1 Introduction
The demand of the lithium batteries (LIBs) is rising rapidly as the scarcity of fossil foil and the destruction of environment. LIBs had been well-popularized in the electric car area over the world such as China, United state and Japan because of its high energy density, non-memory effect and eco-friendly. In 2024, China’s lithium battery exports accounted to US$578,988,210, year-on-year growth of 1.97% [1]. The importance of LIBs in the development of the electric industry is becoming increasingly prominent. LIBs consist of cathode material, anode material, electrolyte and separator, and the cathode materials are the most important component in the LIBs, which account for 50% of the total value. Cathode material has a direct impact on the safety, stability, and capacity of the LIBs [2]. The scientists are now studying various type of cathode materials, such as LiCoO₂, LiMn₂O₄, and LiFePO₄ cathode materials etc.
LiNixCoyMnzO₂ (NCM) displays an outstanding amalgamation of various cathode materials. In comparison to other cathode materials exhibits numerous advantages such as high energy density, extended cycling life, and superior thermal resistance. However, structural instability, cation mixing, and the elevated cost of cathode materials have consistently impeded the further advancement of NCM ternary batteries. In addition to modifying the configuration of the transition metals in NCM, doping technique is another effective method of enhancing the electrochemical performance of LIBs. Generally, there are two major doping techniques that usually use in the research. Figure 1 illustrates the structure of cathode materials employing two distinct doping methods. One method is bulk doping, which involves incorporating additional elements into the materials; the other is surface doping, achieved by coating different elements to the appearance of electrodes.
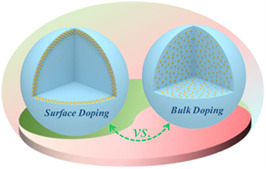
Figure 1. Visual illustration comparing the structural differences between surface doping and bulk doping [3].
In this work, we briefly introducing the performance and properties of some particular dopants present in the NCM cathode materials. We also briefly summarizing the morphology and the structure of doped and undoped NCM cathode materials through the characterization techniques. Our results provide insights into optimizing cathode materials for enhanced battery performance and longevity. Meanwhile, this research has provided a concise overview of the features of each doping technique, discussed future prospect for improving high-quality cathode materials, and suggested a feasible strategy for combining high-tech into the doping experiment.
2 Different doping elements
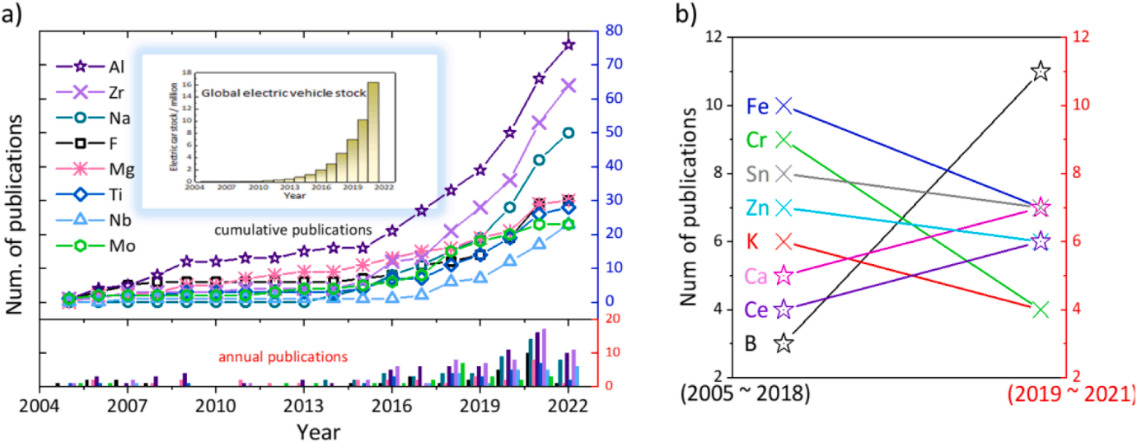
Figure 2. (a) Publication trends on key doping elements in the NCM structure and the global shift towards electric vehicle. (b) A line graphs of public trends on other doping elements [4].
Generally, a large number of elements have been used as a dopant, which can effectively occupy the transition metal (TM) or Li sites in the lattice layered NCM structure. Accounted about 168 different doped spinel systems with 20 dopant variation [3]. The evidences of Improving the electrochemical performance throughout the doping strategies is rooted in Figure 2, the first diagram shows that the yearly publications had increased significantly since 2016, the background of it might because of the huge growth in electric industry. the second diagram indicates the change in the number of publications specific on several doping elements from two separately period [4]. Next, we classify and summarize the different doping elements for improving the performance of Li-ion batteries.
2.1 Transition metals (Zr, Ti, V)
Transition metals (TMs) have been extensively researched because of their outstanding compatibility. Among groups 3, 4, and 5, Zr, Ti, Y, Nb and V have been identified as a superior dopant for improving the characteristics of NCM cathode materials. Herein, it will be mainly focusing on the introducing of the doping research on Zr, Ti, and V in chronological order. In 2011, Lin et al. synthesized Zr-doped NCM 111 using a variety of sample’s compositions. Zr-doped is shown to be effective in improving the electrochemical performance of LiCoO2, Li4Ti5O12, and LiNixCo1-x-yMnyO2. Through the polymerization process, it can enlarge the lattice parameters of cathode materials but also can promote high Li-ion diffusion due to it large ionic radius. The results show that additive Zr-0.01 has the best electrochemical characteristic that can help the electrode resistance, improve the cycling performance and increase the rate capability of the electrochemical cells. The examination of LiNi1/3Co1/3Mn1/3–0.01Zr0.01O2 exhibits more superior results, with a capacity retention of 92.7% over 100 cycles and a capacity of 133.9 mAh·g-1 at 8 C rate [5]. In 2016, Kou et al. successfully synthesized the Titanium (Ti) dopant into NCM. The involvement of Ti shows positive effect on NCM, which benefits the improvement of particle strength and better cycling. They applied two methods to obtain Li1.167Ni0.4-xMn0.383Co0.05TixO2, Co-precipitation and Solid-state reaction. The results show that the addition of Ti effectively enhances the cycling performance and the retention of Li1.167Ni0.4Mn0.383Co0.05O2 [6]. In 2016, Sim et al. synthesized V-doped LiNi0.84Co0.10Mn0.06O2 through the solid-state reaction. Numerous researchers have identified vanadium (V) as a promising dopant owing to its capacity to assume various valence states, thereby augmenting the stability of the NCM crystals and improving the Li-ion diffusion coefficient. The NCM sample displays enhanced cyclic stability and rate capacity (at 2C) in the presence of addition V, compared to pristine NCM. The V-0.005 doped exhibits more excellent electrochemical performance and structural stability. Nevertheless, it was deduced that there is a limit to the quantity of V that may be substituted into the TM sites, and any remaining V results in the harmful impurity of Li3VO4. Figure 3 depicts the rate performance of both pristine and V-doped samples across a range of charge-discharge rates from 0.1C to 2C. At lower current rates of 0.1C to 1C, the capacity of pristine NCM exceeded that of the V-doped samples marginally. When the electronic conductivity and lattice parameters are increased to produce the highest rate capability, V-doped is beneficially leading to rapid Li-ion kinetics [7]. In a nutshell, although the presence transition metals (Zr, Ti, V) present a very excellent and positive result in the doping of NCM cathode materials, the performance really depends on the quantity of dopant is added, which means that excess amount of added dopant may cause a side-effect on the capacity of NCM. The publications reflect that the initial discharge capacity of LNCMZ decreases as Zr’s content increasing. This unexpected reduction might be due to the generation of electrochemically inactive Li2ZrO3 [5]. In the study of dopant Ti. It was also observed that when Ti concentration increases continuously, the morphology of the Ti-0.06 and 0.08 samples drastically changed during the process as the amounts of Ti is added. Agglomeration or severe fragmentation hinders lithium-ion diffusion. The structural morphology of the 0.04 Ti-doped variant is superior to the others [6]. Furthermore, Sim et al. demonstrated that severe vanadium doping beyond 0.01mol% results in a diminished I(003)/(104) ratio, signifying significant Li+/Ni+ cation mixing, which consequently contributes to the distortion in the pristine structure of NCM materials [7].
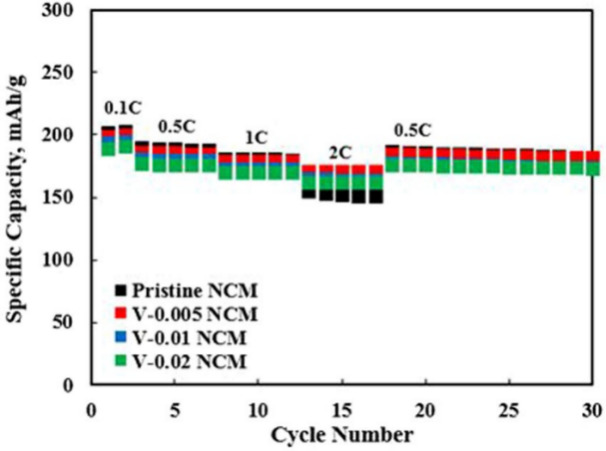
Figure 3. The amount of V in both pristine and added samples ranges from V-0.005 to 0.02 in terms of rate capability [7].
2.2 Alkali metals (Na, K, Rb)
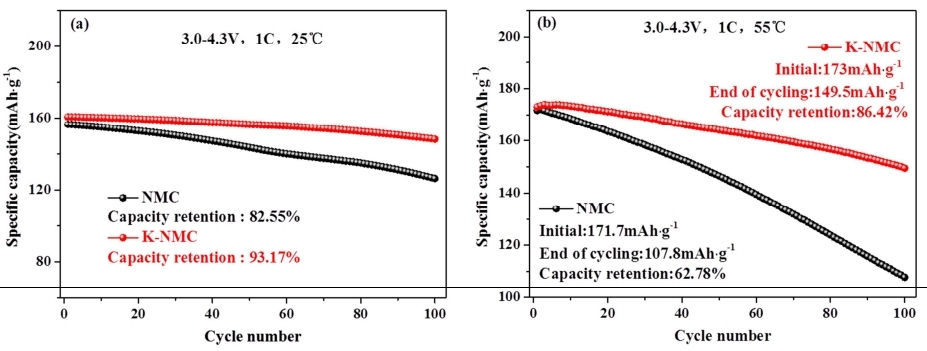
Figure 4. (a) The cycling capacity of NMC and K-doped NCM under the temperature condition of 25 ℃. (b) The cycling efficiency of NMC and K-NMC at a rate of 1 C under the temperature condition of 50 ℃ [9].
The study of alkali metals doping strategies have widely emerged in recent years. In 2021, Gao et al. synthesized Li1−xNaxNi1/3Co1/3Mn1/3O2 by coprecipitation reaction integrate with solid-phase sintering. They utilized Density Functional Theory (DFT) calculations to evaluate the electrochemical properties of Na-doped NCM111. According to the results, the structure of LiNi1/3Co1/3Mn1/3O2 can be stabilized by the Na doping. When the amount Na increases, the electronic potential energy is decreasing. The NCM111 has better conductivity and rate performance when Na-added ranges from 0.03-0.06 mol [8]. Potassium (K), sharing similar radius and structure compare with Li, also can incorporate into the lattice structural NCM to improve the properties. In 2016, Yang et al. incorporated K+ into LiNi0.5Co0.2Mn0.3O2 and successfully synthesized the K-doped into NCM523, by the process of the hydroxide co-precipitation reaction combined with solid-state sintering method [9]. The findings indicate that the enhancement of electrochemical properties can be achieved through the presence of K+ on the Li, which extends the interlayer space of O-Li-O and lessens the rate of cation mixing. Figure 4a illustrates the cycling performance of NCM and K-NCM at 1 C rate, spanning from 3 V to 4.3 V at 25 ℃, while figure 4b presents the cycling performance of NCM and K-NCM at the same 1 C rate at 55 ℃, but at a temperature of 55 ℃. The results indicate a huge improvement in K-NCM’s capacity retention, achieving 93.17%, along with a charge-discharge capacity of 148.4 mAh·g-1 after 100 cycles at 1 C, surpassing NCM’s performance [9]. In 2018, Li et al. applied the co-precipitation method to synthesis the Rb-doped into LiNi0.4Co0.2Mn0.4O2, and made the conclusion about its morphology, structure and electrochemical properties with the existing of Rb dopant. The undoped sample NCM 424 had a first discharge capacity of 153 mAh·g-1. However, the Rb-doped NCM 424 demonstrated superior initial discharge capacity, measuring 168 mAh·g-1. The coulombic efficiency (CE) of the undoped sample was calculated to be 79.05%, while NCMZr-0.03 was 84.74%. the performance of NCM samples during charge-discharge cycles at various rates is presented in Figure 5. The electrochemical properties of LiNi0.4Co0.2Mn0.4O2 were significantly improved under different contents. Among them, Rb-0.03 shows the best rate performance, and the charge-discharge capacity is close to 5C 130 mAh·g-1, which was nearly 170 mAh·g-1 when returning to 0.2C. It may cause by the doping of Rb that expanding and stabilizing the lattice structure of cathode material [10]. To summarize, proper amount of Alkali Metal dopants (Na, K, Rb) can greatly enhance the performance of NCM cathode materials. However, it also refers that excess Na doping will result in the reduction of the NCM’s conductivity [8]. Also, the disadvantage of doped Rb can be revealed in its high cost, it also will affect the lattice structure of the NCM cathode materials when it is excessed [10].
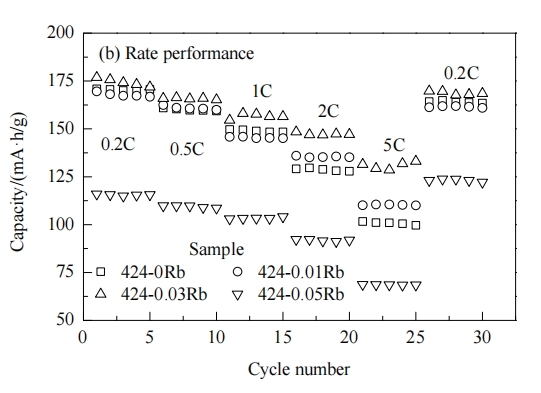
Figure 5. Rate performance of Li1xRbxNi0.4Co0.2Mn0.4O2 samples under different currents [10].
2.3 Metalloid (B, La)
The structural stability and the success of efficient pathways for the diffusion of Li+ ions are both enhanced by processing a series of metalloids. Park et al. made a big progress in the B doping of LiNi0.90Co0.05Mn0.05O2 in 2018. Boron stands out as an excellent doping material that significantly reforms the microstructure of cathode materials. The B1.0-NCM90 sample achieves a discharge capacity of 237 mAh·g-1, maintaining an impressive 91% capacity retention after 100 cycles at 55 ℃, which is 15% higher than that of the pure NCM90 cathode material. Figure 6 illustrates that B doping alters the surface energy of the NCM cathode material, resulting in a microstructure where primary particles are aligned directionally. This configuration improves their ability to manage stress from volume changes, thereby further enhancing both cycling stability and electrochemical properties [11]. Moreover, some publications show that the presence of La dopant results in enhancing the discharge rate, cyclability, and the rate capability of Li-rich NCM. Yu et al. initially synthesized La-doped into Li1.2Mn0.54-xNi0.13Co0.13LaxO2, using a solvothermal method followed by a high-temperature calcination technique. The Li1.2Mn0.52Ni0.13Co0.13La0.02O2 material exhibits exceptional electrochemical performance compared to other La-doped samples, achieving a specific capacity of 286.4 mAh·g-1 at 0.1 C, along with a coulombic efficiency of 90.4% and a capacity retention of 93.2%. Meanwhile, the shift from a layer to a spinel structure and the decrease in voltage can be effectively lessened by partially replacing Mn with La. La-doped can effectively mitigate the decline in average discharge voltage during cycling, addressing a common drawback in current Li-rich cathode materials. [12]. Park et al. applied Galvano-static intermittent titration technique to determine the conductivity of LNCMB in different samples. It shows that the increasing amount of boron doping will inevitably deteriorate the rate capability of the cathodes, which is due to the low conductivity of boron-doped cathodes [11]. Therefore, although the presence of metalloid in cathodes might lessen the structural degradation during cyclic process, it cannot resolve the problems of LIBs once for all.
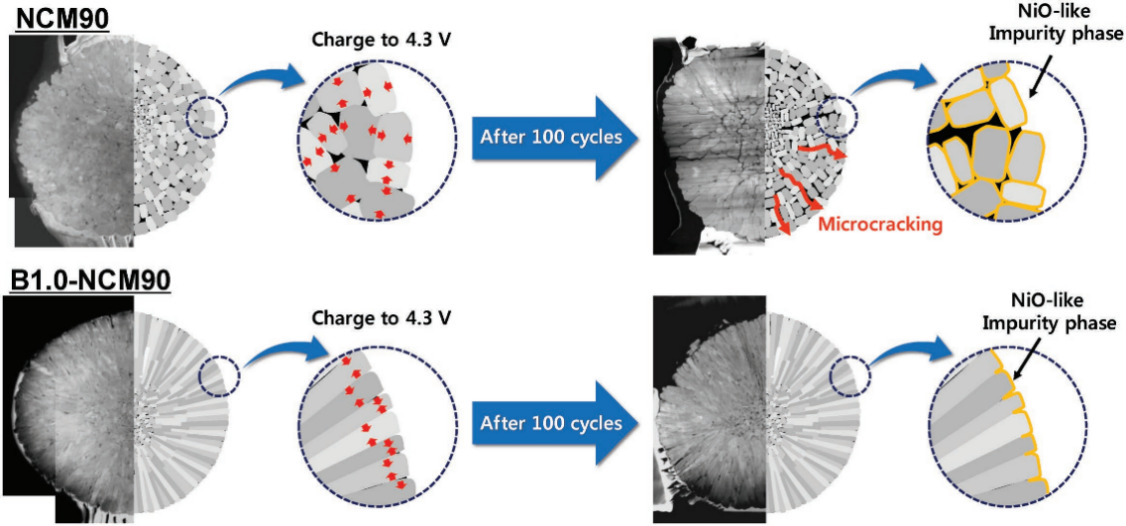
Figure 6. Mechanical stability of the NCM90 cathode materials over charge-discharge cycles as affected by boron doping [12].
3 Characterization of NCM cathode materials
With the development of the LIBs cathode materials, in order to obtain a large amount of effective information to evaluate in terms of cathode material’s properties, morphology, and distribution status. Researchers carry out various characteristic techniques on different cathode materials to examine those aspects that have been referred above. [13].
3.1 Morphology characterization

Figure 7. SEM images (a) SEM image of NCM90 under 1 µm and 10 µm, (b) image of B0.4-NCM90 (c) image of B1.0-NCM90; STEM images of as-prepared (d) image pf NCM90 under 2 µm (e) image of B1.0-NCM90 under 2 µm (f) TEM image of textured pristine particles from B1.0-NCM90, along with electron diffraction pattern in the area highlighted by yellow circle, and a schematic illustration of the crystal structure of oriented primary particle [11].
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) are powerful characterization methods. SEM provides detailed surface topography and composition, ideal for studying surface structures and textures at the micron and sub-micron levels. TEM, on the other hand, delves deeper into internal structures, offering high-resolution images at the atomic scale, enabling detailed analysis of crystallography and defects [14]. Figure 7a-c displays SEM images showing the particle morphologies of typical NCM 90, B0.4-NCM 90, and B1.0-NCM 90 cathodes. All three cathodes have roughly the same size distribution, a spherical particle. Figure 7a and 7b show that each secondary particle has smaller main particles whose size decreases as the B’s content increasing, while no obvious differences are seen in the secondary particle morphology. Figure 7d and 7e display cross-sectional TEM images of secondary particles from the NCM90 and B1.0-NCM90 cathodes, respectively, distinctly illustrating that the incorporation of boron markedly modifies the spatial properties and the constitution of the primary particles. Li et al. have successfully observed and identified the structure of Li1xRbxNi0.4Co0.2Mn0.4O2 in different samples through the diagram of SEM and TEM. Figure 8 shows the TEM diagram of LNCM-Rb materials with different composition. The crystal layer spacing is slightly enlarged, indicating that Rb replaces the part of Li+ into the crystal during the synthetic process, which is beneficial to the stability of the crystal lattice structure and also enhances the electrochemical properties of the cathode materials [10].
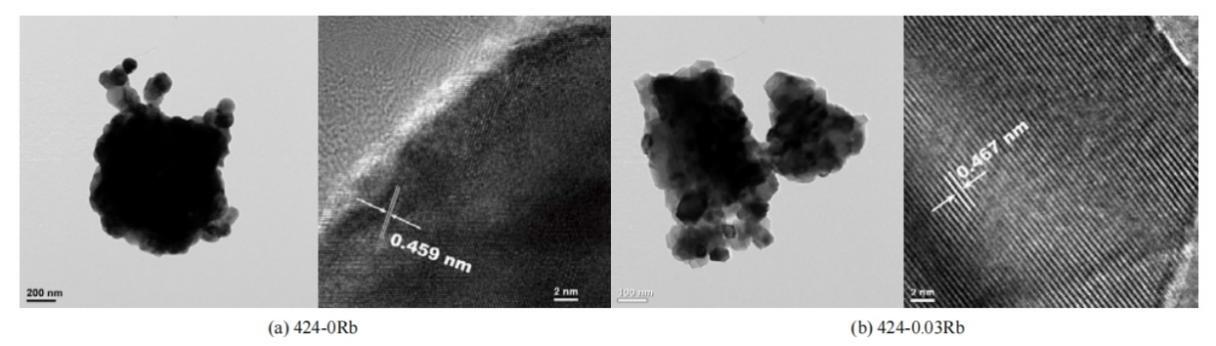
Figure 8. TEM images of Li1xRbxNi0.4Co0.2Mn0.4O2 with varying content of Rb-doped [10].
X-ray diffraction (XRD) is a non-destructive technique that provides insights into the crystal structure, crystallinity, stress, crystal orientation, superstructure and other characteristics of materials. it also reflects the average crystal structure properties of the bulk materials, allowing for the observation of changes in average unit cell structure parameters and the extraction of atomic occupancy information through fitting [15]. La, Yu et al. effectively identified the phase composition and crystal structure of the La-NCM cathode materials through XRD. All samples are illustrated in Figure 9, and they all indicate a typical hexagonal a-NaFeO2 structure with some weak diffraction peaks between 20° and 25°. The brief range of the Li and Mn in the Li2MnO3 layers might be the cause of these. The corresponding crystal structures of these two phases are depicted in Figure 9a and 9b. It suggests that a well-defined layered structure was revealed in a two pairs of the well-split (006)/(102) and (018)/(110) peaks. Furthermore, low-intensity peaks appear in the L3-LLo samples as the La concentration increase substantially. This could be used to suggest the development of a La-rated impurity phase and the breakdown of the composite’s electrochemical characteristics [12].
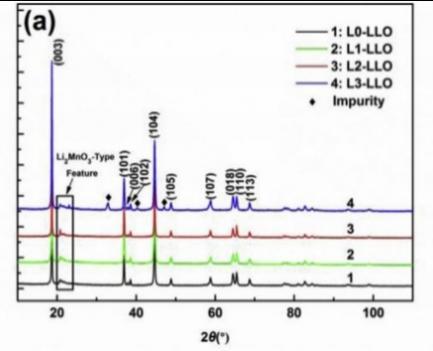
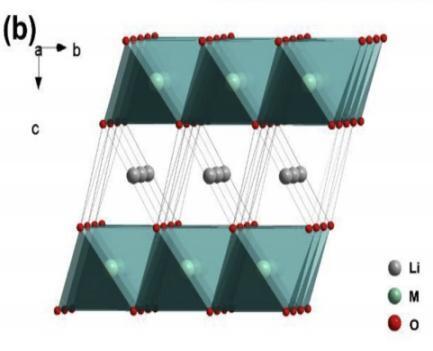
Figure 9. Structural details for prepared samples LLOs both with and without La-added. (a) XRD patterns of 4 prepared samples; (b) crystal structure of the rhombohedral LiMO2 [12].
3.2 Composition characterization
Table 1. Illustration of the average chemical composition of the samples that were produced and the experimental results that were planned [12].
Sample |
Designed molar content of Li: Mn: Ni: Co: La |
Experimental molar content of Li: Mn: Ni: Co: La |
Li1.2Mn0.54Ni0.13CO0.13O2 |
1.2:0.54:0.13:0.13:0 |
1.198:0.541:0.130:0.129:0 |
Li1.2Mn0.53Ni0.13CO0.13La0.01O2 |
1.2:0.53:0.13:0.13:0.01 |
1.200:0.528:0.129:0.131:0.012 |
Li1.2Mn0.52Ni0.13CO0.13La0.02O2 |
1.2:0.52:0.13:0.13:0.02 |
1.201:0.521:0.128:0.130:0.021 |
Li1.2Mn0.51Ni0.13CO0.13La0.03O2 |
1.2:0.51:0.13:0.13:0.03 |
1.201:0.508:0.132:0.128:0.031 |
Inductively coupled plasma (ICP) is one of the significant composition characterization methods, which is mainly used to analyze the elemental composition and content in samples. Yu et al. applied ICP-OES technique to verify the chemical composition of different prepared LiNi0.90Co0.05Mn0.05BxO2 cathode materials. Figure 10 displays the ICP-OES results of the obtained materials. it can be seen from the elemental analysis that the sample compositions are basically the same as the designed compositions. Notably, the La concentration in each sample corresponds accurately with the nominal stoichiometry, while the Mn content decreases as the La concentration increases [12]. Additionally, X-ray photoelectron spectroscope (XPS) is employed to confirm the oxidation states of the metal materials. Yu et al. utilized the XPS technique to verify the oxidation states of the dopant La and the transition metals in both undoped and La-doped samples. All binding energies (BEs) in the XPS analysis are calibrated using the C 1s peak, which is set at 284.6 eV, to correct for specimen charging. Figure 11 illustrates that the binding energies of Mn 2p3/2 and Co 2p3/2 for Lo-LLO samples align perfectly with the data presented in the References, confirming the presence of Mn4+ and Co3+ in both samples. Figure 11 f illustrates a peak around 834.08 eV, which is associated with La 3d5/2, suggesting that the La element exists as La3+ in the L2-LLO sample. At the same time, Figure 11 f and e demonstrate that the Ni2p3/2 binding energies for both samples align the standard values for Ni2+ and Ni3+ [12]. Scanning transmission X-ray microscopy (STXM) serves as a potent synchrotron-based technique commonly utilized to examine the electronic and chemical structures of cathode materials. Sun et al. demonstrated that carbon coated Li4Ti5O12 displays better magnification and cycling performance compared to its uncoated counterpart. STXM and high-resolution TEM are used to ascertain that the amorphous carbon layer is uniformly coated on the surface of LTO particles, with a coating thickness of approximately 5 nm. Researchers also obtain the distribution of C, Ti, and O in single LTO particle, with C coated on the particle’s surface [16].
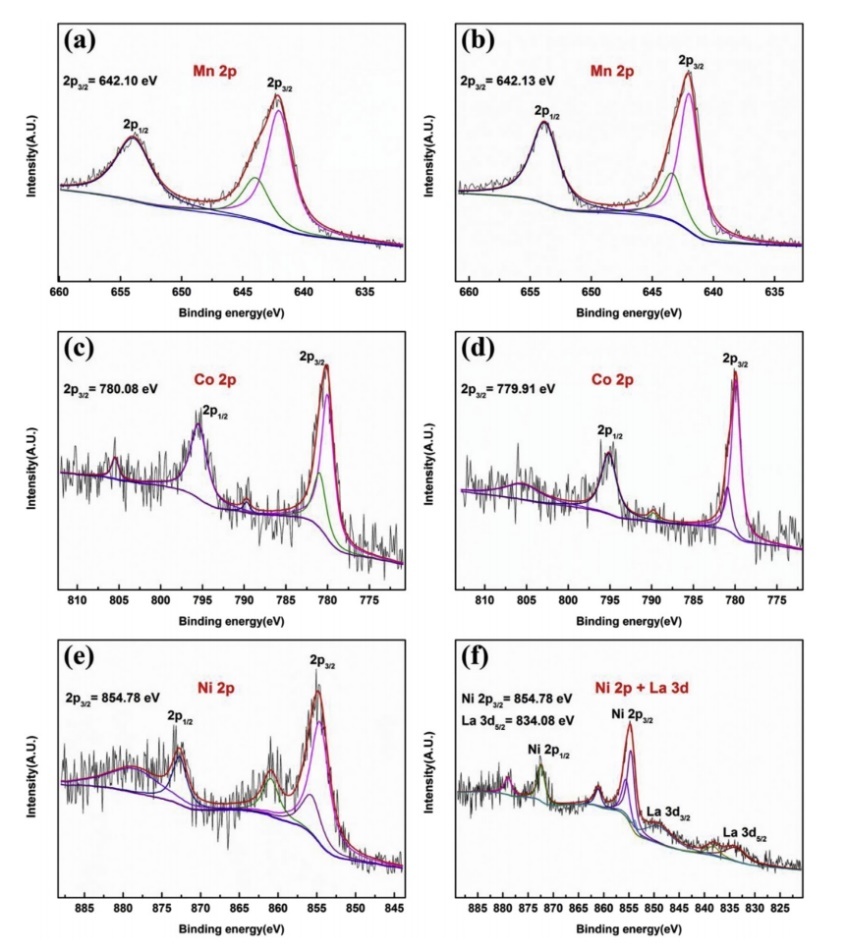
Figure 10. XPS spectra of transition metals specific in different configuration. (a) Mn 2p, (c) Co 2p and (e) Ni 2p for L0-LLO; (b) Mn 2p, (d) Co 2p and (f) Ni 2p and La 3d for L2-LLO [12].
4 Prospect
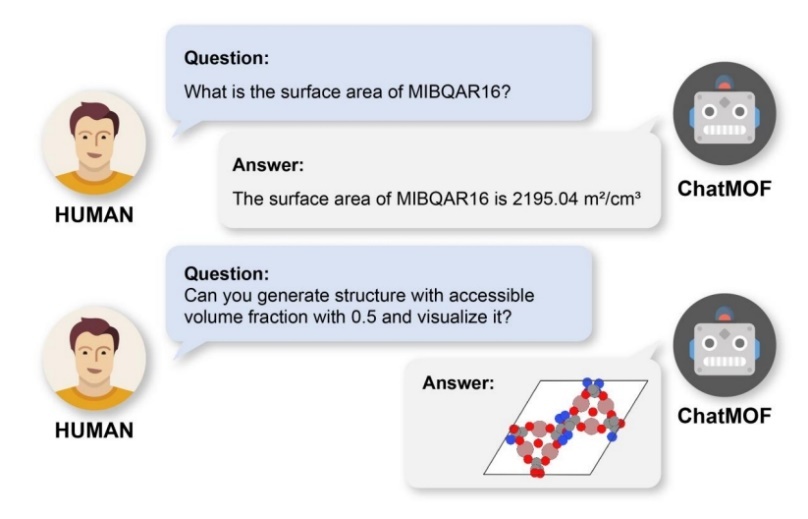
Figure 11. A conceptual image that explains the ChatMOF [18].
The development of Artificial Intelligent (AI) has evolved from simple rule-based systems to sophisticated, learning algorithms capable of mimicking human intelligence and performing complex tasks across various fields. The possibility of combining the doping study and characterization with cutting-edge technology may give us more insight into how to set up a more efficient and accurate method for analyzing and measuring of the doping research. To be specific, ChatMOF may be a tremendous technology that can applying into the study of cathode materials doping. Meng et al. mentioned that ChatMOF serves as an artificial intelligence framework designed for the prediction and generation of potential metal-organic frame materials. This exploration examines the advantages and drawbacks of integrating large language models (LLMs) with databases and machine learning in material sciences, highlighting its potential to significantly accelerating the future revolution and evolvement [17]. Figure 12 elucidates the specific concept of ChatMOF, when a user inquires about the properties of a MOF, ChatMOF provides an appropriate answer. If a user wishes to generate a new MOF, ChatMOF can create one that meets the specified criteria [18]. Furthermore, Sun et al. introduced a kind of automized method, which can automatically extract Pd-based catalyst synthesis routes from the chemical literature. This method avoids a lot of manual data annotation and improves the efficiency of scientific literature information acquisition [19]. Barone et al. applied crystalline materials databases to develop precise machine learning models (ML), facilitating the investigation of materials for energy storage. Through the examination of inorganic materials within the Materials Project and AFLOW databases, a new compilation of more than 190,000 potential cathode materials was established, significantly expanding upon the original database of 5000. This expanded dataset improved prediction models for battery performance by at least 28%, which help to identify more potential electrode candidates with tremendous metrics [20].
5 Conclusion
In conclusion, this study emphasizes the crucial role of LIBs in modern technology and industry, especially focusing on NCM cathode materials. Through detailed characterization and analysis of various dopants, the research mainly aims to introduce several effective dopants base on alkali metals, transition metals as well as metalloids, which present a better electrochemical properties, performance, and stability when they participate in doping process. The doped metals all show a superior performance in terms of cycling capacity and capacity retention after 100 cycles base on particular temperature and specific doped content. The paper also applies other electrochemical test to examine the exactly evidence of doped NCM cathode materials. Nevertheless, the doping of NCM cathode materials continues to encounter significant challenges and hinders. We deem the exorbitant cost of materials as a principal limitation. The instability, unavoidable degradation, and over-thermal effects on the lattice structure represent the foremost issues that the researcher must address moving forward. Furthermore, current research is deficient in investigations aimed at enhancing batteries with a lifespan exceeding 10 years. As the demand for LIBs grows, particularly in the electric vehicle and electric device industry, optimizing NCM cathode materials is essential for enhancing battery efficiency, longevity as well as practicability. In the future, the researcher can leverage AI to replace some of the works and calculations that used to be humans.
References
[1]. Hua, X. (2024, March 2). China’s lithium-ion battery output up 25 pct in 2023. English.www.gov.cn.https://english.www.gov.cn/archive/statistics/202403/02/content_WS65e2e63cc6d0868f4e8e48b0.html. Accessed 2 March 2024.
[2]. Zhang, B., Xu, Y., Silvester, D. S., Banks, C. E., Deng, W., Zou, G., Hou, H., & Ji, X. (2024). Direct Regeneration of Cathode Materials in Spent lithium-ion Batteries toward closed-loop Recycling and Sustainability. Journal of Power Sources, 589, 233728. https://doi.org/10.1016/j.jpowsour.2023.233728. 3 November 2023.
[3]. Qian, Huaming, et al. “Surface Doping vs. Bulk Doping of Cathode Materials for Lithium-Ion Batteries: A Review.” Electrochemical Energy Reviews, vol. 5, no. 4, 10 Nov. 2022, https://doi.org/10.1007/s41918-022-00155-5. Accessed 9 October 2024.
[4]. Ko, G., Jeong, S., Park, S., Lee, J., Kim, S., Shin, Y., Kim, W., & Kwon, K. (2023). Doping strategies for enhancing the performance of lithium nickel manganese cobalt oxide cathode materials in lithium-ion batteries. Energy Storage Materials, 60, 102840. https://doi.org/10.1016/j.ensm.2023.102840. Accessed 7 June 2023.
[5]. Ding, C.X., et al. “Improvement of Electrochemical Properties of Layered LiNi1/3Co1/3Mn1/3O2 Positive Electrode Material by Zirconium Doping.” Solid State Ionics, vol. 189, no. 1, May 2011, pp. 69–73, https://doi.org/10.1016/j.ssi.2011.02.015. Accessed 7 February 2022.
[6]. Kou, Yanjuan, et al. “The Effect of Ti Doping on Electrochemical Properties of Li1.167Ni0.4Mn0.383Co0.05O2 for Lithium-Ion Batteries.” Solid State Ionics, vol. 296, Nov. 2016, pp. 154–157, https://doi.org/10.1016/j.ssi.2016.09.020. Accessed 15 May 2022.
[7]. Sim, Seoung-Ju, et al. “Improving the Electrochemical Performances Using a V-Doped Ni-Rich NCM Cathode.” Scientific Reports, vol. 9, no. 1, 20 June 2019, https://doi.org/10.1038/s41598-019-45556-7. Accessed 11 May 2023.
[8]. Gao, Y., Shen, K., Liu, P., Liu, L., Chi, F., Hou, X., & Yang, W. (2021). First-Principles investigation on electrochemical performance of Na-doped LiNi1/3Co1/3Mn1/3O2. Frontiers in Physics, 8. https://doi.org/10.3389/fphy.2020.616066. Accessed 16 February 2021.
[9]. Yang, Z., Guo, X., Xiang, W., Hua, W., Zhang, J., He, F., Wang, K., Xiao, Y., & Zhong, B. (2017). K-doped layered LiNi0.5Co0.2Mn0.3O2 cathode material: Towards the superior rate capability and cycling performance. Journal of Alloys and Compounds, 699, 358–365. https://doi.org/10.1016/j.jallcom.2016.11.245. Accessed 30 March 2017.
[10]. Yan Li, Wang, X., Zhang, W., He, Y., & Ma, Z. (2018). Synthesis and Electrochemical Characterization of Li1-xRbxNi0.4Co0.2Mn0.4O2 Cathode Materials. Journal of Process Engineering, 18(2), 422–426. https://doi.org/10.12034/j.issn.1009-606x.217296. Accessed 10 April 2018.
[11]. Park, K.-J., Jung, H.-G., Kuo, L.-Y., Kaghazchi, P., Yoon, C. S., & Sun, Y.-K. (2018). Improved Cycling Stability of LiNi0.90 Co0.05 Mn0.05O2 Through Microstructure Modification by Boron Doping for Li-Ion Batteries. Advanced Energy Materials, 8(25), 1801202. https://doi.org/10.1002/aenm.201801202. Accessed 11 July 2018.
[12]. Yu, Ruizhi, Wang. Gang., Liu, Meihong. Zhang, Xiaohui. Wang, Xianyou. Shu, Hongbo. Yang, Xiukang. & Huang, Weihua. (2016). Mitigating voltage and capacity fading of lithium-rich layered cathodes by lanthanum doping. The Journal of Power Sources, 335, 65–75. https://doi.org/10.1016/j.jpowsour.2016.10.042. Accessed 15 December 2016.
[13]. N, B., & H, D. (2016). Review on synthesis, characterizations, and electrochemical properties of cathode materials for lithium-ion batteries. Journal of Material Science & Engineering, 5(4). https://doi.org/10.4172/2169-0022.1000258. Accessed 5 June 2016.
[14]. Ebnesajjad, S. (2011). Surface and material characterization techniques. Handbook of Adhesives and Surface Preparation, 48, 31–48. https://doi.org/10.1016/b978-1-4377-4461-3.10004-5. Accessed 14 January 2011.
[15]. Adams, F. (2005). X-ray absorption and diffraction | Overview. Encyclopedia of Analytical Science, 403, 365–377. https://doi.org/10.1016/b0-12-369397-7/00668-3. Accessed 28 May 2005. Accessed 28 May 2005.
[16]. Sun, X., Hegde, M., Wang, J., Zhang, Y., Liao, J., Radovanovic, P. V., & Cui, B. (2014). Structural Analysis and Electrochemical Studies of Carbon Coated Li4Ti5O12 Particles Used as Anode for Lithium-Ion Battery. ECS Transactions, 58(14), 79–88. https://doi.org/10.1149/05814.0079ecst. Accessed 1 November 2024.
[17]. Meng, K., Bai, K., & Sun, S. (2024). Artificial intelligence driven design of cathode materials for sodium-ion batteries using graph deep learning method. Journal of Energy Storage, 101, 113809–113809. https://doi.org/10.1016/j.est.2024.113809. Accessed 8 October 2024.
[18]. Kang, Y., & Kim, J. (2023). ChatMOF: An Autonomous AI System for Predicting and Generating Metal-Organic Frameworks. ArXiv.org. https://arxiv.org/abs/2308.01423. Accessed 25 August 2023.
[19]. Li, S., Zhang, Y., Fang, Z., Meng, K., Tian, R., He, H., & Sun, S. (2023). Extracting the Synthetic Route of Pd-Based Catalysts in Methanol Steam Reforming from the Scientific Literature. Journal of Chemical Information and Modeling, 63(20), 6249–6260. https://doi.org/10.1021/acs.jcim.3c01442. Accessed 9 October 2023.
[20]. Moses, I. A., Barone, V., & Peralta, J. E. (2022). Accelerating the discovery of battery electrode materials through data mining and deep learning models. Journal of Power Sources, 546, 231977. https://doi.org/10.1016/j.jpowsour.2022.231977. Accessed 27 August 2022.
Cite this article
Luo,Y. (2024). A review study on the modification and characterization of NCM cathode materials through doping strategies for the improvement of Li-ion batteries. Advances in Engineering Innovation,14,39-47.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Journal:Advances in Engineering Innovation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Hua, X. (2024, March 2). China’s lithium-ion battery output up 25 pct in 2023. English.www.gov.cn.https://english.www.gov.cn/archive/statistics/202403/02/content_WS65e2e63cc6d0868f4e8e48b0.html. Accessed 2 March 2024.
[2]. Zhang, B., Xu, Y., Silvester, D. S., Banks, C. E., Deng, W., Zou, G., Hou, H., & Ji, X. (2024). Direct Regeneration of Cathode Materials in Spent lithium-ion Batteries toward closed-loop Recycling and Sustainability. Journal of Power Sources, 589, 233728. https://doi.org/10.1016/j.jpowsour.2023.233728. 3 November 2023.
[3]. Qian, Huaming, et al. “Surface Doping vs. Bulk Doping of Cathode Materials for Lithium-Ion Batteries: A Review.” Electrochemical Energy Reviews, vol. 5, no. 4, 10 Nov. 2022, https://doi.org/10.1007/s41918-022-00155-5. Accessed 9 October 2024.
[4]. Ko, G., Jeong, S., Park, S., Lee, J., Kim, S., Shin, Y., Kim, W., & Kwon, K. (2023). Doping strategies for enhancing the performance of lithium nickel manganese cobalt oxide cathode materials in lithium-ion batteries. Energy Storage Materials, 60, 102840. https://doi.org/10.1016/j.ensm.2023.102840. Accessed 7 June 2023.
[5]. Ding, C.X., et al. “Improvement of Electrochemical Properties of Layered LiNi1/3Co1/3Mn1/3O2 Positive Electrode Material by Zirconium Doping.” Solid State Ionics, vol. 189, no. 1, May 2011, pp. 69–73, https://doi.org/10.1016/j.ssi.2011.02.015. Accessed 7 February 2022.
[6]. Kou, Yanjuan, et al. “The Effect of Ti Doping on Electrochemical Properties of Li1.167Ni0.4Mn0.383Co0.05O2 for Lithium-Ion Batteries.” Solid State Ionics, vol. 296, Nov. 2016, pp. 154–157, https://doi.org/10.1016/j.ssi.2016.09.020. Accessed 15 May 2022.
[7]. Sim, Seoung-Ju, et al. “Improving the Electrochemical Performances Using a V-Doped Ni-Rich NCM Cathode.” Scientific Reports, vol. 9, no. 1, 20 June 2019, https://doi.org/10.1038/s41598-019-45556-7. Accessed 11 May 2023.
[8]. Gao, Y., Shen, K., Liu, P., Liu, L., Chi, F., Hou, X., & Yang, W. (2021). First-Principles investigation on electrochemical performance of Na-doped LiNi1/3Co1/3Mn1/3O2. Frontiers in Physics, 8. https://doi.org/10.3389/fphy.2020.616066. Accessed 16 February 2021.
[9]. Yang, Z., Guo, X., Xiang, W., Hua, W., Zhang, J., He, F., Wang, K., Xiao, Y., & Zhong, B. (2017). K-doped layered LiNi0.5Co0.2Mn0.3O2 cathode material: Towards the superior rate capability and cycling performance. Journal of Alloys and Compounds, 699, 358–365. https://doi.org/10.1016/j.jallcom.2016.11.245. Accessed 30 March 2017.
[10]. Yan Li, Wang, X., Zhang, W., He, Y., & Ma, Z. (2018). Synthesis and Electrochemical Characterization of Li1-xRbxNi0.4Co0.2Mn0.4O2 Cathode Materials. Journal of Process Engineering, 18(2), 422–426. https://doi.org/10.12034/j.issn.1009-606x.217296. Accessed 10 April 2018.
[11]. Park, K.-J., Jung, H.-G., Kuo, L.-Y., Kaghazchi, P., Yoon, C. S., & Sun, Y.-K. (2018). Improved Cycling Stability of LiNi0.90 Co0.05 Mn0.05O2 Through Microstructure Modification by Boron Doping for Li-Ion Batteries. Advanced Energy Materials, 8(25), 1801202. https://doi.org/10.1002/aenm.201801202. Accessed 11 July 2018.
[12]. Yu, Ruizhi, Wang. Gang., Liu, Meihong. Zhang, Xiaohui. Wang, Xianyou. Shu, Hongbo. Yang, Xiukang. & Huang, Weihua. (2016). Mitigating voltage and capacity fading of lithium-rich layered cathodes by lanthanum doping. The Journal of Power Sources, 335, 65–75. https://doi.org/10.1016/j.jpowsour.2016.10.042. Accessed 15 December 2016.
[13]. N, B., & H, D. (2016). Review on synthesis, characterizations, and electrochemical properties of cathode materials for lithium-ion batteries. Journal of Material Science & Engineering, 5(4). https://doi.org/10.4172/2169-0022.1000258. Accessed 5 June 2016.
[14]. Ebnesajjad, S. (2011). Surface and material characterization techniques. Handbook of Adhesives and Surface Preparation, 48, 31–48. https://doi.org/10.1016/b978-1-4377-4461-3.10004-5. Accessed 14 January 2011.
[15]. Adams, F. (2005). X-ray absorption and diffraction | Overview. Encyclopedia of Analytical Science, 403, 365–377. https://doi.org/10.1016/b0-12-369397-7/00668-3. Accessed 28 May 2005. Accessed 28 May 2005.
[16]. Sun, X., Hegde, M., Wang, J., Zhang, Y., Liao, J., Radovanovic, P. V., & Cui, B. (2014). Structural Analysis and Electrochemical Studies of Carbon Coated Li4Ti5O12 Particles Used as Anode for Lithium-Ion Battery. ECS Transactions, 58(14), 79–88. https://doi.org/10.1149/05814.0079ecst. Accessed 1 November 2024.
[17]. Meng, K., Bai, K., & Sun, S. (2024). Artificial intelligence driven design of cathode materials for sodium-ion batteries using graph deep learning method. Journal of Energy Storage, 101, 113809–113809. https://doi.org/10.1016/j.est.2024.113809. Accessed 8 October 2024.
[18]. Kang, Y., & Kim, J. (2023). ChatMOF: An Autonomous AI System for Predicting and Generating Metal-Organic Frameworks. ArXiv.org. https://arxiv.org/abs/2308.01423. Accessed 25 August 2023.
[19]. Li, S., Zhang, Y., Fang, Z., Meng, K., Tian, R., He, H., & Sun, S. (2023). Extracting the Synthetic Route of Pd-Based Catalysts in Methanol Steam Reforming from the Scientific Literature. Journal of Chemical Information and Modeling, 63(20), 6249–6260. https://doi.org/10.1021/acs.jcim.3c01442. Accessed 9 October 2023.
[20]. Moses, I. A., Barone, V., & Peralta, J. E. (2022). Accelerating the discovery of battery electrode materials through data mining and deep learning models. Journal of Power Sources, 546, 231977. https://doi.org/10.1016/j.jpowsour.2022.231977. Accessed 27 August 2022.









