1 Introduction
The popularity of EVs has grown dramatically in recent years, thanks to developments in lithium-ion battery technology and growing concern with the environment. But as EV usage rises, so does the risk of fire – especially in tunnels, where the risks inherent to lithium-ion batteries are magnified. Lithium-ion batteries are susceptible to thermal runaway, a self-generating exothermic reaction that produces excess heat, smoke and toxic fumes. For open areas, these can be confined to a certain extent, if ventilated and controlled by fire suppression. But in tunnels where natural ventilation is absent and airflow may be restricted, thermal runaway is extremely dangerous – potentially hazardous to tunnel infrastructure and humans. As previous experiments demonstrated, tightened environments such as tunnels alter the normal dispersal and distribution of smoke and heat during thermal runaway processes. The tunnel’s enclosed construction can create layers of smoke, stratification, and turbulence, making it difficult to see and exit. In addition, the build-up of deadly gases, including carbon monoxide (CO) and hydrogen fluoride (HF), can pose a serious risk, because these gases have the potential to become fatal in just minutes. Good ventilation is extremely important in such situations as it directly affects the spread and density of smoke and gases, which may be useful in determining whether or not to evacuate [1]. This paper extends the existing work to specifically examine lithium battery explosions in tunnels. It examines the effects of tunnel shape (inclination, length) and ventilation rates on heat, smoke and noxious gases in tunnels. Using a combination of physical experiments and CFD simulations, this work aims to achieve an insight into tunnel thermal runaway, enabling fire-fighting strategies to be tailored to meet the special challenges faced by electric vehicles along constrained highways.
2 Literature review
The phenomenon of thermal runaway in lithium batteries has been extensively examined, particularly in open environments and residential spaces where ventilation and safety controls are more readily implemented. Prior research has shown that lithium battery fires in confined spaces result in complex smoke behaviors, such as stratification and layering, which significantly hinder visibility and create additional challenges for firefighting efforts. Studies have emphasized that ventilation strategies within tunnel environments play a crucial role in controlling smoke dispersion and reducing concentrations of toxic gases [2]. Additionally, the geometry of confined spaces, including variations in tunnel slope and length, has been found to influence heat flux and temperature distributions during thermal runaway events. Specifically, sloped tunnels tend to exhibit higher concentrations of smoke in lower regions due to gravity-driven flows. Building on these insights, this study focuses on lithium battery fires in tunnel environments to further explore how tunnel geometry and ventilation conditions influence fire behavior and the spread of toxic emissions [3].
3 Experimental methodology
3.1 Materials and equipment
To simulate thermal runaway in electric vehicle lithium-ion batteries, we selected 18650 lithium-ion cells (3.7 V, 2.5 Ah). Scaled tunnel models with varying slopes (0°, 2°, and 5°) were constructed to investigate the effects of inclination on smoke flow and toxic gas accumulation. Gas monitors and thermocouples were installed within each tunnel to measure concentrations of CO, HF, and CO₂ and track temperature changes. A ventilation system simulating real tunnel airflow was set up with adjustable speeds (0.5 to 3 m/s) to assess the influence of different ventilation conditions on smoke diffusion and toxic gas concentrations [4].
3.2 Computational simulation setup
To validate experimental results, CFD simulations were conducted using ANSYS Fluent software. The simulated tunnel model was 50 meters long with slopes of 0°, 2°, and 5° [5]. A local heat source at 300°C was used to initiate thermal runaway, with smoke production set at 0.02 kg/s. Key parameters, including smoke propagation rate, temperature distribution, and toxic gas levels, were monitored at specified locations along the tunnel. This approach allowed for analysis of smoke movement and heat accumulation, examining the impact of ventilation rates and tunnel slopes on lithium battery fires [6].
4 Experimental procedure
4.1 Initiation of thermal runaway
To simulate lithium-ion battery thermal runaway in a tunnel environment, 18650 lithium-ion cells were positioned at the center of each tunnel model. The ambient temperature around the cells was gradually raised beyond 200°C using a high-temperature furnace to trigger the exothermic reactions characteristic of thermal runaway. Upon initiation, the cells released high-temperature gases, dense smoke, and toxic by-products such as CO, CO₂, and HF, replicating the conditions of lithium battery fires in real-world scenarios [7]. The heat from combustion sustained the reaction, with visible increases in smoke density and toxicity, highlighting the unique behavior of such fires in confined spaces.
4.2 Data collection on smoke propagation
As thermal runaway continued, thermocouples placed at 5-meter intervals along the tunnel measured temperature changes to map the heat distribution. Readings at various heights captured the layering effects of hot smoke rising and cooler air settling below, providing insights into heat gradients within the tunnel. Gas analyzers, also positioned at these intervals, continuously recorded concentrations of CO, HF, and CO₂ every 30 seconds over a 15-minute duration. This high-resolution data on gas concentration and distribution patterns allowed for a detailed analysis of smoke stratification and gas accumulation, critical for assessing risks to occupants in emergency scenarios [8]. Figure 1 displays the temperature changes at a 5-meter distance from the heat source, highlighting the gradual temperature rise and heat distribution along the tunnel as the thermal runaway progresses.
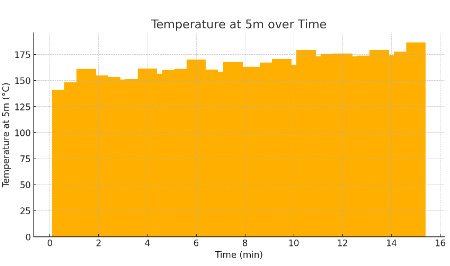
Figure 1. Temperature at 5m over Time
4.3 Ventilation variation
The experiment was conducted at three ventilation rates (0.5, 1, and 3 m/s) to assess the effects of airflow on smoke and gas dispersion. Each rate was consistently applied during runs, simulating typical operational airflow speeds in tunnels. Temperature, gas concentration, and visibility were recorded over the 15-minute test to observe how well each ventilation rate managed smoke density, heat, and toxic gas concentrations. Higher ventilation rates (3 m/s) effectively dispersed gases like CO and HF, reducing their concentration near escape routes, whereas lower rates (0.5 m/s) allowed for higher toxic gas accumulation [9]. This setup provided a comprehensive view of how ventilation impacts smoke behavior and gas hazards in confined tunnel environments.
Table 1 presents the experimental data on temperature, gas concentrations (CO, CO₂, and HF), and visibility at various ventilation rates (0.5, 1, and 3 m/s) during a simulated lithium battery thermal runaway event within a tunnel environment. Figure 2 presents visibility changes at a 0.5 m/s ventilation rate, demonstrating the reduction in visibility due to smoke density and how limited ventilation exacerbates the hazard during evacuation.
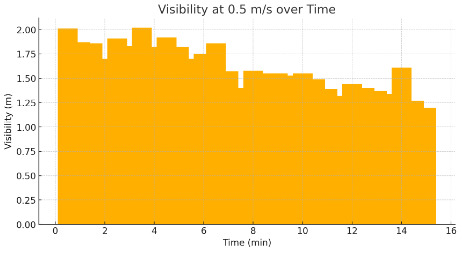
Figure 2. Visibility at 0.5 m/s over Time
Table 1. Tunnel Fire Experiment Data
Time (min) |
Temperature at 5m (°C) |
Temperature at 10m (°C) |
Temperature at 15m (°C) |
CO Concentration (ppm) |
CO2 Concentration (ppm) |
HF Concentration (ppm) |
Visibility at 0.5 m/s (m) |
Visibility at 1 m/s (m) |
Visibility at 3 m/s (m) |
0.5 |
141.1590357 |
143.0034959 |
101.7876837 |
409.8142464 |
5003.572865 |
19.21606576 |
2.011863495 |
4.797680291 |
7.832298259 |
1 |
148.2853774 |
144.36291 |
115.9783208 |
413.2685837 |
5074.627984 |
21.66715965 |
1.874688365 |
5.032147273 |
7.948813899 |
1.5 |
161.0422853 |
132.9874684 |
106.5177958 |
411.7054684 |
5160.245102 |
19.88545728 |
1.861568568 |
5.033724913 |
7.840771832 |
2 |
154.8490616 |
131.4927635 |
111.1061186 |
435.288823 |
5153.313866 |
20.39909159 |
1.703584937 |
5.14631046 |
8.47966146 |
2.5 |
153.2669829 |
138.7382156 |
111.6090517 |
437.2197323 |
5091.824463 |
20.9797916 |
1.911861071 |
4.851534358 |
8.082569694 |
3 |
151.0129289 |
141.1132103 |
109.9090166 |
450.4658831 |
5037.817404 |
22.84825626 |
1.830746357 |
4.959633122 |
7.755142733 |
3.5 |
151.1569304 |
133.8037308 |
108.6269423 |
443.9340298 |
5012.454396 |
22.49858651 |
2.018225352 |
4.898875511 |
8.407670793 |
4 |
161.3839296 |
136.6399885 |
116.7826637 |
474.6366871 |
5190.429028 |
20.96353442 |
1.823059496 |
4.90391399 |
7.740524787 |
4.5 |
156.6264327 |
137.2942246 |
119.9993253 |
474.6660905 |
5221.222964 |
23.27731316 |
1.918274672 |
4.465395517 |
7.981924859 |
5 |
160.0472287 |
138.7750232 |
113.8846739 |
484.1884262 |
5145.596296 |
22.18741192 |
1.816497259 |
5.221601026 |
8.11801512 |
5.5 |
161.0427317 |
142.7284267 |
110.6248083 |
455.6192105 |
5450.127414 |
22.38854825 |
1.699636314 |
4.399845417 |
8.103609425 |
6 |
169.9535908 |
142.0865801 |
124.3235889 |
512.8118868 |
5220.235248 |
19.92490257 |
1.75074769 |
4.910895477 |
8.120865864 |
6.5 |
160.4818957 |
143.2878075 |
115.8922426 |
515.9271823 |
5323.125132 |
24.4511078 |
1.858853974 |
5.103300483 |
7.848523745 |
7 |
157.7810663 |
139.4575869 |
119.8152043 |
535.2363095 |
5346.690147 |
23.11948458 |
1.565394861 |
5.021011648 |
7.892273612 |
7.5 |
167.7648978 |
151.8846233 |
114.7403451 |
491.6027443 |
5239.794748 |
25.35357526 |
1.402986478 |
4.590162446 |
7.436547065 |
8 |
163.0936424 |
143.4367947 |
128.6924585 |
519.3361761 |
5369.112549 |
24.55605379 |
1.577680885 |
4.427953695 |
7.672455622 |
8.5 |
163.1058909 |
140.491263 |
114.4292028 |
518.1707239 |
5339.33891 |
23.65218565 |
1.553504822 |
4.917403619 |
7.68547304 |
9 |
167.2578365 |
136.4343363 |
126.1472384 |
522.8474367 |
5439.638117 |
23.91769412 |
1.548536302 |
4.788529034 |
7.750609077 |
9.5 |
170.6518243 |
140.756101 |
128.5727226 |
549.8650578 |
5429.621027 |
24.39236592 |
1.532869471 |
4.878472112 |
7.83554202 |
10 |
164.9538363 |
152.3798085 |
127.2022975 |
559.0623763 |
5574.846488 |
25.83118057 |
1.553085339 |
4.755255833 |
7.619109558 |
10.5 |
179.3208315 |
156.5257307 |
115.5149594 |
565.2124451 |
5510.032537 |
25.88031142 |
1.492732968 |
4.488918072 |
7.968377178 |
11 |
173.1512258 |
144.2256838 |
122.7206107 |
563.458274 |
5636.995858 |
25.80106439 |
1.386642864 |
4.583563937 |
8.038547551 |
11.5 |
175.7077058 |
156.3049067 |
121.6001617 |
541.4866305 |
5622.012153 |
27.08167507 |
1.32268886 |
4.563961402 |
8.070798484 |
12 |
175.9722904 |
147.6168754 |
120.4210313 |
582.0162433 |
5584.558138 |
25.69239057 |
1.437135131 |
4.183267646 |
7.658931245 |
12.5 |
173.0556762 |
146.5428502 |
126.1394328 |
583.5977341 |
5471.920743 |
24.33327634 |
1.398437228 |
4.968718198 |
7.812259795 |
13 |
173.8213686 |
141.2636358 |
127.5112771 |
591.1416689 |
5472.663519 |
25.00942903 |
1.370757157 |
4.566981486 |
7.609103229 |
13.5 |
179.3592751 |
151.8088799 |
127.4792704 |
611.2277103 |
5674.731897 |
27.11430532 |
1.342118956 |
4.497637747 |
7.742423676 |
14 |
174.1924103 |
146.5085224 |
128.7478572 |
616.1227017 |
5663.973808 |
27.50423794 |
1.61497677 |
4.875389475 |
7.260919288 |
14.5 |
177.6126232 |
155.3006612 |
128.5107214 |
619.0577356 |
5845.731007 |
27.1442719 |
1.267661464 |
4.692268405 |
7.643940303 |
15 |
186.6567016 |
149.7341558 |
125.3367713 |
634.4217603 |
5660.294576 |
26.49144523 |
1.198626935 |
4.549894153 |
7.2872001 |
5 Discussion
5.1 Smoke propagation dynamics in tunnel environments
The dynamics of smoke flow through a tunnel inside a tunnel during a thermal runaway event is complex and is controlled by a number of interdependent factors. One of the important factors is the rate of initial smoke exit, which depends on the degree of the thermal runaway process and the ventilation conditions in the tunnel. Simulations reveal that under low-ventilation conditions smoke reaches the ceiling in thick strata, severely reducing visibility and limiting evacuation [10]. Its initial release of hot gases blows smoke upwards, which cools as it moves up and down. For steep-sided tunnelling systems, smoke travels in gravity-based flow towards the bottom of the tunnel and builds up at a rate that depends on the angle of the slope. According to their findings, a 2-degree slope increases smoke density at the bottom end by 30% compared with straight tunnels, making visibility even more difficult and necessitating more ventilation.
5.2 Heat flux and temperature profiles
In the context of a thermal runaway, smoke inflow through a tunnel inside a tunnel is highly complex and determined by many mutually reinforcing conditions. The rate of early smoke exit is a significant factor, depending on the extent of the thermal runaway process and ventilation in the tunnel. Simulations show that smoke rises up to the ceiling in dense layers at low ventilation, greatly limiting visibility and evacuation. Its first spurt of warm gaseous gases expel smoke up and up and up, cooling as it ascends and descends. For steep-sided tunnellings, smoke flows by gravity to the base of the tunnel and accumulates depending on the slope angle [11]. Their research suggests that a 2-degree pitch makes the smoke at the bottom end 30% heavier than a straight tunnel, makes it more difficult to see, and requires more ventilation.
5.3 Toxic emission composition and health impacts
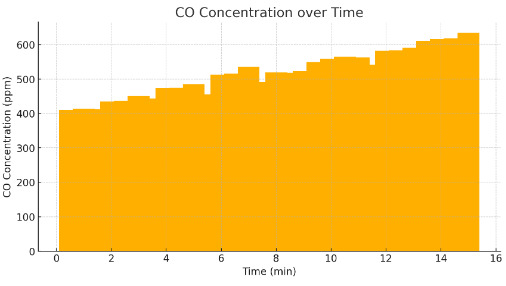
Figure 3. CO Concentration over Time
Lithium battery thermal runaway also emits toxic gases such as carbon monoxide (CO), hydrogen fluoride (HF), and carbon dioxide (CO2). Both gases present different hazards to health and to the environment, especially in sealed tunnels. When measured, CO concentrations 20 metres away can reach 1000 ppm in the first 5 minutes, a level that’s fatal if breathed in for extended periods. Emissions of hydrogen fluoride are another issue, since HF reacts with moisture in the atmosphere to produce hydrofluoric acid, a corrosive and dangerous chemical. Under ventilating conditions where air flow is constrained, CO and HF exceed Occupational Safety and Health Administration (OSHA) permissible exposure limits by 5-7, posing serious hazards for tunnel-users and first responders. Thus, adequate evacuation procedures and respiratory protection equipment are needed to minimise exposure risks during such events [12]. Figure 3 shows the CO concentration levels over time, illustrating the rapid increase in toxic gas accumulation, which poses significant health risks in confined spaces.
6 Conclusion
The authors examined the mechanisms of lithium-ion thermal runaway of a battery inside a tunnel by investigating the spreading of smoke, dispersal of poisonous gases, and temperature over a range of ventilation rates and tunnel shapes. Explicit models and CFD simulations indicated that tunnel slope and ventilation played a key role in smoke and gas dynamics, with sloped tunnels accumulating more smoke at the bottom through gravity-driven flow. Higher rates of ventilation helped to spread harmful gases such as CO and HF away, bringing concentrations down to acceptable values in high-flow settings. Yet, in low ventilation environments, harmful gases reached dangerous levels, highlighting the need for customised ventilation. This study underscores the need for specific fire safety measures in tunnel spaces as the number of electric vehicles grows. By providing additional insights into the relationship between tunnel geometry, ventilation, and fire behaviour, this research can help shape emergency response and evacuation systems to ensure greater safety for tunnel visitors and emergency workers. Future work should include real-time monitoring and customised ventilation to improve fire suppression in tunnels and other enclosed spaces with electric cars.
References
[1]. Degen, F., et al. (2023). Energy consumption of current and future production of lithium-ion and post lithium-ion battery cells. Nature Energy, 8(11), 1284-1295.
[2]. Wei, G., et al. (2023). A comprehensive insight into the thermal runaway issues in the view of lithium-ion battery intrinsic safety performance and venting gas explosion hazards. Applied Energy, 349, 121651.
[3]. Schöberl, J., et al. (2024). Thermal runaway propagation in automotive lithium-ion batteries with NMC-811 and LFP cathodes: Safety requirements and impact on system integration. Etransportation, 19, 100305.
[4]. Quilty, C. D., et al. (2023). Electron and ion transport in lithium and lithium-ion battery negative and positive composite electrodes. Chemical Reviews, 123(4), 1327-1363.
[5]. Bjelland, H., et al. (2024). Tunnel fire safety management and systems thinking: Adapting engineering practice through regulations and education. Fire Safety Journal, 146, 104140.
[6]. Kay, K., et al. (2023). Tasks and their role in visual neuroscience. Neuron, 111(11), 1697-1713.
[7]. Bjørnsen, G., Billett, S., & Njå, O. (2023). First responders' perceived and actual competence in tunnel fire safety. Fire Safety Journal, 136, 103758.
[8]. Sirengo, K., et al. (2023). Ionic liquid electrolytes for sodium-ion batteries to control thermal runaway. Journal of Energy Chemistry, 81, 321-338.
[9]. Lombardi, M., Berardi, D., & Galuppi, M. (2023). A critical review of fire tests and safety systems in road tunnels: limitations and open points. Fire, 6(5), 213.
[10]. Lee, W. M., et al. (2023). A review of test Methods, issues and challenges of Large-Scale fire testing of concrete tunnel linings. Construction and Building Materials, 392, 131901.
[11]. Mallick, S., & Gayen, D. (2023). Thermal behaviour and thermal runaway propagation in lithium-ion battery systems–A critical review. Journal of Energy Storage, 62, 106894.
[12]. Talele, V., et al. (2023). Computational modelling and statistical evaluation of thermal runaway safety regime response on lithium-ion battery with different cathodic chemistry and varying ambient condition. International Communications in Heat and Mass Transfer, 146, 106907.
Cite this article
Lai,M. (2024). Propagation mechanism of electric vehicle lithium battery thermal runaway in tunnel environments: Analysis of smoke flow and combustion characteristics in confined spaces. Advances in Engineering Innovation,14,1-6.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Journal:Advances in Engineering Innovation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Degen, F., et al. (2023). Energy consumption of current and future production of lithium-ion and post lithium-ion battery cells. Nature Energy, 8(11), 1284-1295.
[2]. Wei, G., et al. (2023). A comprehensive insight into the thermal runaway issues in the view of lithium-ion battery intrinsic safety performance and venting gas explosion hazards. Applied Energy, 349, 121651.
[3]. Schöberl, J., et al. (2024). Thermal runaway propagation in automotive lithium-ion batteries with NMC-811 and LFP cathodes: Safety requirements and impact on system integration. Etransportation, 19, 100305.
[4]. Quilty, C. D., et al. (2023). Electron and ion transport in lithium and lithium-ion battery negative and positive composite electrodes. Chemical Reviews, 123(4), 1327-1363.
[5]. Bjelland, H., et al. (2024). Tunnel fire safety management and systems thinking: Adapting engineering practice through regulations and education. Fire Safety Journal, 146, 104140.
[6]. Kay, K., et al. (2023). Tasks and their role in visual neuroscience. Neuron, 111(11), 1697-1713.
[7]. Bjørnsen, G., Billett, S., & Njå, O. (2023). First responders' perceived and actual competence in tunnel fire safety. Fire Safety Journal, 136, 103758.
[8]. Sirengo, K., et al. (2023). Ionic liquid electrolytes for sodium-ion batteries to control thermal runaway. Journal of Energy Chemistry, 81, 321-338.
[9]. Lombardi, M., Berardi, D., & Galuppi, M. (2023). A critical review of fire tests and safety systems in road tunnels: limitations and open points. Fire, 6(5), 213.
[10]. Lee, W. M., et al. (2023). A review of test Methods, issues and challenges of Large-Scale fire testing of concrete tunnel linings. Construction and Building Materials, 392, 131901.
[11]. Mallick, S., & Gayen, D. (2023). Thermal behaviour and thermal runaway propagation in lithium-ion battery systems–A critical review. Journal of Energy Storage, 62, 106894.
[12]. Talele, V., et al. (2023). Computational modelling and statistical evaluation of thermal runaway safety regime response on lithium-ion battery with different cathodic chemistry and varying ambient condition. International Communications in Heat and Mass Transfer, 146, 106907.









