-surface-area reduced graphene sheet, resulting in a uniform distribution of metal oxide [7]. By utilising metal oxides to maintain a gap between graphene layers, the hindrance effect for pure graphene can be minimised where Coulombic efficiency is greater than 98% with a specific capacity of up to 96 mAhg-1 by 100 cycles. There is, however, a lack of kinetic understanding of the synergistic effect of electron and Li-ion transfer within this combination structure. Therefore, it is necessary to carry out further experiments to confirm the existence of those nanostructures created from metal oxides and graphene.
The charging cycle stability, in addition to the high charging or discharging rates, plays an important role in determining how long Li-ion batteries will last. It is possible to overcome the reversibility of Li-ion batteries using a graphene and MXene anode, where the MXene is a layer of transition metal carbides that suppress graphene agglomeration without significantly altering electrochemical properties [8]. Under the thermal reduction of dispersion mixing with graphene oxides and MXene, the fabrication process of graphene and MXene porous films can be achieved. By improving the fabrication process, this type of anode had a better after-cycle capacity of up to 700 mAhg-1, making it an ideal Li-ion battery anode due to the precision control of the nanostructure dimensions.
As discussed above, the trend of graphene-typed anodes is not simply manufactured as single elements, more complex graphene-based anodes like γ-MnOOH-graphene nanocomposite are under research to increase the energy density of Li-ion batteries [9]. Graphene, in conjunction with other elements and structures, may be a trend that takes advantage of graphene's recent advances in electric conductivity and nanostructure stability for Li-ion storage.
2. Carbon nanotubes
Carbon nanotubes are one form of carbon allotropes but exhibit different anode performance than graphene. As carbon nanotubes are commonly made by the chemical vapour decomposition of hydrocarbon gas, different levels of graphene are used to fabricate the final products, so the number of cylindrical walls will vary depending on the amount of graphene used in fabrication. A single-wall carbon nanotube is the most difficult one to produce due to the difficulty of manufacturing raw single-layer graphene, which is why it is considered an addictive material to modify other anode materials rather than to be used solely as an anode material. Moreover, according to research examining silicon anodes with additives, carbon nanotubes may deteriorate electrical conductivity once a critical amount is reached [10]. This may be due to the difficulty of controlling the number of walls during the manufacturing process. Consequently, the uneven distribution of carbon nanotubes from single to multi-walls results in poorer electrochemical performance than carbon nanofibers in silicon anodes.
In addition to being an additive to silicon anodes, carbon nanotubes can also be used to modify other anode types due to their flexibility and electron conductivity. Among the candidate materials for the fabrication of lithium-ion batteries with long life and reasonable stability is lithium titanate [11]. It is possible to support the microstructure of this type of anode using carbon nanotubes of relatively high strength, which are able to overcome the issue of low conductivity and lithium-ion storage inherent to this type of anode. Therefore, carbon nanotubes can be considered an effective additive to structures with poor electrochemical performance in order to vary their electric conductivity as anode candidates for lithium-ion batteries.
Furthermore, carbon nanotubes can also achieve similar performance by forming composites with transition metal oxalates, similar to graphene, which is capable of combining both stability and conductivity [12]. A porous composite was created by electrostatic attraction of metal oxalates and multiwall carbon nanotubes, which are then nucleated and grown by electrostatic attraction. A hollow carbon nanotube can easily transfer ions when the metal oxalates are stable, and the capacity can remain at 983 mAhg-1 after 600 cycles. Thus, rob-like porous composite structures may be considered potential candidates for the production of anodes with high performance and stability.
Accordingly, carbon nanotubes can significantly improve electrochemical performance and structural stability over other candidate anode materials. A further study has demonstrated that carbon nanotubes and graphene sheets complement each other, and a molybdenum disulphide layered composite structure could be constructed by combining these two materials [13]. However, carbon nanotubes remain challenging to manufacture, specifically in terms of qualifying the number of walls within the tube.
3. Si-based materials
In spite of its unique atomic structure and bond layout, silicon has a much higher theoretical capacity than graphite anode as a group IV element. Since silicon suffers large volume changes during charging/discharging cycles, it is unlikely to replace graphite anodes as a method for advanced lithium-ion battery exploration. Despite research on alumina-coated tin particle layers to restore crack formation during the silicon-based anode charge cycle, bulk materials still fail rapidly and refer back to the volumetric change issue [14].
It has been observed that while manipulating surface properties is less effective in resolving the cracking problem for bulk silicon-based anodes, the demonstration of complex nano-structured anodes may result in a more stable anode structure. A typical interlayer of silver nanoparticles and amorphous silicon thin film was prepared by depositing silicon thin film under inert conditions and thermally evaporating silver particles into the gap between the films [15]. During the charging cycle, silver nanoparticles inhibited the formation of lithium silicon alloys by condensing electric fields and increasing formation activation energy, which reduced the effect of volumetric change and lithium-ion penetration during charging.
Moreover, nanostructure silicon materials can be a potential solution to make silicon-based anodes applicable. Using silicon carbides as a coating, silicon nanofibers formed by chemical vapour decomposition on graphite were firmly fixed [16]. Carbide provided relative strength and forced silicon to maintain its shape, while silicon nanowires provided a large diffusion channel and storage capacity for lithium ions, that specific capacity can exabit to 510 mAhg-1 after 100 cycles of variant current densities. As a result of the combined structure of the silicon-based anode and the nano-composite structure, the possibility for volume expansion to be reduced had the potential to be extended to a greater number of lithium-ion battery applications.
Besides different nanostructures of silicon being examined as anode materials, single-phase compounds containing silicon and liquid metal are being investigated for their electrochemical potential to replace the current anode material in lithium-ion batteries [17]. By strengthening the bond between silicon and liquid metal, anti-volumetric distortion of crystal structure may result, while electric conductivity actually increases as a result of overcoming increased nano-silicon particle resistance caused by size variations.
Overall, the integration of silicon nanostructures with addictive elements may provide a method for extending silicon anode life. As a result, energy storage would be more efficient, as charge and discharge rates would be increased, and cycle life would be extended.
4. Others
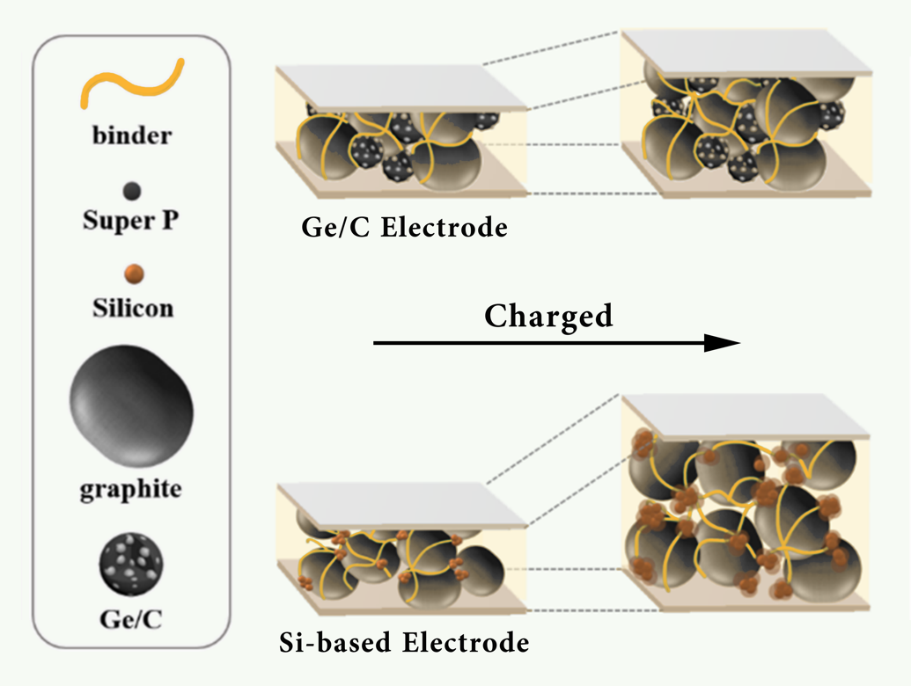
In order to overcome the limitations of the currently popular materials such as shrinkage under high charging rates, and a much lower practical energy density compared to theory, some similar bond or band gap structures and excellent conductivity elements have been utilized to modify the electrochemical properties of those anodes. In the manufacturing of advanced anodes, germanium is one of the group IV elements which is structurally stable and electrically conductive, making it an excellent alternative to silicon [18]. The germanium and carbon composite are shown in Figure 2, which was created through the spinodal decomposition of germanium microparticles into a carbon substrate, the volumetric expansion was significantly reduced through the strong ionic bond maintaining attraction throughout the electrode structure.
Figure 2. Comparison of graphite germanium and silicon electrodes [18].
Other complex intermetallic compounds and structures are also present, along with enhanced kinetics of lithium-ion diffusion and storage within anode compounds [19]. Although fewer kinetic and mechanical explorations have been conducted under these structures, it is unclear what the future development roadmap will entail. Researchers need to have a better understanding of the underlying chemical and physical properties of these materials in order to determine the best approach for developing safer, more efficient, and more cost-effective lithium-ion batteries.
3. Cathode
1. Lithium cobalt oxide (LCO)
Among the popular cathode choices for portable devices, LCO is a popular choice due to its volumetric energy density and good performance at a rapid rate. During operation, however, the heat generated will dramatically degrade lithium ions, and electrons will diffuse throughout the cathodes. Due to the electrochemical kinetic slowdown nature of LCO at high operating temperatures and its structural instability, this may occur. A potential solution may be to dope and coat the bulk of the material in order to improve its structural properties [20]. For example, using lanthanum and magnesium to modify the bulk material can improve the conductivity, and around 6 nm thick aluminium oxide coating can suppress deformation caused by lithium-ion and electron transfer. By spontaneous fabrication, lanthanum and magnesium were present in LCO that form ions which maintained the crystal structure at extreme charging rates and operating temperatures. By using relatively strong metal oxide as the outer coating surface, electron penetration can be minimized, and massive volumetric deformation can be prevented during operation.
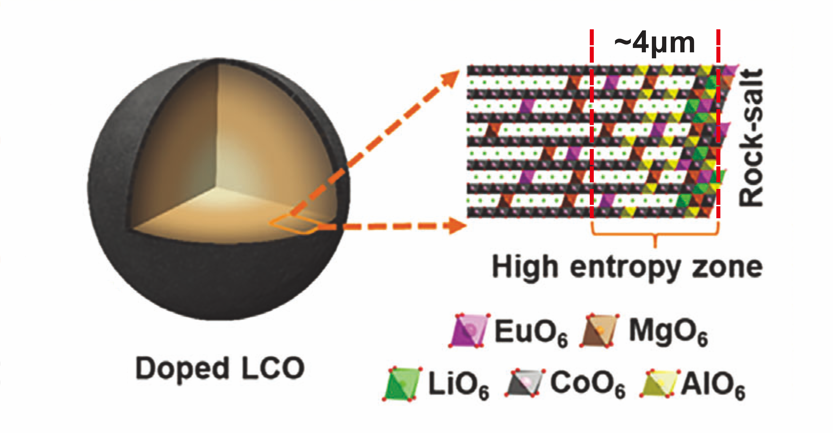
However, the cathode has an uneven distribution of surface thickness with an unbonded interface structure that may adversely affect performance under normal conditions. Additionally, the cathode may suffer from electrode decomposition and irreversible capacity loss when subjected to high-voltage cycling. To enhance cycling performance, a magnesium-aluminium-europium co-doped cathode was examined for its impact on kinetics [21]. The synthesis of the cathode by calcined resulted in a Rock-salt structure accumulating few nanometres thick high entropy zone near the surface as shown in Figure 3, which suppressed the formation of a lithium-ion blocking phase under high voltage. Therefore, the performance of LCO cathode is quite stable and can consist of numerous cycles.
Figure 3. Schematics of doped LCO structure [21].
In summary, LCO cathodes may require further development in order to reach their theoretical capacity under a wide range of operating conditions. There are also different approaches, such as doping with nickel and phosphorus [22], to increase voltages and decrease internal resistances, which results in higher efficiency and better capacity. This battery is a leading choice for many applications due to its high energy density and excellent cycle performance.
2. Lithium iron phosphate (LFP)
It is widely used on electric vehicles lithium-ion batteries since the LFP cathode is relatively stable and safe, or to put it another way, more reliable under complex operating conditions. Due to the poor electrochemical properties, particularly the low energy density, the applications are limited. The low diffusive mechanism of lithium ions, which is heavily influenced by surface kinetics, is responsible for both advantages as well as drawbacks. Therefore, the attempt to modify the surface electrochemical property may be beneficial to overcome the kinetic limitations. For example, a very thin layer of rare earth oxide coatings can be used to accelerate the diffusion of lithium ions [23]. An LFP powder was synthesized by re-carbothermic reduction, and an approximate 3 nm thick lanthanum oxide slurry was sintered on its surface after the powder had been synthesized. By accelerating the diffusivity of lithium ions, the lanthanum oxide coating had erosion resistance and improved the conductivity between bulk materials and lithium ions.
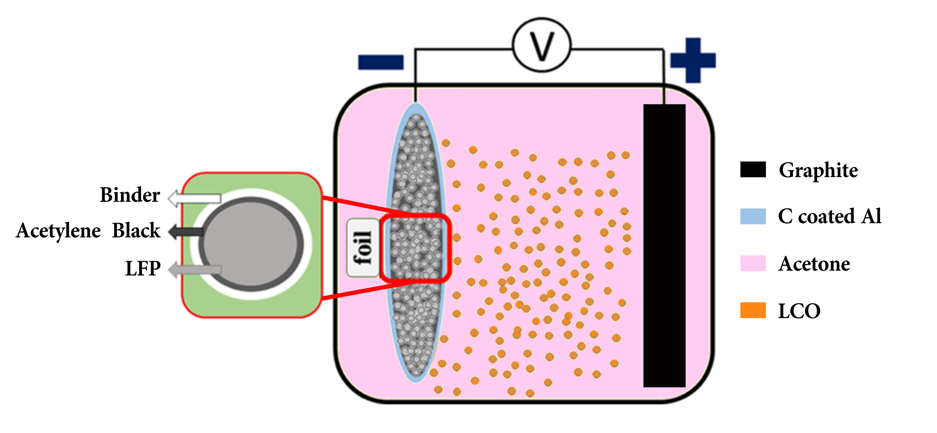
Furthermore, the nanostructure can also provide a larger surface area for the diffusion of lithium ions or electrons. In Figure 4, the LFP base is electrophoresed on carbon-coated aluminium foil and polyvinylpyrrolidone is used as a binder in order to create an evenly layered cathode thin film [24]. As the cathode surface was firmly held in its structure, the diffusion of lithium ions and electrons was dramatically increased. Thus, stability was maintained that specific capacity remained around 85% with 99% Coulombic efficiency after 200 cycles, while electrochemical properties were improved by increasing the cathode's effective interface.
Figure 4. Schematics of carbon-coated aluminium foil and polyvinylpyrrolidone setup [24].
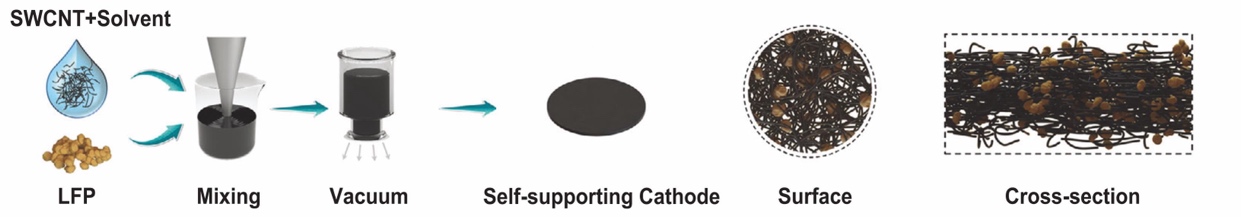
Besides the changes in size and dimension of bulk materials, the other nanostructure may improve the conductivity of the cathode. In addition to strengthening or modifying the electronic properties of the anode using single-walled carbon nanotubes [25], a larger effective diffuse surface with self-supporting properties can be achieved by using single-walled carbon nanotubes. In Figure 5, LFP nanoparticles are attracted by solvent to carbon nanotubes, and when the solvent is removed, the particles are evenly deposited on the nanostructure. This mechanism of modification is similar to that of thin films, where carbon nanotubes provide strength to structures and electronic conductivity to nanoparticles. Therefore, by using a simple solution-based method, it is possible to modify the LFP cathode with a nanostructure or form a nano-composite, which could be useful for determining new cathodes with interesting and unique properties.
Figure 5. LFP nanoparticles attracted carbon nanotubes preparing process schematics [25].
3. Others
A nickel-based cathode is an alternative to the cathodes discussed in previous sections for constructing advanced lithium-ion batteries [26]. By combining nickel and oxygen bonds, nickel-rich cathodes are able to maintain high reversibility under numerous cycles, which prevents side reactions and results in better stability than LCO cathodes. Hence, there are no limitations to the type of cathode material available today.
Additionally, the lithium metal oxide layer structure combined with nanoparticles has impressive storage capacity and mechanical stability [27]. However, the synthesis of these complex structure materials is not fully understood, and the characterization of these cathodes is far from their theoretical value. Therefore, development under the manufacturer process in those nano-composites is required to evaluate their potential to replace the current mature cathodes. There is a possibility that this could lead to the development of cathodes with a higher energy density, improved cyclability, and improved safety characteristics. Ultimately, this may help to revolutionize the energy industry and facilitate the development of cheaper, more efficient, and more sustainable energy sources.
4. Conclusion
In summary, both the anodes including carbon-based and silicon-based nanomaterials, and the cathodes including LCO or LFP, have met their physical property limits for bulk material. However, the nanostructure or nano-technic modification requires precision-controlled manufacturing processes, which increases the cost of those materials and challenges the massive production. Future developments are likely to focus on the synthesis of nanostructure materials or fabrication of nanocomposites with other elements, as there is greater potential for development in these areas. As a consequence, it is necessary to explore novel materials or nanostructures with enhanced performance. As part of the research, batteries may also be improved in terms of their safety and stability, as well as their energy density and cycle life.
N. Armaroli and V. Balzani, 2011 Chem Asian J, 6 768–784.
V. Etacheri, R. Marom, R. Elazari, G. Salitra, and D. Aurbach, 2011 Energy Environ Sci, 4 3243.
G. Buticchi, P. Wheeler, and D. Boroyevich, 2023 Proceedings of the IEEE, 111, 356–370, .
S. Choi and G. Wang, 2018 Adv Mater Technol, 3 1700376.
J. Zhang et al., 2022 J Power Sources, 522, 231007.
Q. Yang et al., 2019 Diam Relat Mater, 91 54–63.
D. Wang et al., 2009 ACS Nano, 3. 907–914.
S. Xu, Y. Dall’Agnese, J. Li, Y. Gogotsi, and W. Han, 2018 Chemistry – A European Journal, 24 18556–18563.
S. P. Varghese, B. Babu, V. Surendran, D. Damien, R. Antony, and M. M. Shaijumon, 2022 J Energy Storage, 47 103636.
J. W. Yap, T. Wang, H. Cho, and J.-H. Kim, 2023 Electrochim Acta, 446 14210.
F. Baltazar Iniguez et al., 2022 Journal of Industrial and Engineering Chemistry, 112 125–133.
F.-F. Xing et al., 2023 J Electron Mater, 52, 4179–4190.
H. Jian et al., 2023 Materials, 16 3218.
X. Zhou et al., 2023 Nanotechnology, 34 235705.
Y.-X. Chen, H.-C. Liao, Y.-W. Cheng, J.-H. Huang, and C.-P. Liu,2023 ACS Appl Mater Interfaces, 15 18845–18856.
M. Liu, B. Liu, R. Zhang, Z. Xie, P. Huang, and J. Zhang, 2023 Journal of Electrochemical Energy Conversion and Storage, 20 021008.
Y. Li et al., 2023 Nanomicro Lett, 15 63.
C. Jo, B. Wen, H. Jeong, S. K. Park, Y. Son, and M. De Volder, 2023 ACS Nano, 17 8403–8410.
Y. Li et al., 2023 Chemical Engineering Journal, 462, 142208.
J. Ren et al., 2023EcoMat, 5, e12344.
X. Tan et al., 2023 Adv Energy Mater, 13, 2300147.
Z. Zhuang et al., 2023 Advanced Materials, 35, 2212059.
L. Tong, Z. Hu, Z. Long, M. Tang, and X. Qiu, 2023J Alloys Compd, 947 169581.
N. Tolganbek, N. Zhalgas, Y. Kadyrov, N. Umirov, Z. Bakenov, and A. Mentbayeva, 2023 ACS Omega, 8 8045–8051.
M. Guo et al., 2023 Advanced Science, 10, 2207355.
M. Qi et al., 2023 Small Methods, 7 2300280.
M. Solzi et al., 2023 Journal of Physics: Materials 6 034002.
References
[1]. N. Armaroli and V. Balzani, 2011 Chem Asian J, 6 768–784.
[2]. V. Etacheri, R. Marom, R. Elazari, G. Salitra, and D. Aurbach, 2011 Energy Environ Sci, 4 3243.
[3]. G. Buticchi, P. Wheeler, and D. Boroyevich, 2023 Proceedings of the IEEE, 111, 356–370, .
[4]. S. Choi and G. Wang, 2018 Adv Mater Technol, 3 1700376.
[5]. J. Zhang et al., 2022 J Power Sources, 522, 231007.
[6]. Q. Yang et al., 2019 Diam Relat Mater, 91 54–63.
[7]. D. Wang et al., 2009 ACS Nano, 3. 907–914.
[8]. S. Xu, Y. Dall’Agnese, J. Li, Y. Gogotsi, and W. Han, 2018 Chemistry – A European Journal, 24 18556–18563.
[9]. S. P. Varghese, B. Babu, V. Surendran, D. Damien, R. Antony, and M. M. Shaijumon, 2022 J Energy Storage, 47 103636.
[10]. J. W. Yap, T. Wang, H. Cho, and J.-H. Kim, 2023 Electrochim Acta, 446 14210.
[11]. F. Baltazar Iniguez et al., 2022 Journal of Industrial and Engineering Chemistry, 112 125–133.
[12]. F.-F. Xing et al., 2023 J Electron Mater, 52, 4179–4190.
[13]. H. Jian et al., 2023 Materials, 16 3218.
[14]. X. Zhou et al., 2023 Nanotechnology, 34 235705.
[15]. Y.-X. Chen, H.-C. Liao, Y.-W. Cheng, J.-H. Huang, and C.-P. Liu,2023 ACS Appl Mater Interfaces, 15 18845–18856.
[16]. M. Liu, B. Liu, R. Zhang, Z. Xie, P. Huang, and J. Zhang, 2023 Journal of Electrochemical Energy Conversion and Storage, 20 021008.
[17]. Y. Li et al., 2023 Nanomicro Lett, 15 63.
[18]. C. Jo, B. Wen, H. Jeong, S. K. Park, Y. Son, and M. De Volder, 2023 ACS Nano, 17 8403–8410.
[19]. Y. Li et al., 2023 Chemical Engineering Journal, 462, 142208.
[20]. J. Ren et al., 2023EcoMat, 5, e12344.
[21]. X. Tan et al., 2023 Adv Energy Mater, 13, 2300147.
[22]. Z. Zhuang et al., 2023 Advanced Materials, 35, 2212059.
[23]. L. Tong, Z. Hu, Z. Long, M. Tang, and X. Qiu, 2023J Alloys Compd, 947 169581.
[24]. N. Tolganbek, N. Zhalgas, Y. Kadyrov, N. Umirov, Z. Bakenov, and A. Mentbayeva, 2023 ACS Omega, 8 8045–8051.
[25]. M. Guo et al., 2023 Advanced Science, 10, 2207355.
[26]. M. Qi et al., 2023 Small Methods, 7 2300280.
[27]. M. Solzi et al., 2023 Journal of Physics: Materials 6 034002.
Cite this article
Li,L. (2023). Advancements in anode and cathode nanomaterials for high-performance Li-ion batteries. Applied and Computational Engineering,26,193-200.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2023 International Conference on Functional Materials and Civil Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. N. Armaroli and V. Balzani, 2011 Chem Asian J, 6 768–784.
[2]. V. Etacheri, R. Marom, R. Elazari, G. Salitra, and D. Aurbach, 2011 Energy Environ Sci, 4 3243.
[3]. G. Buticchi, P. Wheeler, and D. Boroyevich, 2023 Proceedings of the IEEE, 111, 356–370, .
[4]. S. Choi and G. Wang, 2018 Adv Mater Technol, 3 1700376.
[5]. J. Zhang et al., 2022 J Power Sources, 522, 231007.
[6]. Q. Yang et al., 2019 Diam Relat Mater, 91 54–63.
[7]. D. Wang et al., 2009 ACS Nano, 3. 907–914.
[8]. S. Xu, Y. Dall’Agnese, J. Li, Y. Gogotsi, and W. Han, 2018 Chemistry – A European Journal, 24 18556–18563.
[9]. S. P. Varghese, B. Babu, V. Surendran, D. Damien, R. Antony, and M. M. Shaijumon, 2022 J Energy Storage, 47 103636.
[10]. J. W. Yap, T. Wang, H. Cho, and J.-H. Kim, 2023 Electrochim Acta, 446 14210.
[11]. F. Baltazar Iniguez et al., 2022 Journal of Industrial and Engineering Chemistry, 112 125–133.
[12]. F.-F. Xing et al., 2023 J Electron Mater, 52, 4179–4190.
[13]. H. Jian et al., 2023 Materials, 16 3218.
[14]. X. Zhou et al., 2023 Nanotechnology, 34 235705.
[15]. Y.-X. Chen, H.-C. Liao, Y.-W. Cheng, J.-H. Huang, and C.-P. Liu,2023 ACS Appl Mater Interfaces, 15 18845–18856.
[16]. M. Liu, B. Liu, R. Zhang, Z. Xie, P. Huang, and J. Zhang, 2023 Journal of Electrochemical Energy Conversion and Storage, 20 021008.
[17]. Y. Li et al., 2023 Nanomicro Lett, 15 63.
[18]. C. Jo, B. Wen, H. Jeong, S. K. Park, Y. Son, and M. De Volder, 2023 ACS Nano, 17 8403–8410.
[19]. Y. Li et al., 2023 Chemical Engineering Journal, 462, 142208.
[20]. J. Ren et al., 2023EcoMat, 5, e12344.
[21]. X. Tan et al., 2023 Adv Energy Mater, 13, 2300147.
[22]. Z. Zhuang et al., 2023 Advanced Materials, 35, 2212059.
[23]. L. Tong, Z. Hu, Z. Long, M. Tang, and X. Qiu, 2023J Alloys Compd, 947 169581.
[24]. N. Tolganbek, N. Zhalgas, Y. Kadyrov, N. Umirov, Z. Bakenov, and A. Mentbayeva, 2023 ACS Omega, 8 8045–8051.
[25]. M. Guo et al., 2023 Advanced Science, 10, 2207355.
[26]. M. Qi et al., 2023 Small Methods, 7 2300280.
[27]. M. Solzi et al., 2023 Journal of Physics: Materials 6 034002.