1. Introduction
Organophosphorus flame retardants (OPFRs) are an excellent alternative to traditional brominated flame retardants, offering superior flame retardancy, environmental friendliness, compatibility, safety, and cost-effectiveness. Compared to banned or restricted brominated flame retardants such as polybrominated diphenyl ethers (PBDEs), OPFRs exhibit lower bioaccumulation and better degradability [1]. However, due to their widespread use and persistence, residual levels of OPFRs have been detected in various environmental matrices such as indoor dust, fine particulate matter, seawater, and sediments, exceeding those of brominated flame retardants [2-4]. It has been reported that the total concentration levels of OPFRs in surface water can reach μg/L, with tris-(1-chloro-2-propyl) phosphate (TCIPP) having the highest detected concentration [5]. The highest concentration levels of OPFRs in soil and sediments range from μg/g to mg/g, with TCIPP being one of the most commonly detected OPFRs [6]. OPFRs can enter the human body through multiple pathways and accumulate within it, such as dietary intake, inhalation of polluted air and dust, and skin absorption after contact with OPFR-containing objects [7]. Recent studies have suggested that OPFRs possess endocrine-disrupting properties, capable of interfering with the binding of endocrine hormones to receptors. However, current research on their interactions with endocrine nuclear receptors and their toxic mechanisms remains limited. This study employs molecular docking technology, selecting TCIPP—one of the most commonly detected OPFRs—as a ligand molecule to investigate its interaction patterns with endocrine nuclear receptors, thereby elucidating its endocrine-disrupting mechanisms. This provides a basis for research into the endocrine-disrupting effects and toxicological mechanisms of organophosphorus flame retardants, and offers new insights into the risk assessment and potential hazards of OPFRs in the environment.
2. Materials and methods
2.1. Preparation of ligand molecules
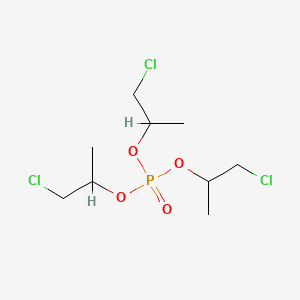
OPFRs are one of the most widely studied emerging pollutants. Although they are considered safer than brominated flame retardants, their potential threats to human health have gradually been confirmed. Currently, most studies suggest that they possess endocrine-disrupting properties, potentially affecting hormone secretion by binding to endocrine nuclear receptors, thereby leading to endocrine disorders. Therefore, this experiment selected TCIPP, one of the representative compounds of OPFRs, as the ligand molecule. Its CAS number is 13674-84-5, with a relative molecular mass of 327.6 g/mol, and its molecular structure is shown in Figure 1. The molecular conformation of the ligand molecule was obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/ ) and exported in SDF format. The Open Babel software (https://open-babel.readthedocs. io/ ) to further convert the conformation, forming the target conformation of the ligand molecule required for docking. This was then converted to MOL2 format and imported into the visualization tool AutoDockTools (Olson Laboratory, Scripps Research Institute, USA) within the AutoDock 1.5.7 software for preprocessing, enabling rotation of the ligand during docking to form a crystal conformation suitable for editing; Save the conformation file in PDBQT format for further molecular docking studies.
2.2. Receptor molecule preparation
Search for receptor molecules on the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/ ), retrieve the amino acid sequences of the receptor molecules ER (6V8T), TR (1Q4X), AR (2PNU), MR (5HCV), GR (3E7C), PR (1A28), PPAR (2ZK0), and RAR (1XAP), and compare them with the Protein Data Bank (PDB, https://www.rcsb.org/ ) to obtain the three-dimensional conformation of the proteins; use PyMOL software (https://pymol.org/2/ ) to display their three-dimensional conformations, remove excess chains, water molecules, and small molecules from the conformations, and save them in PDB format. Open the PDB format file in Autodock, perform hydrogenation, calculate the charge count, determine the rigid structure, and save it in PDBQT format for subsequent molecular docking steps.
2.3. Molecular docking
Import the pre-prepared ligand and protein into Autodock, use the Grid module to set the docking region, select all possible docking sites, save it in GPF format, and run the Autogrid module. In the Docking module, select the receptor and ligand, set the automatic docking runs to 50, determine the most likely conformation of TCIPP binding to the nuclear receptor, save in DPF format, and run Autodock for computation. After computation, in the Analyze module, sort the docking results by rank and export the data, then save the molecular file in PDBQT format. After docking is complete, select the optimal docking conformation based on the principles of low energy and reasonable conformation.
2.4. Analysis of docking results
In Open Babel, convert the PDBQT file of the docking results to a PDB format file, open it in Pymol software, set the parameters, display the optimal docking sites involved in the interaction between TCIPP and the eight nuclear receptors, the interaction forces, and the amino acid sites, and save the analysis results. In the three-dimensional interaction diagram of the receptor-ligand complex, the ligand small molecules are marked in yellow, the docking amino acid sites are annotated in green, and the remaining parts are made transparent and blurred. The amino acids designed in this study include Ala (alanine), Leu (leucine), Lys (lysine), His (histidine), Phe (phenylalanine), Asn (asparagine), Trp (tryptophan), Met (methionine), Pro (proline), Tyr (tyrosine), Ile (isoleucine).
3. Results
The molecular docking results of the eight receptor molecules with TCIPP (see Table 1) are as follows:
TCIPP binds to ER in the cavity near Ala350. TCIPP forms a hydrophobic interaction with the Ala350 residue of ER at a distance of 3.32 Å, and also forms a halogen bond with the Leu387 residue of ER at a distance of 3.26 Å.
TCIPP binds to TR in the cavity near His412. TCIPP forms three hydrogen bonds with TR, located at Lys211, His412, and Phe417, with hydrogen bond distances of 2.05, 3.31, and 2.99 Å, respectively. It also forms a halogen bond with Asn408 via a Cl-O bond, with a distance of 3.50 Å, and a salt bridge at Lys211, with a distance of 3.64 Å.
TCIPP binds to AR in the cavity near Leu704. TCIPP forms hydrophobic interactions with AR at Leu704, Leu707, TRP741, and Met780, with distances of 3.97, 3.79, 3.27, and 3.72 Å, respectively, and forms a halogen bond with Asn705 via a Cl-O bond, with a distance of 3.53 Å.
TCIPP binds to MR near the cavity at Pro788. TCIPP forms hydrophobic interactions with MR at Pro788, Asn898, and Tyr899, with distances of 3.91, 3.67, and 3.87 Å, respectively, and forms a hydrogen bond with Asn898 at a distance of 4.10 Å.
TCIPP binds to GR in the cavity near Phe623. TCIPP forms hydrophobic interactions with GR at Leu563 and Phe623, with distances of 3.71 and 3.41 Å, respectively.
TCIPP binds to RAR in the cavity near Ile623. TCIPP forms hydrophobic interactions with RAR at Leu259, Ile263, and Phe295, with distances of 3.36, 3.69, and 3.91 Å, respectively.
TCIPP binds to PR in the cavity near Phe905. TCIPP forms hydrophobic interactions with PR at Leu797, Tyr890, and Phe905, with distances of 3.83, 3.33, and 3.30 Å, respectively.
TCIPP binds to PPAR near the cavity at Phe264. TCIPP forms hydrophobic interactions with PPAR at Phe262 and Ile281, with distances of 3.60 and 3.53 Å, respectively.
|
Receptor molecule |
Best binding site |
Best binding energy |
Hydrophobic amino acids |
Other amino acid residues |
|
ER |
Ala350 |
-4.36 |
Ala350,Leu387 |
Leu387 |
|
TR AR MR GR RAR PR PPAR |
His412 Leu704 Pro788 Phe623 Ile263 Phe905 Phe264 |
-4.76 -4.48 -3.06 -4.14 -3.76 -4.40 -4.12 |
Leu704,Leu707,Met780,Trp741 Pro788,Asn898,Tyr899 Leu563,Phe623 Leu259,Ile263,Phe295 Leu797,Tyr890,Phe905 Phe264,Ile281 |
Lys211,His412,Phe417 Asn705 Asn898 |
4. Discussion
This study investigated the molecular docking of TCIPP, one of the most representative OPFRs, with eight endocrine-related nuclear receptors to analyze their interaction and toxicity mechanisms. The results showed that TCIPP mainly interacts with nuclear receptors through hydrogen bonds and hydrophobic interactions. The minimum binding energies of TCIPP with ER, TR, AR, MR, GR, RAR, PR, and PPAR were -4.36, -4.76, -4.48, -3.06, -4.14, -3.76, -4.40, and -4.12 kcal/mol, respectively. Based on the comparison of molecular binding energies, the binding affinities of TCIPP with the aforementioned eight nuclear receptors, from highest to lowest, are as follows: TR >AR >PR> ER >GR> PPAR > RAR > MR. Analysis suggests that although TCIPP exhibits lower binding affinity with RAR and MR, TR, AR, PR, and ER demonstrate higher affinity, including GR and PPAR, all of which exhibit certain endocrine-disrupting effects. Due to differences in the protein and its ligand-binding domain (LBD), the binding affinity is influenced by energy matching with the ligand, resulting in significant variations in binding strength. Additionally, similar binding sites exist in different proteins. In molecular docking information between TCIPP and multiple nuclear receptors, Leu is widely present in the docking structures, including: Leu387 when TCIPP binds to ER, Leu704 and Leu707 when binding to AR, Leu563 when binding to GR, Leu259 when binding to RAR, and Leu797 when binding to PR. This suggests that the stability of the binding conformation may be influenced by electronic effects, stereochemical structure, and the composition of surrounding amino acids, indicating a strong correlation with spatial stereochemical matching. It also implies that the endocrine-disrupting effects of TCIPP may vary among different receptors, with specific effects potentially related to the underlying mechanisms of action of the respective receptors.
Studies have shown that endocrine disruptors such as TCIPP and its analogues can interfere with the synthesis of testosterone and estradiol by altering the expression of genes encoding key enzymes involved in steroid biosynthesis. Research has also demonstrated that high doses of TPHP and TCEP can downregulate the expression of genes encoding low-density lipoprotein receptors, StAR, CYP11A1, and CYP17 in male mouse testes, thereby interfering with cholesterol transport and conversion and inhibiting testosterone synthesis in the testes [8]. On the other hand, TCIPP and other OPFRs can exert agonist or antagonist effects by binding to sex hormone receptors, exhibiting hormone-like activity or anti-hormone activity. Liu et al. found that both 0.02 mg/L and 2 mg/L TDCPP significantly upregulated the mRNA levels of the ER gene and the vitellogenin (VTG) gene controlled by ER in zebrafish embryos, indicating that TDCPP has estrogenic activity [9]. However, recent studies have identified a G protein-coupled estrogen receptor that can bind to estrogen and mediate rapid non-genomic effects. The interference of OPFRs with this receptor may be a key mechanism underlying their complex estrogen-disrupting effects [10]. Additionally, emerging studies indicate that TCIPP and TCEP may be associated with the disruption of thyroid hormone homeostasis, potentially through interference with the synthesis, transport, and metabolism of thyroid hormones. This interference may further induce alterations in thyroid hormone circulating levels and perturb their downstream signaling pathways. Potential mechanisms include inhibiting thyroid hormone metabolic enzymes, disrupting thyroid hormone transporters, and regulating thyroid hormone receptor activity [11]. Research also indicates that TCIPP and TCEP may interact with peroxisome proliferator-activated receptors (PPARs), which are involved in lipid and glucose metabolism as well as inflammatory responses, potentially disrupting metabolic homeostasis and contributing to the development of metabolic disorders. Disruption of estrogen, androgen, and thyroid hormone signaling pathways can interfere with normal tissue and organ development, potentially leading to congenital malformations, neurodevelopmental defects, and metabolic programming. Interactions with other nuclear receptors may further exacerbate metabolic dysfunction and increase the risk of metabolic syndrome [12].
Due to their flame-retardant properties, flame retardants are widely used in various industries such as automotive, chemical, and construction. However, with the acceleration of global industrialization, the use of flame retardants has surged, and their potential health risks have increasingly drawn public attention. Studies have shown that flame retardants enter environmental media through various pathways, including indoor volatilization, furniture wear, dust dispersion, outdoor industrial emissions, electronic waste disposal, and landfill, and subsequently accumulate in the human body [13]. According to reports, multiple OPFRs and their metabolites have been detected in various biological samples, including human blood, semen, urine, breast milk, hair, and nails [14]. OPFRs are easily metabolized in the human body and excreted through urine, but they can still be detected in blood. Bio-monitoring has found that the total OPFR levels in whole blood (2.61–79.2 ng/mL) are higher than those in urine (0.106–11.6 ng/mL), possibly because the affinity of OPFRs with plasma proteins reduces the human body's clearance rate [15,16]. High levels of OPFRs (10–604 ng/g) have been detected in human hair, with TCIPP, TEHP, and TPHP being the primary OPFRs [17]. Toxicological investigations on organophosphate flame retardants demonstrate that these compounds exhibit endocrine-disrupting properties, which interfere with hormone biosynthesis in the hypothalamic-pituitary-gonadal, hypothalamic-pituitary-thyroid, and hypothalamic-pituitary-adrenal axes, thereby impairing the physiological functionalities of these three endocrine axes [18].
5. Conclusion
There is limited research on the endocrine-disrupting effects of TCIPP and OPFRs in the existing literature, and there is a lack of theoretical basis. This study employed molecular docking technology to analyze the binding interactions between TCIPP and endocrine nuclear receptors, while investigating the binding energies and associated amino acid residues involved in the binding process. The results indicate that the binding affinity of TCIPP with nuclear receptors is primarily driven by hydrophobic interactions with non-polar residues of the receptor, as well as hydrogen bonds or halogen bonds formed with key residues. Among these, TR, AR, ER, and PR exhibit higher affinities, which may lead to more significant endocrine-disrupting effects. This study reveals that phosphorus-based flame retardants represented by TCIPP may exhibit different biological toxicities toward various hormone receptors in organisms, providing a basis for subsequent experiments and corresponding risk management. However, this study only analyzed the endocrine-disrupting mechanism of TCIPP through molecular docking simulations. The specific differences in toxic effects and the formulation of regulatory measures require further investigation and validation through in vitro experiments.
References
[1]. Jing-yi, Z., Zi-xiang, Z., Meng-juan, L., et al. (2022). A systematic scoping review of epidemiological studies on the association between organophosphate flame retardants and neurotoxicity.Ecotoxicology and Environmental Safety, 243, 113973–113973.
[2]. Ting, W., Mi, T., Nan, D., et al. (2018). Semivolatile Organic Compounds (SOCs) in Fine Particulate Matter (PM2.5) during Clear, Fog, and Haze Episodes in Winter in Beijing, China.Environmental Science & Technology, 52(9), 5199–5207.
[3]. Torre, L. D. A., Navarro, I., Sanz, P., et al. (2020). Organophosphate compounds, polybrominated diphenyl ethers and novel brominated flame retardants in European indoor house dust: Use, evidence for replacements and assessment of human exposure.Journal of Hazardous Materials, 382, 121009.
[4]. Chen, M., Gan, Z., Qu, B., et al. (2019). Temporal and seasonal variation and ecological risk evaluation of flame retardants in seawater and sediments from Bohai Bay near Tianjin, China during 2014 to 2017.Marine Pollution Bulletin, 146, 874–883.
[5]. Li, W., Wang, Y., & Kannan, K. (2019). Occurrence, distribution and human exposure to 20 organophosphate esters in air, soil, pine needles, river water, and dust samples collected around an airport in New York state, United States.Environment International, 131, 105054.
[6]. Chi, Y., Hanpei, Y., & Ying, L. (2021). A review on organophosphate flame retardants in the environment: Occurrence, accumulation, metabolism and toxicity.The Science of the Total Environment, 795, 148837–148837.
[7]. Wei, G., Li, D., Zhuo, M., et al. (2015). Organophosphorus flame retardants and plasticizers: Sources, occurrence, toxicity and human exposure.Environmental Pollution, 196, 29–46.
[8]. Chen, G., Jin, Y., Wu, Y., et al. (2015). Exposure of male mice to two kinds of organophosphate flame retardants (OPFRs) induced oxidative stress and endocrine disruption.Environmental Toxicology and Pharmacology, 40(1), 310–318.
[9]. Feng, Yixing, Duan, Hejun, & Cui, Xia. (2024). Effects of triphenyl phosphate and tri (1, 3- dichloro -2- propyl) phosphate on DNA damage and cell cycle of mouse spermatocytes.Journal of Environmental Hygiene, 14(03), 226–232+246+273.
[10]. Xiaoya, J., Na, L., Mei, M., et al. (2020). Tricresyl phosphate isomers exert estrogenic effects via G protein-coupled estrogen receptor-mediated pathways.Environmental Pollution, 264(prepublish), 114747.
[11]. D. J. M., & M. H. S. (2010). House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters.Environmental Health Perspectives, 118(3), 318–323.
[12]. Kojima, H., Takeuchi, S., Eede, D. V. N., et al. (2016). Effects of primary metabolites of organophosphate flame retardants on transcriptional activity via human nuclear receptors.Toxicology Letters, 245, 31–39.
[13]. Zhou, L., & Püttmann, W. (2019). Distributions of organophosphate flame retardants (OPFRs) in three dust size fractions from homes and building material markets.Environmental Pollution, 245, 343–352.
[14]. Zhao, manqi, & Liu, Jing. (n.d.). Research progress on endocrine disrupting effects of organophosphate flame retardants.Journal of Ecotoxicology, 1–13. Retrieved from https: //doi.org/ [Accessed: 2025-05-17]
[15]. Wang, X., Liu, Q., Zhong, W., et al. (2020). Estimating renal and hepatic clearance rates of organophosphate esters in humans: Impacts of intrinsic metabolism and binding affinity with plasma proteins.Environment International, 134, 105321.
[16]. Hou, M., Shi, Y., Jin, Q., et al. (2020). Organophosphate esters and their metabolites in paired human whole blood, serum, and urine as biomarkers of exposure.Environment International, 139, 105698.
[17]. Qiao, L., Zheng, X., Zheng, J., et al. (2016). Analysis of human hair to assess exposure to organophosphate flame retardants: Influence of hair segments and gender differences.Environmental Research, 148, 177–183.
[18]. Hou, R., Xu, Y., & Wang, Z. (2016). Review of OPFRs in animals and humans: Absorption, bioaccumulation, metabolism, and internal exposure research.Chemosphere, 153, 78–90.
Cite this article
Liu,Y. (2025). Probing the endocrine disrupting effect of flame retardants based on molecular docking technique - an example of tris (1-chloro-2-propyl) phosphate. Advances in Engineering Innovation,16(10),39-43.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Journal:Advances in Engineering Innovation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Jing-yi, Z., Zi-xiang, Z., Meng-juan, L., et al. (2022). A systematic scoping review of epidemiological studies on the association between organophosphate flame retardants and neurotoxicity.Ecotoxicology and Environmental Safety, 243, 113973–113973.
[2]. Ting, W., Mi, T., Nan, D., et al. (2018). Semivolatile Organic Compounds (SOCs) in Fine Particulate Matter (PM2.5) during Clear, Fog, and Haze Episodes in Winter in Beijing, China.Environmental Science & Technology, 52(9), 5199–5207.
[3]. Torre, L. D. A., Navarro, I., Sanz, P., et al. (2020). Organophosphate compounds, polybrominated diphenyl ethers and novel brominated flame retardants in European indoor house dust: Use, evidence for replacements and assessment of human exposure.Journal of Hazardous Materials, 382, 121009.
[4]. Chen, M., Gan, Z., Qu, B., et al. (2019). Temporal and seasonal variation and ecological risk evaluation of flame retardants in seawater and sediments from Bohai Bay near Tianjin, China during 2014 to 2017.Marine Pollution Bulletin, 146, 874–883.
[5]. Li, W., Wang, Y., & Kannan, K. (2019). Occurrence, distribution and human exposure to 20 organophosphate esters in air, soil, pine needles, river water, and dust samples collected around an airport in New York state, United States.Environment International, 131, 105054.
[6]. Chi, Y., Hanpei, Y., & Ying, L. (2021). A review on organophosphate flame retardants in the environment: Occurrence, accumulation, metabolism and toxicity.The Science of the Total Environment, 795, 148837–148837.
[7]. Wei, G., Li, D., Zhuo, M., et al. (2015). Organophosphorus flame retardants and plasticizers: Sources, occurrence, toxicity and human exposure.Environmental Pollution, 196, 29–46.
[8]. Chen, G., Jin, Y., Wu, Y., et al. (2015). Exposure of male mice to two kinds of organophosphate flame retardants (OPFRs) induced oxidative stress and endocrine disruption.Environmental Toxicology and Pharmacology, 40(1), 310–318.
[9]. Feng, Yixing, Duan, Hejun, & Cui, Xia. (2024). Effects of triphenyl phosphate and tri (1, 3- dichloro -2- propyl) phosphate on DNA damage and cell cycle of mouse spermatocytes.Journal of Environmental Hygiene, 14(03), 226–232+246+273.
[10]. Xiaoya, J., Na, L., Mei, M., et al. (2020). Tricresyl phosphate isomers exert estrogenic effects via G protein-coupled estrogen receptor-mediated pathways.Environmental Pollution, 264(prepublish), 114747.
[11]. D. J. M., & M. H. S. (2010). House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters.Environmental Health Perspectives, 118(3), 318–323.
[12]. Kojima, H., Takeuchi, S., Eede, D. V. N., et al. (2016). Effects of primary metabolites of organophosphate flame retardants on transcriptional activity via human nuclear receptors.Toxicology Letters, 245, 31–39.
[13]. Zhou, L., & Püttmann, W. (2019). Distributions of organophosphate flame retardants (OPFRs) in three dust size fractions from homes and building material markets.Environmental Pollution, 245, 343–352.
[14]. Zhao, manqi, & Liu, Jing. (n.d.). Research progress on endocrine disrupting effects of organophosphate flame retardants.Journal of Ecotoxicology, 1–13. Retrieved from https: //doi.org/ [Accessed: 2025-05-17]
[15]. Wang, X., Liu, Q., Zhong, W., et al. (2020). Estimating renal and hepatic clearance rates of organophosphate esters in humans: Impacts of intrinsic metabolism and binding affinity with plasma proteins.Environment International, 134, 105321.
[16]. Hou, M., Shi, Y., Jin, Q., et al. (2020). Organophosphate esters and their metabolites in paired human whole blood, serum, and urine as biomarkers of exposure.Environment International, 139, 105698.
[17]. Qiao, L., Zheng, X., Zheng, J., et al. (2016). Analysis of human hair to assess exposure to organophosphate flame retardants: Influence of hair segments and gender differences.Environmental Research, 148, 177–183.
[18]. Hou, R., Xu, Y., & Wang, Z. (2016). Review of OPFRs in animals and humans: Absorption, bioaccumulation, metabolism, and internal exposure research.Chemosphere, 153, 78–90.









