1. Introduction
According to the embodied simulation hypothesis, language processing involves activation of sensory-motor brain regions, rejecting the standard view that language understanding only relies on amodal symbol representations [1, 2, 3]. While direct causal evidence for the hypothesis from motor system was abundant, there was a lack of evidence for the functional role of perception system in corresponding semantic processing.
1.1. Causal Evidence from Motor System
Most of the direct evidence for the embodiment of language processing comes from studies on the role of somatotopically-organized motor areas in processing language about motor actions. Multiple brain imaging studies have shown that somatotopic motor activity correlates with action language processing [1, 2, 4]. In addition, there is experimental evidence that motor areas are rapidly activated during language processing, providing evidence that this activity is simply a result of motor imagery [5, 6]. However, testing the causal role of motor cortices in language processing requires direct manipulation of motor brain regions using non-invasive brain stimulation. A study has shown that applying Transcranial Magnetic Stimulation (TMS) to the left “hand areas” of premotor cortex increased the response speed to manual-action words, indicating a causal relationship between premotor cortex and motor word processing [7], and multiple similar studies using transcranial Direct Current Stimulation (tDCS) have provided further evidences for such a causal relationship [8, 9, 10].
1.2. Correlational Evidence from Perceptual System
Beyond the motor system, evidence for embodied simulation is less clear. Numerous studies have tried to use behavioral methods for testing embodied simulation hypothesis [11, 12, 13]. For example, studies have tested for the compatibility between pictures and the sentences describing those pictures and claim that they provide evidence of visual simulation [11, 12, 13]. However, these studies failed to test the embodied simulation hypothesis directly because they did not provide direct evidence for the activation of modality-specific brain regions, as opposed to multifunctional or amodal brain regions, during language processing.
A small number of studies have used brain imaging to demonstrate a correlation between activity in the visual areas of the brain and language about the visible world. For example, reading color property words predicts activation in regions of visual cortex that support color perception [11], and processing words from different semantic categories (e.g. color and form) activates the corresponding cortical areas to different degrees. Similar correlational studies have been carried out for other perception-specific words. For example, reading olfactory-associated words selectively activates primary olfactory cortex [12], and reading taste-related words predicted stronger activation of primary and secondary gustatory cortices [13]. Likewise, reading words with highly relevant acoustic features predicted activation of auditory brain regions responsible for sound perception [14]. However, no studies that demonstrate a causal role for perceptual system in corresponding semantic processing were found.
1.3. Experimental Objective
The experimental objective of the paper was to test for the causal role of visual cortices in processing the meanings of words that refer to visible entities, therefore adding evidence to the embodied simulation hypothesis. The experimental hypothesis was that the visual cortex plays a functional role in semantic processing of visual-associated words.
2. Method
2.1. Design
The experiment will utilize tDCS to stimulate the primary visual cortex and test for its influence on the response time in lexical decision task.
The stimulation method of tDCS was chosen for several reasons. First, it involves a simple procedure and has effects that last long enough to conduct a behavioral experiment [15, 16]. Second, it provides a more reliable sham condition than TMS, therefore providing a more convincing double-blind experimental design [17]. Compared to TMS, tDCS stimulates target areas less focally, primarily due to relatively larger tDCS electrodes [18]. Although this lack of spatial resolution could be a disadvantage in some experiments, it is not a concern in the present experiment because the experiment aimed to stimulate the whole primary visual cortex, not the specific parts of it.
Each participant will take part in two experimental sessions, in which they will receive either sham or true tDCS. This within-subjects design is in line with research demonstrating participants’ inability to distinguish sham tDCS from true tDCS [19, 20]. The sham tDCS condition was motivated by previous studies that revealed potential placebo effect of tDCS, that the sense of being stimulated may influence participants’ performance [21].
Unlike motor cortex, where right or left body parts are strictly related with corresponding lateralized brain regions [7, 8], laterality of visual cortex is not related directly to the right or left eyes. Therefore, in normal participants, there should not be lateralized activation of primary visual cortex when receiving actual visual stimulation. Therefore, according to embodied simulation hypothesis [1, 2, 3], visual-associated semantics processing should bilaterally activate primary visual cortex. Therefore, anodal electrode will be applied over Oz, but not lateralized visual brain regions.
Applying the Electroencephalography 10–20 system, one of the electrodes (anode/target) will be placed over Oz and the other (cathode/reference) placed over Fpz based on previous work which showed that applying anodal tDCS has an excitatory effect [8]. The Fpz region was selected as the reference point to maximize the anodal–cathodal stimulation distance, in line with previous studies [22].
During lexical decision task, word stimuli will be presented acoustically, not visually, to prevent actual visual stimulation. A previous fMRI study has shown that listening to action-related word stimuli elicits activation of motor brain regions [23]. While studies have shown that stimulating primary visual cortex affects visual perception in various ways, including visual acuity and visual processing speed [22], visual working memory [24], and visual motion perception [25], whether anodal-tDCS or cathodal-tDCS will enhance or impair specific visual perception and processing ability remained unclear (i.e., while anodal-tDCS may improve visual working memory, cathodal-tDCS may enhance visual acuity and processing speed potentially by denoising related neurons [22, 24]).
2.2. Participants
Adult participants (N = 70) will be recruited. Participants will be monolingual native English speakers and right-handed, assessed by Edinburgh Handedness Inventory-Short Form [26], with no history of psychiatric or neurological illness, no history of visual impairment, and are not taking medication or having any electronic implants at the time of test. All participants will provide informed consent.
2.3. Materials
List of 100 adjectives with strong visual connotations (e.g., cloudy, dazzling, and dim) will be selected from the Modality Exclusivity Norms for 423 Object Properties [27]. A second list of 100 adjectives with weak visual connotations (e.g., acidic, beeping, and bitter) will be selected from the same set of norms [27]. 50 pseudowords (e.g., babones, baresion, and cerf) will be generated by Random Word Generator website (Random Word Generator; https://randomwordgenerator.com).
2.4. Procedure
Participants will receive anodal-tDCS stimulation or sham tDCS to the occipital in two experimental sessions, then perform an auditory lexical decision task after each session [28]. The effects of the type of stimulation will be assessed by the response times (RTs). The response times (RTs) to words describing visible entities (e.g. “cloudy”, “Bronze”) will be compared with its effects on RTs to words describing non-visible entities (e.g. “aching”, “loud”).
2.4.1. Anodal-Transcranial Direct Current Stimulation
The tDCS procedure will follow the protocols in the previous studies [21]. A dual channel midline double monopolar montage will be used [29]. The sites of stimulation were identified using the Electroencephalography 10–20 system, with one of the electrodes (anode/target) placed over Oz and the other (cathode/ reference) placed over Fpz, both covered by saline-soaked sponges.
Participants will be treated with anodal tDCS in one session and sham tDCS in a second session on a different day, with the order of sessions counterbalanced. Following previous study[8], participants will receive anodal stimulation for 20 minutes (2 mA) immediately prior to each day’s behavioral testing session (or sham stimulation for the same duration). In the sham condition, stimulation will be maintained for only the first and last 10 seconds to evoke the sensation of being stimulated, without causing neurophysiological changes that may influence performance [30]. In the true tDCS condition, participants will receive 20 minutes of stimulation at 2 mA, which will slowly ramp up from 0 mA at stimulation onset, and ramp down to 0 mA at stimulation offset, with both ramping up and ramping down happening over the course of 20 seconds [8].
2.4.2. Lexical-Decision Task
After receiving tDCS, participants will perform an auditory lexical decision task [28]. Words will be presented auditorily. The stimulus words will be pronounced by one speaker and be digitized and stored for use in the experiment. Participants will be instructed to hold a button box and press one button to indicate that the stimulus is a word in English and another button to indicate that it is a pseudoword word, using the left and right thumbs. A flexible response-mapping scheme will be used, so that the left/right position of the response options will vary randomly [7]. The stimuli will be presented in a random order, with word types counterbalanced across participants. Participants will be instructed to respond as quickly and accurately as possible.
2.4.3. Data Analysis
The accuracy data and RTs for target trials will be analyzed with mixed effects models. The independent variables for both models will consist of three two-level fixed effects: tDCS type (sham vs. true) and verb type (visible entities vs. nonvisual entities). Random effects for participants and words will be included. Nonce trials will be excluded before analysis.
Accuracy data will be analyzed using a general linear model with a binomial linking function [8]. The dependent variable for this model is whether the response for each trial was correct or incorrect. For the RT model, all incorrect trials will be discarded.
3. Predicted Results
If the embodied simulation hypothesis is supported, then compared to sham tDCS, anodal tDCS will change the RTs in response to the visual-associated word stimuli significantly more than to the nonvisual-associated word stimuli (Figure 1a). By contrast, if the embodied simulation hypothesis is not supported, then anodal tDCS will not selectively affect RTs for visual-associated words; anodal tDCS may have no effect, or it may affect RTs for both visual-associated and nonvisual-associated words nondifferentially (Figure 1b).
Figure 1a shows a facilitating effect of anodal-tDCS to primary visual cortex (Oz), yet a priori, it is difficult to predict whether the effect of anodal tDCS will improve or impair RTs[8]. However, regardless of whether the anodal stimulation selectively improves or impairs visual semantics processing, finding a 2-way interaction in which RTs for visual words are selectively modulated (as opposed to RTs for non-visual words) would support a casual role or visual cortex in processing visual semantics.
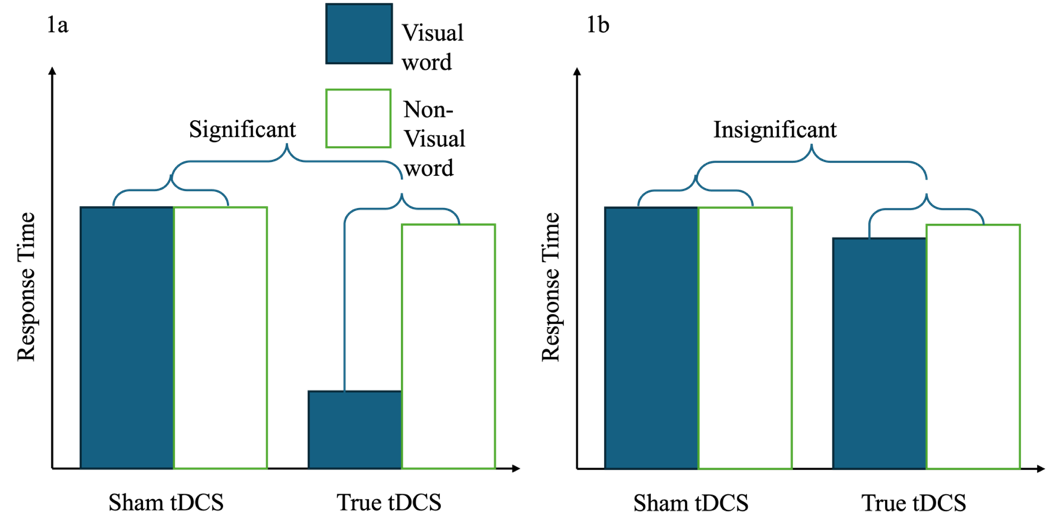
Figure 1: predictions of the proposed study. 1a. (Left): Predictions that follows from the embodied simulation hypothesis of visual semantics processing. 1b. (Right): Predictions that follows from disembodied cognition model.
4. Discussion
4.1. Implications
Our proposed experiment aims to test for a causal role of visual cortex in processing visual semantics. If the experimental result support the hypothesis that visual cortex is selectively involved in visual semantics (as opposed to non-visual semantics), then then this experiment would provide additional empirical evidence for the embodied simulation hypothesis, and it would provide the first direct evidence for a causal relationship between visual words and visual cortex activation. Moreover, the experiment establishing a causal role for visual cortex in visual semantic processing would encourage further studies to test causal roles for other sensory cortices in word meaning.
By contrast, if the experiment does not show that visual cortex selectively affects visual semantic processing (e.g., if anodal-tDCS influences RTs or accuracy for both visual and non-visual words nondifferentially), then the experiment will fail to proof the causal role of visual cortex in visual semantic processing. This will encourage researchers to repeat the study with the addition of a visual manipulation check to test whether stimulating the primary visual cortex (Oz) influences performances on visual perception tasks. This could be done by following previous studies examining how tDCS to visual cortex affects visual acuity and visual processing speed [22] or visual working memory [24].
4.2. Future Studies
If the proposed experiment supports our experimental hypothesis, then several future study designs can happen.
First, as the experiment verifies the causal role of visual cortex in processing visual semantics, experiments investigating whether other perception systems have a similar causal role in processing corresponding semantics might provide further evidence for the embodiment of semantic processing in various modality-specific brain regions [9]. Second, to generalize the predicted findings to a different type of neurostimulation, a similar lexical decision study could be run using repetitive TMS, rather than tDCS [7, 8]. Finally, the proposed experiment will only involve stimulating the primary visual cortex, whereas previous studies have revealed correlations between higher visual areas and processing words for specific visual properties such as color [11]. Future studies could investigate whether more focal brain stimulation to specific regions within visual cortex can selectively affect processing of words for specific visual properties (e.g., color, motion).
5. Conclusion
The proposed experiment aimed to investigate the causal role of visual cortex in processing of words referring to visible entities. Whether true anodal tDCS selectively affect RTs to visual-associated words compared to sham tDCS would provide evidence to perceive whether the primary visual cortex plays a causal role in visual-associated semantic processing. If our result revealed such a causal relationship, it will be the first evidence for the embodiment of visual-associated semantic processing. Furthermore, it will provide additional empirical evidence for the embodied simulation hypothesis, encouraging future studies testing for similar causal relationship in perception system.
Acknowledgments
Kei Xie and Bert Zhang contributed equally to this work and should be considered co-first authors.
References
[1]. O. Hauk, I. Johnsrude, and F. Pulvermuller, Somatotopic representation of action words in human motor and premotor cortex. Neuron 41 (2004) 301-7.
[2]. F. Pulvermuller, Brain mechanisms linking language and action. Nat Rev Neurosci 6 (2005) 576-82.
[3]. L.W. Barsalou, Grounded cognition. Annu Rev Psychol 59 (2008) 617-45.
[4]. L. Aziz-Zadeh, S.M. Wilson, G. Rizzolatti, and M. Iacoboni, Congruent embodied representations for visually presented actions and linguistic phrases describing actions. Curr Biol 16 (2006) 1818-23.
[5]. O. Hauk, and F. Pulvermuller, Neurophysiological distinction of action words in the fronto-central cortex. Hum Brain Mapp 21 (2004) 191-201.
[6]. F. Pulvermuller, Y. Shtyrov, and R. Ilmoniemi, Brain signatures of meaning access in action word recognition. J Cogn Neurosci 17 (2005) 884-92.
[7]. R.M. Willems, L. Labruna, M. D'Esposito, R. Ivry, and D. Casasanto, A functional role for the motor system in language understanding: evidence from theta-burst transcranial magnetic stimulation. Psychol Sci 22 (2011) 849-54.
[8]. T. Gijssels, R.B. Ivry, and D. Casasanto, tDCS to premotor cortex changes action verb understanding: Complementary effects of inhibitory and excitatory stimulation. Sci Rep 8 (2018) 11452.
[9]. F. Vitale, I. Padron, A. Avenanti, and M. de Vega, Enhancing Motor Brain Activity Improves Memory for Action Language: A tDCS Study. Cereb Cortex 31 (2021) 1569-1581.
[10]. K. Johari, N. Riccardi, S. Malyutina, M. Modi, and R.H. Desai, HD-tDCS of primary and higher-order motor cortex affects action word processing. Front Hum Neurosci 16 (2022) 959455.
[11]. W.K. Simmons, V. Ramjee, M.S. Beauchamp, K. McRae, A. Martin, and L.W. Barsalou, A common neural substrate for perceiving and knowing about color. Neuropsychologia 45 (2007) 2802-10.
[12]. J. Gonzalez, A. Barros-Loscertales, F. Pulvermuller, V. Meseguer, A. Sanjuan, V. Belloch, and C. Avila, Reading cinnamon activates olfactory brain regions. Neuroimage 32 (2006) 906-12.
[13]. A. Barros-Loscertales, J. Gonzalez, F. Pulvermuller, N. Ventura-Campos, J.C. Bustamante, V. Costumero, M.A. Parcet, and C. Avila, Reading salt activates gustatory brain regions: fMRI evidence for semantic grounding in a novel sensory modality. Cereb Cortex 22 (2012) 2554-63.
[14]. M. Kiefer, E.J. Sim, B. Herrnberger, J. Grothe, and K. Hoenig, The sound of concepts: four markers for a link between auditory and conceptual brain systems. J Neurosci 28 (2008) 12224-30.
[15]. M.A. Nitsche, and W. Paulus, Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527 Pt 3 (2000) 633-9.
[16]. M.A. Nitsche, and W. Paulus, Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57 (2001) 1899-901.
[17]. P.C. Gandiga, F.C. Hummel, and L.G. Cohen, Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol 117 (2006) 845-50.
[18]. M.A. Nitsche, S. Doemkes, T. Karakose, A. Antal, D. Liebetanz, N. Lang, F. Tergau, and W. Paulus, Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol 97 (2007) 3109-17.
[19]. R.M. Reinhart, and G.F. Woodman, Causal control of medial-frontal cortex governs electrophysiological and behavioral indices of performance monitoring and learning. J Neurosci 34 (2014) 4214-27.
[20]. R.M. Reinhart, and G.F. Woodman, Enhancing long-term memory with stimulation tunes visual attention in one trial. Proc Natl Acad Sci U S A 112 (2015) 625-30.
[21]. R.M. Reinhart, J.D. Cosman, K. Fukuda, and G.F. Woodman, Using transcranial direct-current stimulation (tDCS) to understand cognitive processing. Atten Percept Psychophys 79 (2017) 3-23.
[22]. A.B.F. de Venecia, 3rd, and S.M. Fresnoza, Visual Cortex Transcranial Direct Current Stimulation for Proliferative Diabetic Retinopathy Patients: A Double-Blinded Randomized Exploratory Trial. Brain Sci 11 (2021).
[23]. M. Tettamanti, G. Buccino, M.C. Saccuman, V. Gallese, M. Danna, P. Scifo, F. Fazio, G. Rizzolatti, S.F. Cappa, and D. Perani, Listening to action-related sentences activates fronto-parietal motor circuits. J Cogn Neurosci 17 (2005) 273-81.
[24]. T. Makovski, and M. Lavidor, Stimulating occipital cortex enhances visual working memory consolidation. Behav Brain Res 275 (2014) 84-7.
[25]. D. Wu, C. Li, N. Liu, P. Xu, and W. Xiao, Visual motion perception improvements following direct current stimulation over V5 are dependent on initial performance. Exp Brain Res 238 (2020) 2409-2416.
[26]. J.F. Veale, Edinburgh Handedness Inventory - Short Form: a revised version based on confirmatory factor analysis. Laterality 19 (2014) 164-77.
[27]. D. Lynott, and L. Connell, Modality exclusivity norms for 423 object properties. Behav Res Methods 41 (2009) 558-64.
[28]. M. Mimura, M. Verfaellie, and W.P. Milberg, Repetition priming in an auditory lexical decision task: effects of lexical status. Mem Cognit 25 (1997) 819-25.
[29]. P. Nasseri, M.A. Nitsche, and H. Ekhtiari, A framework for categorizing electrode montages in transcranial direct current stimulation. Front Hum Neurosci 9 (2015) 54.
[30]. M. Barbieri, M. Negrini, M.A. Nitsche, and D. Rivolta, Anodal-tDCS over the human right occipital cortex enhances the perception and memory of both faces and objects. Neuropsychologia 81 (2016) 238-244.
Cite this article
Xie,K.;Zhang,B. (2025). Causal Role for Visual Cortex in Processing Visual Semantics. Communications in Humanities Research,55,27-33.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of 3rd International Conference on Interdisciplinary Humanities and Communication Studies
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. O. Hauk, I. Johnsrude, and F. Pulvermuller, Somatotopic representation of action words in human motor and premotor cortex. Neuron 41 (2004) 301-7.
[2]. F. Pulvermuller, Brain mechanisms linking language and action. Nat Rev Neurosci 6 (2005) 576-82.
[3]. L.W. Barsalou, Grounded cognition. Annu Rev Psychol 59 (2008) 617-45.
[4]. L. Aziz-Zadeh, S.M. Wilson, G. Rizzolatti, and M. Iacoboni, Congruent embodied representations for visually presented actions and linguistic phrases describing actions. Curr Biol 16 (2006) 1818-23.
[5]. O. Hauk, and F. Pulvermuller, Neurophysiological distinction of action words in the fronto-central cortex. Hum Brain Mapp 21 (2004) 191-201.
[6]. F. Pulvermuller, Y. Shtyrov, and R. Ilmoniemi, Brain signatures of meaning access in action word recognition. J Cogn Neurosci 17 (2005) 884-92.
[7]. R.M. Willems, L. Labruna, M. D'Esposito, R. Ivry, and D. Casasanto, A functional role for the motor system in language understanding: evidence from theta-burst transcranial magnetic stimulation. Psychol Sci 22 (2011) 849-54.
[8]. T. Gijssels, R.B. Ivry, and D. Casasanto, tDCS to premotor cortex changes action verb understanding: Complementary effects of inhibitory and excitatory stimulation. Sci Rep 8 (2018) 11452.
[9]. F. Vitale, I. Padron, A. Avenanti, and M. de Vega, Enhancing Motor Brain Activity Improves Memory for Action Language: A tDCS Study. Cereb Cortex 31 (2021) 1569-1581.
[10]. K. Johari, N. Riccardi, S. Malyutina, M. Modi, and R.H. Desai, HD-tDCS of primary and higher-order motor cortex affects action word processing. Front Hum Neurosci 16 (2022) 959455.
[11]. W.K. Simmons, V. Ramjee, M.S. Beauchamp, K. McRae, A. Martin, and L.W. Barsalou, A common neural substrate for perceiving and knowing about color. Neuropsychologia 45 (2007) 2802-10.
[12]. J. Gonzalez, A. Barros-Loscertales, F. Pulvermuller, V. Meseguer, A. Sanjuan, V. Belloch, and C. Avila, Reading cinnamon activates olfactory brain regions. Neuroimage 32 (2006) 906-12.
[13]. A. Barros-Loscertales, J. Gonzalez, F. Pulvermuller, N. Ventura-Campos, J.C. Bustamante, V. Costumero, M.A. Parcet, and C. Avila, Reading salt activates gustatory brain regions: fMRI evidence for semantic grounding in a novel sensory modality. Cereb Cortex 22 (2012) 2554-63.
[14]. M. Kiefer, E.J. Sim, B. Herrnberger, J. Grothe, and K. Hoenig, The sound of concepts: four markers for a link between auditory and conceptual brain systems. J Neurosci 28 (2008) 12224-30.
[15]. M.A. Nitsche, and W. Paulus, Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527 Pt 3 (2000) 633-9.
[16]. M.A. Nitsche, and W. Paulus, Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57 (2001) 1899-901.
[17]. P.C. Gandiga, F.C. Hummel, and L.G. Cohen, Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol 117 (2006) 845-50.
[18]. M.A. Nitsche, S. Doemkes, T. Karakose, A. Antal, D. Liebetanz, N. Lang, F. Tergau, and W. Paulus, Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol 97 (2007) 3109-17.
[19]. R.M. Reinhart, and G.F. Woodman, Causal control of medial-frontal cortex governs electrophysiological and behavioral indices of performance monitoring and learning. J Neurosci 34 (2014) 4214-27.
[20]. R.M. Reinhart, and G.F. Woodman, Enhancing long-term memory with stimulation tunes visual attention in one trial. Proc Natl Acad Sci U S A 112 (2015) 625-30.
[21]. R.M. Reinhart, J.D. Cosman, K. Fukuda, and G.F. Woodman, Using transcranial direct-current stimulation (tDCS) to understand cognitive processing. Atten Percept Psychophys 79 (2017) 3-23.
[22]. A.B.F. de Venecia, 3rd, and S.M. Fresnoza, Visual Cortex Transcranial Direct Current Stimulation for Proliferative Diabetic Retinopathy Patients: A Double-Blinded Randomized Exploratory Trial. Brain Sci 11 (2021).
[23]. M. Tettamanti, G. Buccino, M.C. Saccuman, V. Gallese, M. Danna, P. Scifo, F. Fazio, G. Rizzolatti, S.F. Cappa, and D. Perani, Listening to action-related sentences activates fronto-parietal motor circuits. J Cogn Neurosci 17 (2005) 273-81.
[24]. T. Makovski, and M. Lavidor, Stimulating occipital cortex enhances visual working memory consolidation. Behav Brain Res 275 (2014) 84-7.
[25]. D. Wu, C. Li, N. Liu, P. Xu, and W. Xiao, Visual motion perception improvements following direct current stimulation over V5 are dependent on initial performance. Exp Brain Res 238 (2020) 2409-2416.
[26]. J.F. Veale, Edinburgh Handedness Inventory - Short Form: a revised version based on confirmatory factor analysis. Laterality 19 (2014) 164-77.
[27]. D. Lynott, and L. Connell, Modality exclusivity norms for 423 object properties. Behav Res Methods 41 (2009) 558-64.
[28]. M. Mimura, M. Verfaellie, and W.P. Milberg, Repetition priming in an auditory lexical decision task: effects of lexical status. Mem Cognit 25 (1997) 819-25.
[29]. P. Nasseri, M.A. Nitsche, and H. Ekhtiari, A framework for categorizing electrode montages in transcranial direct current stimulation. Front Hum Neurosci 9 (2015) 54.
[30]. M. Barbieri, M. Negrini, M.A. Nitsche, and D. Rivolta, Anodal-tDCS over the human right occipital cortex enhances the perception and memory of both faces and objects. Neuropsychologia 81 (2016) 238-244.









