1. Letting the brain "talk" directly to machines: From BCI to bimodal neural probes
1.1. BCI: The technology that enables the brain to "speak"
Have you ever imagined typing, moving a robotic hand, or even playing a game using only your thoughts? Brain-Computer Interface (BCI) is precisely this kind of magical technology! It allows the human brain to communicate directly with computers or mechanical devices without the need for a keyboard, mouse, or voice input—relying solely on brain signals to execute commands.
1.1.1. The history of BCI development
This technology is not just a product of science fiction; research on BCI began as early as the 1970s. Scientists discovered that the brain generates weak electrical signals that can convey thoughts and motor intentions. They then attempted to "listen" to these signals using electrodes and convert them into control commands. With advancements in computing, artificial intelligence, and neuroscience, BCI technology has made rapid progress [1].
1970s-1980s: Scientists experimentally demonstrated for the first time that brain electrical signals could be used to control devices.
1990s: Monkeys successfully controlled robotic arms using BCI, bringing the technology closer to human applications.
2000s: BCI was applied to assist paralyzed patients, allowing them to type using brain signals.
Present: BCI is making a significant impact not only in medicine but also in gaming, virtual reality, smart homes, and even military research [2][3].
1.1.2. The wide applications of BCI
BCI is not just futuristic "black technology"—it is actively transforming the world:
Medical Field: Helping paralyzed patients control wheelchairs and prosthetics with their thoughts; assisting in the treatment of neurological diseases such as Parkinson’s, epilepsy, and depression [4].
Human-Machine Interaction: Enabling people to control computers, games, and smart homes with their minds, even achieving "immersive experiences" in virtual reality (VR) [5].
Neuroscience Research: Scientists use BCI to study how the human brain functions, aiding the development of smarter AI and treatments for neurological disorders.
However, despite its impressive potential, BCI still faces a critical challenge—how to read brain signals more accurately? This leads to the concept of neural probes.
1.2. Neural probes: The "listening device" for brain signals
If the brain is compared to a complex "city," then neural probes serve as its "listening devices," designed to receive brain signals and transmit this information to computers or other devices [6].
1.2.1. How the brain generates signals
Neurons in the brain communicate in two primary ways:
·Electrical Signals (The Brain’s "Waves")
Neurons in the brain generate action potentials, which function like tiny "electric currents" jumping between neurons.
These electrical signals instruct the body on how to move, such as raising a hand, walking, or remembering something [9].
·Chemical Signals (Neurotransmitters)
When electrical signals reach the end of a neuron, they trigger the release of neurotransmitters, such as dopamine and glutamate [10].
These chemical messengers transmit more subtle information between neurons, such as regulating emotions and determining attention levels.
1.2.2. How neural probes work
Traditional neural probes primarily detect electrical signals. They function by placing a series of tiny "antennas" (Microelectrode Arrays, MEA) inside the brain, capturing neurons' "electrical waves," and transmitting this data to computers for analysis [7][8]. However, this method has a major limitation: It can only monitor electrical signals but cannot obtain information about brain chemicals. If chemical data is needed, an additional probe must be implanted, which increases the risk of brain tissue damage.
To solve this problem, scientists have developed a more advanced technology—bimodal neural probes.
1.3. Bimodal neural probes: Making brain signals clearer
1.3.1. What are bimodal neural probes?
Bimodal neural probes are an upgraded version of traditional probes. They can not only monitor the brain’s electrical signals but also detect its chemical signals. This is equivalent to equipping machines with a more complete set of "sensors," allowing for a more accurate understanding of how the brain works.
1.3.2. How bimodal probes capture brain signals
Microelectrode Array (MEA): Monitoring Electrical Signals
This component acts like an ultra-sensitive "microphone," capturing brain electrical signals through multiple tiny electrodes and recording neuronal activity.
These microelectrodes, only a few micrometers in size, can penetrate different brain regions and listen to multiple neurons communicating simultaneously [9].
1.3.3. Microfluidic channel: Detecting chemical signals
This is the probe’s "chemical sensor." It functions like a straw, drawing in brain chemicals such as neurotransmitters (dopamine, glutamate, etc.) [10].
By analyzing the concentration of these chemicals, scientists can determine whether the brain is in a state of excitement, stress, relaxation, or focus.
1.3.4. Advantages of bimodal neural probes [12]
·Dual-Signal Acquisition: Simultaneously obtaining both electrical and chemical signals for a more comprehensive understanding.
·Reduced Tissue Damage: Compared to traditional probes that require multiple implants, bimodal probes minimize brain tissue damage.
·More Accurate Neural Decoding: Scientists can better interpret brain signals, improving the precision of BCI-controlled devices.
1.3.5. Practical applications of bimodal neural probes [11][13]
·Restoring Mobility in Paralyzed Patients: More accurately decoding motor intentions, enhancing the precision of brain-controlled robotic limbs.
·Optimizing Neurological Disorder Treatments: Monitoring brain signals in Parkinson’s patients to refine deep brain stimulation (DBS) therapy.
·Enhancing Human-Machine Interaction: In the future, we may be able to control VR devices, smart homes, or even interact with AI more naturally using only our brains.
2. Innovative design: Making brain signals "fully visible"
Bimodal neural probes represent a breakthrough in neuroscience by simultaneously capturing the brain's electrical signals ("brain waves") and detecting neurotransmitter chemical changes ("chemical language"), ensuring precise temporal and spatial alignment. This integration allows scientists to better understand brain activity—similar to replacing a blurry black-and-white surveillance camera with a high-definition one, making brain communication clearer.
To achieve this, bimodal neural probes incorporate advanced electrode arrays, microfluidic channels, and intelligent materials. This revolutionary design overcomes two major limitations of traditional neural probes:
Incomplete Information from Single-Modal Probes: Conventional probes can only detect electrical or chemical signals, failing to provide a complete picture of neural activity.
Tissue Damage from Multiple Probe Combinations: Using multiple probes increases brain tissue damage, compromising the stability of long-term implants [14].
Next, we break down the core technologies of bimodal neural probes and explore how they enhance Brain-Computer Interface (BCI) performance.
2.1. Capturing electrical signals: Precisely detecting brain "waves"
2.1.1. Why measure electrical signals?
Neurons communicate through action potentials, which function like the "Morse code of the brain," governing movement, thought, and learning. The brain's electrical activity is a crucial source of information for BCI systems to decode thoughts and control external devices [15].
2.1.2. How electrical signals are collected [13]
Bimodal neural probes use Microelectrode Arrays (MEA) to monitor these signals. Think of them as an array of ultra-small antennas that precisely capture neural "waves."
The main differences between conventional MEA and bimodal neural probes are summarized in Table 1.
Table 1. Comparison of data between conventional MEA and bimodal neural probe[13]
Parameter | Conventional MEA | Advanced Bimodal Probe |
Number of Electrodes | 16-32 | 64-128 |
Electrode Spacing | 100-200μm | 50-100μm |
Impedance | >100kΩ | <10kΩ |
Temporal Resolution | 1-10ms | 1ms |
Spatial Resolution | 50-100μm | 10-50μm |
Core Technology: Microelectrode Array (MEA)
Function: Acts as an array of ultra-sensitive miniature "antennas" that directly listen to neuronal firing activity.
2.1.3. Features of microelectrode arrays [16]
·Composed of dozens to hundreds of tiny electrodes, each with a diameter of just 10-50 micrometers (about one-tenth the width of a human hair).
·The spacing between electrodes typically ranges from 20-200 micrometers, ensuring broad brain region coverage while preventing signal interference.
·High Sensitivity: Capable of recording multiple neurons' firing patterns, enabling precise neural signal decoding.
2.1.4. Innovations
High-density electrodes combined with low-impedance optimization enable BCI systems to accurately decode motor intentions, recognize more complex neural activity patterns, and enhance device control sensitivity [17].
2.2. Monitoring chemical signals: Decoding the Brain’s "chemical language"
2.2.1. Why measure chemical signals?
The brain does not rely solely on electrical signals; it also releases neurotransmitters (such as dopamine, glutamate, and GABA), which regulate emotions, attention, and memory. For example:
Glutamate concentration increases significantly when the brain is in an excited or learning state.
Dopamine release levels determine mood and motivation.
GABA (Gamma-Aminobutyric Acid) functions primarily to inhibit neural activity, maintaining a balance between excitation and inhibition in the brain [18].
2.2.2. How neurotransmitters are detected
Bimodal neural probes are equipped with Microfluidic Channels, which function like ultra-microscopic straws that extract cerebrospinal fluid samples from the surrounding neurons to analyze neurotransmitter content [19].
2.2.3. Features of microfluidic channels [20][21]
Channel width is less than 100 micrometers, ensuring no interference with normal neural activity.
Air-separation structure prevents sample cross-contamination, improving measurement accuracy.
Integrated sensors allow real-time monitoring of neurotransmitter concentration changes.The differences in microfluidic channel performance between conventional probes and bimodal neural probes are summarized in Table 2.
Table 2. Comparison of data from microfluidic channels in conventional probes and bimodal neural probes[22]
Parameter | Conventional Microfluidic Probe | Bimodal Neural Probe |
Channel Width | 100-200μm | <100μm |
Neurotransmitter Detection Sensitivity | 1μM | 100nM |
Sampling Time | 10s | <5s |
Sample Cross-Contamination | High contamination risk | Air-separation design, contamination rate reduced by 40% |
Parameter | Conventional Microfluidic Probe | Bimodal Neural Probe |
2.2.4. Innovations
Optimized microfluidic technology enables BCI systems not only to detect whether the brain is "active" but also to analyze "what it is doing," providing richer biological information for emotion decoding and disease monitoring (e.g., Parkinson’s disease) [23].
2.3. Multimodal signal integration: Achieving precise alignment
2.3.1. Why integrate electrical and chemical signals?
Imagine watching a movie with only sound but no picture or vice versa—you would struggle to fully understand the story. Similarly, brain electrical signals and chemical signals are closely interconnected, and observing only one provides incomplete information.
In traditional neural probes, researchers typically measure either electrical signals (neuronal firing) or chemical signals (neurotransmitter release), but both are essential for understanding brain function. Measuring only one can lead to data gaps or even misinterpretation of brain activity.
For example:
·Measuring only electrical signals: Captures neuron activity patterns but does not confirm whether neurotransmitter release occurs, making it difficult to determine functional significance.
·Measuring only chemical signals: Detects neurotransmitter concentration changes but cannot trace their neuronal origins or pinpoint the timing of their release.
To achieve more comprehensive and precise neural signal monitoring, the key innovation of bimodal neural probes is their ability to simultaneously record electrical and chemical signals while ensuring their temporal and spatial alignment [24].
2.3.2. Why is spatial alignment critical?
To synchronize electrical and chemical signal acquisition, microelectrodes and microfluidic channels must be positioned as closely as possible—achieving spatial alignment.
This is similar to filmmaking: if the camera and microphone are not aligned with the actor, the audio and video will not match.
Brain activity involves dynamic interactions between electrical and chemical signals:
Electrical signals reveal when neurons fire (action potentials).
Chemical signals reveal whether firing is accompanied by neurotransmitter release (e.g., dopamine fluctuations).
If signals are not synchronized, misinterpretations of brain activity may occur.
For example:
High electrical activity but low neurotransmitter levels might indicate neurological dysfunction.
Only synchronized electrical and chemical signals provide a complete picture of brain function [25].
2.3.3. How bimodal probes achieve spatial alignment
·Integrated microelectrode and microfluidic channel design
·Electrode-to-channel spacing controlled within 100-200μm to ensure signals originate from the same neuron group.
·3D flexible structure allowing probes to adjust within tissue, ensuring both modules remain aligned with the same neuron cluster.
·Multilayer Sensor Structure
Microelectrode arrays are placed at the probe tip to maximize proximity to neuron bodies (soma). Microfluidic channels are positioned 100-200μm away from the electrode array to collect neurotransmitters diffusing from synaptic spaces.
·Enhanced Probe Flexibility for Reduced Tissue Response
Traditional rigid probes may shift after implantation due to brain tissue movement, causing misalignment. However, using flexible materials (e.g., PI, PDMS) allows probes to adapt to brain motion, maintaining spatial precision over long-term implantation [26].
The improvements in spatial localization accuracy provided by bimodal neural probes compared to traditional single-modality probes are summarized in Table 3.
Table 3. Comparison of spatial localization effects between traditional single-modality neural probes and dual-modality neural probes [28]
Parameter | Traditional Single-Modal Probe | Bimodal Neural Probe |
Electrode-to-Channel Spacing | 500μm-1mm | 100-200μm |
Signal Source Collection Error | 30-50% | <10% |
Tissue Damage | High | Low (Flexible Materials) |
2.3.4. Benefits of this approach [27]
·Provides scientists with a complete view of neural activity, eliminating the limitations of single-signal monitoring.
·Enhances BCI decoding accuracy, improving response speed and precision of brain-controlled devices.
·Offers new research tools to better understand complex mechanisms of brain disorders.
2.3.5. Innovations
The optimized design of bimodal neural probes ensures that the sources of electrical and chemical signal acquisition are almost perfectly aligned, preventing data mismatches and making multimodal data more biologically meaningful [29].
3. Material innovation: Pushing the performance limits of neural probes
The success of bimodal neural probes relies on breakthroughs in material science. To achieve high sensitivity, low damage, and long-term stability, researchers have introduced graphene and polymers (e.g., PI, PDMS) to optimize electrode conductivity, probe flexibility, and tissue compatibility. This section focuses on graphene and polymers while briefly mentioning other materials to establish a comprehensive material selection framework [30].
3.1. Graphene: Enabling ultra-high sensitivity neural probes
3.1.1. Why choose graphene? [31]
Graphene is a two-dimensional material composed of a single layer of carbon atoms. Due to its ultra-high conductivity, flexibility, transparency, and biocompatibility, it is considered an ideal candidate for next-generation neural electrodes. Compared to traditional metal electrodes (e.g., platinum, gold), graphene offers distinct advantages:
·High Conductivity: Electron mobility exceeds 15,000 cm²/Vs, more than 10 times faster than silicon.
·Ultra-Low Impedance: 1-2 orders of magnitude lower than traditional metal electrodes, enhancing signal-to-noise ratio (SNR).
·Ultra-Thin Flexibility: Can bend to nanometer-scale thickness, significantly reducing brain tissue damage.
·Biocompatibility: Less likely to trigger immune reactions, enabling long-term stable recording.
3.1.2. How graphene enhances neural probes [32]
·Reduces Electrode Impedance, Improving Signal Quality
Traditional metal electrodes have high impedance (>100 kΩ), limiting the detection of low-amplitude neural signals.
Graphene electrodes have impedance as low as <10 kΩ, enabling more stable signal transmission and clearer neural recordings.
·Enhances Neurotransmitter Detection via Microfluidic Integration
Studies show that graphene-coated microfluidic channels improve neurotransmitter adsorption and detection sensitivity, reaching a detection threshold in the nanomolar (nM) range.
·Ultra-Thin Flexibility Improves Implant Stability
With a thickness of <5nm, graphene adheres like a "sticker" to the brain surface, minimizing tissue damage and extending probe longevity.
Figure 1 shows the structure of graphene used in neural electrodes.
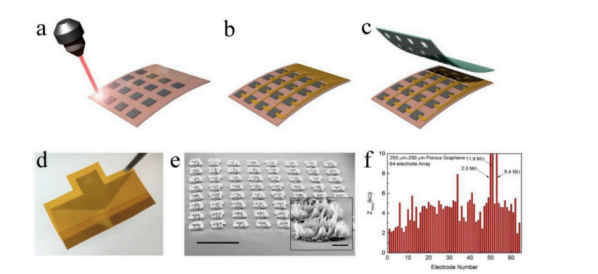
Figure 1. Structure diagram of graphene used in nerve electrode [35]
3.2. Polymer materials: Enabling flexibility and biocompatibility
While graphene provides exceptional electrical performance, it requires structural support. This is where polymer materials—such as polyimide (PI), polydimethylsiloxane (PDMS), and PEDOT (poly(3,4-ethylenedioxythiophene))—come into play.
3.2.1. Why choose polymers? [33]
Enhances Flexibility:
Polymers are bendable, matching the mechanical properties of brain tissue and preventing damage caused by rigid probes.
Increases Stability:
Prevents electrode material degradation and improves long-term implant performance.
Optimizes the Electrode-Tissue Interface:
Polymer coatings reduce electrode-tissue impedance, enhancing signal transmission quality.
The functions and properties of commonly used polymers in neural probes are summarized in Table 4.
Table 4. Functions and characteristics of three polymers: PI, PDMS, and PEDOT[33]
Material | Function | Properties |
Polyimide (PI) | Probe Substrate Material | High flexibility, heat-resistant, chemically stable |
PDMS | Flexible Protective Layer | Reduces immune response, prevents probe breakage |
PEDOT | Electrode Surface Modification | Enhances conductivity, improves neural signal recording accuracy |
To further illustrate how flexible materials contribute to neural probe design, Figure 2 shows the structure of a flexible neural probe.
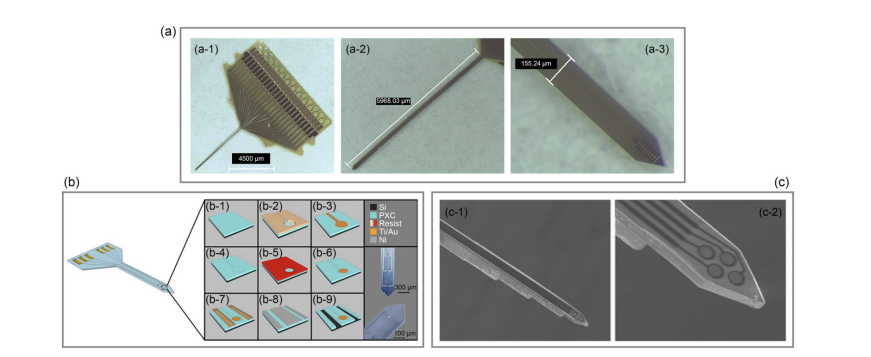
Figure 2. Structure diagram of the flexible neural probe [36]
3.3. Material interactions and optimization strategies
3.3.1. How to combine graphene and polymers for the optimal probe [34]
To fully leverage graphene's conductivity and polymers' flexibility, researchers employ a composite material strategy:
·Depositing Graphene Electrodes on a PI Substrate → Ensures high conductivity and enhanced signal acquisition.
·Doping Graphene Nanoplatelets into the PDMS Layer → Increases flexibility and reduces tissue rejection.
·Modifying Graphene Electrodes with PEDOT → Enhances electrochemical activity and improves neurotransmitter detection sensitivity.
3.3.2. Visual example: Effects of material optimization
Figure 1 shows the structure of graphene used in neural electrodes, while Figure 2 illustrates the flexible neural probe incorporating advanced materials. The key advancements in bimodal neural probe materials—including graphene electrodes, polymer substrates, and flexible coatings—have significantly enhanced their electrical conductivity, mechanical flexibility, and long-term biocompatibility. Table5 provides a comprehensive comparison of these material optimizations and their benefits.
Table 5. Improvements in the internal components of bimodal neural probes[34]
Optimization | Performance Enhancement |
Graphene Electrodes | Low impedance, high signal sensitivity |
PI Substrate | High flexibility, long-term stability |
PDMS Protective Layer | Reduces tissue reaction |
PEDOT Coating | Enhances electrochemical performance |
4. Bimodal neural probes: How they can change our lives
Bimodal neural probes are not just scientific tools—they help us better understand the brain and improve treatments for neurological disorders. Imagine if we could capture brain signals more precisely—this could unlock incredible applications, such as enhancing learning abilities, restoring movement in paralyzed individuals, and precisely treating neurological diseases.
So, how do these probes achieve this? Let’s explore some real-world examples.
4.1. Enhancing learning and memory: the Brain’s super booster
4.1.1. Question: Can we "upgrade" memory?
When learning new information, neurons adjust their connections—a process known as synaptic plasticity. As we acquire new knowledge, neuronal firing patterns change, and the brain releases glutamate, a neurotransmitter essential for memory storage.
However, traditional methods only detect electrical signals, making it impossible to monitor neurotransmitter fluctuations. This limitation has hindered research on how memory formation actually works [37].
4.1.2. How bimodal neural probes help
·Record brain electrical activity to identify the "learning" neurons' activity patterns.
·Monitor glutamate release to determine if the brain is forming new memories.
4.1.3. Real case study: Enhancing learning in mice
Real Case Study: Enhancing Learning in Mice
Scientists conducted an experiment where mice were trained to navigate a maze, while using bimodal neural probes to record activity in their hippocampus (the brain region responsible for memory formation).
As the mice learned to navigate the maze, the probe detected:
·Increased neuronal firing
·Higher glutamate levels
Researchers then mildly stimulated these neurons and boosted glutamate release, and the results showed: These mice learned faster, memorizing the correct path 30% more efficiently than unstimulated mice[38].
4.1.4. How this technology could be applied in real life
With this technology, scientists could develop wearable brain-enhancing devices that optimize learning and memory performance.
This could also lead to new treatments for Alzheimer’s disease, helping patients restore memory function.
4.2. Helping paralyzed patients "stand up again"
4.2.1. Question: How can paralyzed patients control prosthetics?
When a person is paralyzed due to spinal cord injury or stroke, the brain still generates movement commands, but these signals cannot reach the muscles.
Current Brain-Computer Interface (BCI) technology attempts to decode movement intentions using electrical signals, but it remains imprecise. This is because movement control is not just electrical signals—dopamine and acetylcholine also influence movement fluidity [39].
4.2.2. How bimodal neural probes help
·Decode the brain’s "walking" signals and transmit the information to prosthetics.
·Simultaneously monitor neurotransmitters to ensure the movement signals are accurate.
4.2.3. Real case study: Brain-controlled exoskeleton
Scientists implanted bimodal neural probes into the motor cortex of paralyzed patients.
When the patients imagined moving their arms, the probe recorded the corresponding electrical signals.
However, electrical signals alone were not accurate enough, so the probe also measured dopamine levels to ensure the movement signals were real and effective.
These signals were then transmitted to a mechanical exoskeleton, allowing the patients to control it with their thoughts.
After several weeks of practice, the patients successfully used their brain signals to control a robotic arm, achieving 40% greater accuracy than traditional single-signal decoding methods [40].
4.2.4. How this technology could be applied in real life
In the future, paralyzed patients could wear smart prosthetics and perform daily actions just like ordinary people.
Beyond medical applications, healthy individuals could also use brain-controlled electronic devices, such as typing with thoughts or operating drones using only brain signals.
4.3. Precision treatment for Parkinson’s disease
4.3.1. Question: Why does Parkinson’s disease cause tremors?
Patients with Parkinson’s disease suffer from damage to substantia nigra neurons, leading to a dopamine deficiency, which disrupts motor control. This results in tremors, stiffness, and difficulty walking.
The traditional treatment method, Deep Brain Stimulation (DBS), uses implanted electrodes to stimulate the brain. However, doctors can only rely on electrical signals to determine when to stimulate, without real-time dopamine monitoring, making it difficult to optimize treatment [41].
4.3.2. How bimodal neural probes help
·Record neural activity in the basal ganglia to detect abnormal movement signals.
·Monitor dopamine levels in real time to ensure optimal stimulation timing.
4.3.3. Real case study: Precision-adjusted deep brain stimulation
Scientists implanted bimodal neural probes in the basal ganglia of Parkinson’s disease patients and made an important discovery:
Before a tremor episode, the patient's β-wave activity increased abnormally.
At the same time, the probe detected a drop in dopamine levels.
When β-waves exceeded a threshold and dopamine fell below a certain level, the probe automatically triggered Deep Brain Stimulation (DBS) and simultaneously released microdoses of dopamine.
Compared to traditional DBS therapy, this precision-targeted stimulation reduced tremor frequency by 60%, significantly improving patients’ movement fluidity [42].
4.3.4. How this technology could be applied in real life
If this technology is further developed and adopted for routine treatment, Parkinson’s disease patients could reduce their reliance on medication, avoiding long-term side effects.
In the future, smart neural modulation implants could be developed, allowing personalized treatment for all neurological disorders.
4.4. A "firewall" for epilepsy patients
4.4.1. Question: Can we predict epileptic seizures?
Epileptic seizures are typically caused by abnormal synchronized neuronal firing. However, EEG monitoring alone cannot accurately predict seizure onset, as chemical signal changes often occur earlier [43].
4.4.2. How bimodal neural probes help
·Monitor neuronal firing patterns at the seizure onset location.
·Detect glutamate and GABA concentrations to identify early signs of chemical imbalance.
4.4.3. Real case study: Precision epilepsy prediction
Scientists implanted bimodal neural probes in the hippocampus of epilepsy patients and discovered:
Before a seizure, glutamate (an excitatory neurotransmitter) increased by 200%.
GABA (an inhibitory neurotransmitter) significantly decreased.
The probe used this data to issue a seizure warning 30 seconds in advance and automatically triggered neurostimulation, preventing the seizure from occurring [44].
4.4.4. How this technology could be applied in real life
In the future, scientists may develop implantable devices that continuously monitor brain activity and prevent seizures in real-time.
This technology could also be used to regulate emotional fluctuations, potentially helping to prevent anxiety and depression.
5. Conclusion: The future and challenges of bimodal neural probes
The emergence of bimodal neural probes has brought revolutionary breakthroughs to neuroscience, Brain-Computer Interfaces (BCI), and neurological disease treatment.
This technology not only enables the simultaneous recording of electrical and chemical signals but also offers a new way to understand and regulate brain activity.
However, as bimodal probes move toward clinical applications, it is essential to address ethical, societal, and technological challenges to ensure their development remains responsible and sustainable.
5.1. Key breakthroughs and future potential
5.1.1. The significance of bimodal probes
·Precisely decoding neural signals, making BCI more intelligent, allowing paralyzed patients to control devices with their thoughts.
·Optimizing neurological disease treatments, such as Parkinson’s disease and epilepsy, by accurately monitoring and regulating neurotransmitters, enabling personalized therapy.
·Advancing neuroscience research, helping scientists gain deeper insights into the mechanisms of learning, memory, emotions, and cognitive functions.
5.1.2. Future directions
·Smaller, more flexible, and more stable probes:
Graphene, nanomaterials, and biocompatible polymers will further optimize probes, making them smaller, more flexible, reducing post-implantation tissue reactions, and improving long-term stability.
·Integrating Artificial Intelligence (AI) to optimize neural signal decoding:
AI-driven real-time data analysis will enable more accurate decoding of movement intentions, emotional states, and even predictions of disease progression.
·Developing wireless implantable systems for "non-invasive BCI":
Most current BCIs still rely on wired connections, but future research will focus on wireless neural probes, reducing external device interference and making implants safer and more convenient.
5.2. Ethical and societal impact: How to advance responsibly
While bimodal neural probes offer exciting possibilities, they also come with significant ethical and societal challenges. Here are some key concerns:
5.2.1. Data privacy and security
In the future, probes may be able to read brain signals, meaning that users' "thoughts" could be decoded and stored. This raises concerns about neural data privacy. Therefore, strict privacy protection regulations need to be established to clearly define which data can be stored, how it should be encrypted, and who has the right to access it, ensuring that users retain control over their own data.
5.2.2. Neuroenhancement and fairness
If bimodal probes are used not only for medical purposes but also to enhance cognitive abilities (such as improving memory and accelerating learning), they may contribute to technological inequality. Therefore, research institutions and policymakers need to establish fair use guidelines to ensure that this technology does not exacerbate social disparities but instead benefits everyone. For example, governments or foundations could support the widespread adoption of this technology in the medical field, prioritizing patients in need rather than limiting access to the wealthy.
5.2.3. Long-term safety
There are concerns about whether future implantable devices might have unknown long-term effects on the brain, as current long-term biocompatibility data for most neural probes remain limited. Therefore, longer-term clinical trials will be needed in the future to assess the stability and biosafety of neural probes. Additionally, degradable or removable probes could be used to allow for extraction or replacement when necessary.
5.3. Making this technology more accessible: Reducing costs and scalable manufacturing
5.3.1. How to reduce manufacturing costs
Currently, advanced neural probes still rely on high-precision microfabrication, leading to high costs that limit their widespread adoption. To make this technology accessible to more people, the following measures can be taken:
Mass Production: Combining photolithography with 3D printing to enable large-scale production of flexible probes, reducing the manufacturing cost per unit.
Optimizing Materials: Using low-cost polymers to replace some expensive materials in the future, such as developing new low-cost probes based on PEDOT or conductive nanomaterials.
Open Technology Standards: Promoting open-source hardware and software to allow more research institutions and companies to develop compatible probe systems, accelerating industrialization.
5.3.2. How to promote clinical applications
·Establishing Standardized Implantation Procedures: Reducing the learning curve for doctors and increasing surgical success rates.
·Government or Insurance Support: Ensuring that patients can afford this technology rather than it being limited to high-end private hospitals.
5.4. Conclusion: Advancing bimodal neural probes responsibly
Bimodal neural probes bring us closer to the goal of "decoding the brain." They not only help us unravel the mysteries of neuroscience but also improve the quality of life for many people. However, the challenges posed by new technologies cannot be ignored.
Actionable Future Development Recommendations:
·Strengthening neural data privacy protection to ensure user data security.
·Optimizing manufacturing processes to lower costs and make the technology accessible to more patients.
·Promoting long-term safety research to ensure device stability and biocompatibility.
·Establishing ethical guidelines for responsible neuro-enhancement to prevent technology misuse.
In the future, bimodal neural probes may revolutionize the way humans interact with the brain. They could enable paralyzed individuals to walk again, help Parkinson’s patients reduce tremors, and deepen our understanding of brain function. As the technology evolves, we have a responsibility to ensure its advancement in the safest, fairest, and most sustainable way—creating a better future for all.
References
[1]. Bhansali, V., Rawal, V. T., Sachin, A., Arora, H., & Manjila, T. (2024). Implications and ethical considerations of brain-computer interface: A mini-review. ResearchGate. https://www.researchgate.net/publication/381232392_Implications_and_Ethical_Considerations_of_Brain-Computer_Interface_A_Mini-Review
[2]. Ming, X. (2024). Research on ethical challenges and rational regulation of brain-computer interface technology. Information and Communications Technology and Policy. http://ictp.caict.ac.cn/EN/abstract/abstract1236.shtml
[3]. Vindhya, G., Parveen, S., Sowmya, P., et al. (2024). Neuralink: Reclassifying the limits of human insight. IEEE Xplore. https://ieeexplore.ieee.org/abstract/document/10724926/
[4]. Trott, J., Slaymaker, C., Niznik, G., & Althoff, T. (2024). Brain-computer interfaces: An introduction for clinical neurodiagnostic technologists. The Neurodiagnostic Journal. https://www.tandfonline.com/doi/abs/10.1080/21646821.2024.2408501
[5]. Lotte, F., & Jeunet-Kelway, C. (2024). Brain-computer interaction and neuroergonomics. In Mind, Body, and Digital Brains (pp. 1-20). Springer. https://link.springer.com/chapter/10.1007/978-3-031-58363-6_10
[6]. Rostami, B. (2024). Technological and computational approaches for large count high-density neural probes. Deep Blue Library, University of Michigan. https://deepblue.lib.umich.edu/handle/2027.42/193333
[7]. Zhou, Z., Sun, L., et al. (2024). Ultraflexible PEDOT:PSS/IrOx-modified electrodes: Applications in behavioral modulation and neural signal recording in mice. Micromachines, 15(4), 447. https://www.mdpi.com/2072-666X/15/4/447
[8]. Hao, Y., Han, L., Jin, Y., et al. (2024). A 64-channel fully implantable brain-computer microsystem with custom microelectrode array. IEEE Circuits and Systems. https://ieeexplore.ieee.org/abstract/document/10798410/
[9]. Wang, T., Chen, Y., Wang, Y., & Lee, S. H. (2024). Advanced neural probe sensors toward multi-modal sensing and modulation: Design, integration, and applications. Advanced Sensor Research. https://advanced.onlinelibrary.wiley.com/doi/abs/10.1002/adsr.202400142
[10]. Xu, M., Zhao, Y., Xu, G., Zhang, Y., et al. (2022). Recent development of neural microelectrodes with dual-mode detection. Biosensors, 13(1), 59. https://www.mdpi.com/2079-6374/13/1/59
[11]. Chae, U., Shin, H., Choi, N., et al. (2021). Bimodal neural probe for highly co-located chemical and electrical monitoring of neural activities in vivo. Biosensors and Bioelectronics. https://www.sciencedirect.com/science/article/pii/S0956566321005108
[12]. Dalrymple, A. N., Huynh, M., & Nayagam, B. A. (2020). Electrochemical and biological characterization of thin-film platinum-iridium alloy electrode coatings: A chronic in vivo study. Journal of Neural Engineering. https://iopscience.iop.org/article/10.1088/1741-2552/ab933d/meta
[13]. Drakopoulou, S., Varkevisser, F., Sohail, L., et al. (2023). Hybrid neuroelectronics: towards a solution-centric way of thinking about complex problems in neurostimulation tools. Frontiers in Electronics. https://www.frontiersin.org/articles/10.3389/felec.2023.1250655/full
[14]. Cui, X. T., Siwakoti, U., Jones, S. A., & Kumbhare, D. (2025). Recent progress in flexible microelectrode arrays for combined electrophysiological and electrochemical sensing. Preprints. https://www.preprints.org/frontend/manuscript/08e841067cfadf1d6c555fe14f87ac7c/download_pub
[15]. Parashiva, P. K., Gangadaran, S., & Vinod, A. P. (2025). Subject specific deep learning model for motor imagery direction decoding. arXiv preprint arXiv:2501.01725. https://arxiv.org/pdf/2501.01725
[16]. Duvan, F. T., Cunquero, M., Masvidal-Codina, E., et al. (2024). Graphene-based microelectrodes with bidirectional functionality for next-generation retinal electronic interfaces. Nanoscale Horizons. https://pubs.rsc.org/en/content/articlehtml/2024/nh/d4nh00282b
[17]. Wang, L., Suo, Y., Wang, J., et al. (2024). High-density implantable neural electrodes and chips for massive neural recordings. Brain-X. https://onlinelibrary.wiley.com/doi/abs/10.1002/brx2.65
[18]. Sahin, K., Korkusuz, A. K., Sahin, E., & Orhan, C. (2024). The effect of water-soluble Alpinia Galanga extract on sleep and the activation of the GABAAergic/serotonergic pathway in mice. Pharmaceuticals, 17(12), 1649. https://www.mdpi.com/1424-8247/17/12/1649
[19]. Shin, H., Lee, H. J., Choi, N., et al. (2015). Neural probes with multi-drug delivery capability. Lab on a Chip. https://pubs.rsc.org/en/content/articlehtml/2015/lc/c5lc00582e
[20]. Sahasrabudhe, A. (2024). Multifunctional wireless gut-brain neurotechnology. Massachusetts Institute of Technology. https://dspace.mit.edu/handle/1721.1/157069
[21]. Billa, S. (2024). Advancing electrochemical microsensors for multiplexed neurotransmitters and environmental toxins detection. Louisiana Tech University. https://digitalcommons.latech.edu/dissertations/1022/
[22]. Celshia, S., Selvamani, M., & Suresh, V. (2024). Synthesis and characterization of Mn₂O₃ and its electrochemical properties in relation to dopamine. Cureus. https://pmc.ncbi.nlm.nih.gov/articles/PMC11426951/
[23]. Xu, S., Liu, Y., Yang, Y., Zhang, K., Liang, W., Xu, Z., & Wu, Y. (2023). Recent progress and perspectives on neural chip platforms integrating PDMS-based microfluidic devices and microelectrode arrays. Micromachines, 14(4), 709. https://www.mdpi.com/2072-666X/14/4/709
[24]. Mathieu, F., Marty, F. H., & Blatche, M. C. (2024). Real-time and high-resolution monitoring of neuronal electrical activity and pH variations based on the co-integration of nanoelectrodes and Chem-FinFETs. Small. https://onlinelibrary.wiley.com/doi/abs/10.1002/smll.202309055
[25]. Jonsson, A., Inal, S., & Uguz, I. (2016). Bioelectronic neural pixel: Chemical stimulation and electrical sensing at the same site. Proceedings of the National Academy of Sciences, 113(34), 9440-9445. https://www.pnas.org/doi/abs/10.1073/pnas.1604231113
[26]. Takeuchi, S., Ziegler, D., Yoshida, Y., Mabuchi, K., & Suzuki, T. (2005). Parylene flexible neural probes integrated with microfluidic channels. Lab on a Chip, 5(5), 519-523. https://pubs.rsc.org/en/content/articlehtml/2005/lc/b417497f
[27]. Jiao, Y., Lei, M., Zhu, J., Chang, R., & Qu, X. (2023). Advances in electrode interface materials and modification technologies for brain-computer interfaces. Biomaterials Translational. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10817795/
[28]. Wen, R., Tao, C., Ji, H., & Qiu, J. (2024). Dual-modal fusion PRI-SWT model for eddy current detection of cracks, delamination, and impact damage in carbon fiber-reinforced plastic materials. Applied Sciences, 14(22), 10282. https://www.mdpi.com/2076-3417/14/22/10282
[29]. Wang, X., Han, S., Yan, P., Lin, Y., & Wang, C. (2024). A 1024-channel simultaneous electrophysiological and electrochemical neural recording system with in-pixel digitization and crosstalk compensation. IEEE Transactions on Circuits and Systems. https://ieeexplore.ieee.org/abstract/document/10680349/
[30]. Cui, X. T., Siwakoti, U., Jones, S. A., & Kumbhare, D. (2025). Recent progress in flexible microelectrode arrays for combined electrophysiological and electrochemical sensing. Preprints. https://www.preprints.org/frontend/manuscript/08e841067cfadf1d6c555fe14f87ac7c/download_pub
[31]. Cho, Y., Park, S., Lee, J., & Yu, K. J. (2021). Emerging materials and technologies with applications in flexible neural implants: A comprehensive review of current issues with neural devices. Advanced Materials, 33(9), 2005786. https://onlinelibrary.wiley.com/doi/abs/10.1002/adma.202005786
[32]. Xu, M., Zhao, Y., Xu, G., Zhang, Y., Sun, S., Sun, Y., & Wang, J. (2022). Recent development of neural microelectrodes with dual-mode detection. Biosensors, 13(1), 59. https://www.mdpi.com/2079-6374/13/1/59
[33]. He, L., Feng, H., & Li, Y. (2020). Recent development of implantable and flexible nerve electrodes. Smart Materials in Medicine, 1, 1-12. https://www.sciencedirect.com/science/article/pii/S2590183420300132
[34]. Zhang, F., Dong, S., & Zhang, S. (2024). Soft electronics for neural engineering. In Advanced Soft Electronics in Biomedical Applications (pp. 75-98). Taylor & Francis. https://www.taylorfrancis.com/chapters/edit/10.1201/9781003493631-5/soft-electronics-neural-engineering-fan-zhang-shurong-dong-shaomin-zhang
[35]. Wei, W., & Wang, X. (2021). Graphene-based electrode materials for neural activity detection. Materials, 14(20), 6170. https://doi.org/10.3390/ma14206170
[36]. Pimenta, S. F. R., Freitas, J. R. M., & Correia, J. H. (2024). Flexible neural probes: a review of the current advantages, drawbacks, and future demands. Journal of Zhejiang University. B. Science, 25(2), 153-167. https://doi.org/10.1631/jzus.B2300337
[37]. Robinson, J., Chawla, N., Patel, S., Spey, E., & McNulty, O. (2024). Neurodevelopmental abnormalities in Down syndrome: assessing structural and functional deficits. Cureus. https://pmc.ncbi.nlm.nih.gov/articles/PMC11750628/
[38]. Deshetty, U. M., & Periyasamy, P. (2023). Potential biomarkers in experimental animal models for traumatic brain injury. Journal of Clinical Medicine, 12(12), 3923. https://www.mdpi.com/2077-0383/12/12/3923
[39]. Jackson, A., & Zimmermann, J. B. (2012). Neural interfaces for the brain and spinal cord—restoring motor function. Nature Reviews Neurology, 8(12), 690-699. https://www.nature.com/articles/nrneurol.2012.219
[40]. Zhang, H., Jiao, L., Yang, S., Li, H., & Jiang, X. (2024). Brain–computer interfaces: the innovative key to unlocking neurological conditions. International Journal of Surgery. https://journals.lww.com/international-journal-of-surgery/fulltext/2024/09000/brain_computer_interfaces__the_innovative_key_to.43.aspx
[41]. Wei, K., Ping, H., Tang, X., Li, D., Zhan, S., Sun, B., & Kong, X. (2025). The effect of L-dopa and DBS on cortical oscillations in Parkinson's disease analyzed by hidden Markov model algorithm. NeuroImage. https://www.sciencedirect.com/science/article/pii/S1053811924004890
[42]. Zakaria, Z., Idris, Z., Halim, S. A., Ghani, A. R. I., & Abdullah, J. M. (2023). STN-deep brain stimulation reduces the power of mu and beta rhythms and enhances synchrony at the motor cortices in Parkinson's disease: a report of … Cureus. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10024512/
[43]. Litt, B., Esteller, R., Echauz, J., D'Alessandro, M., Shor, R., & Vachtsevanos, G. (2001). Epileptic seizures may begin hours in advance of clinical onset: a report of five patients. Neuron, 30(1), 51-64. https://www.cell.com/neuron/fulltext/S0896-6273(01)00262-8
[44]. Jiménez-Sánchez, L., Wong, T. P., & Ouro, A. (2024). Regulation of AMPA receptors in brain diseases, from the genetic to the functional level, volume II. Frontiers in Synaptic Neuroscience. https://www.frontiersin.org/journals/synaptic-neuroscience/articles/10.3389/fnsyn.2024.1470791/full
Cite this article
He,M. (2025). Letting the brain connect directly to machines: Innovations and challenges of bimodal neural probes. Advances in Engineering Innovation,16(1),56-68.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Journal:Advances in Engineering Innovation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Bhansali, V., Rawal, V. T., Sachin, A., Arora, H., & Manjila, T. (2024). Implications and ethical considerations of brain-computer interface: A mini-review. ResearchGate. https://www.researchgate.net/publication/381232392_Implications_and_Ethical_Considerations_of_Brain-Computer_Interface_A_Mini-Review
[2]. Ming, X. (2024). Research on ethical challenges and rational regulation of brain-computer interface technology. Information and Communications Technology and Policy. http://ictp.caict.ac.cn/EN/abstract/abstract1236.shtml
[3]. Vindhya, G., Parveen, S., Sowmya, P., et al. (2024). Neuralink: Reclassifying the limits of human insight. IEEE Xplore. https://ieeexplore.ieee.org/abstract/document/10724926/
[4]. Trott, J., Slaymaker, C., Niznik, G., & Althoff, T. (2024). Brain-computer interfaces: An introduction for clinical neurodiagnostic technologists. The Neurodiagnostic Journal. https://www.tandfonline.com/doi/abs/10.1080/21646821.2024.2408501
[5]. Lotte, F., & Jeunet-Kelway, C. (2024). Brain-computer interaction and neuroergonomics. In Mind, Body, and Digital Brains (pp. 1-20). Springer. https://link.springer.com/chapter/10.1007/978-3-031-58363-6_10
[6]. Rostami, B. (2024). Technological and computational approaches for large count high-density neural probes. Deep Blue Library, University of Michigan. https://deepblue.lib.umich.edu/handle/2027.42/193333
[7]. Zhou, Z., Sun, L., et al. (2024). Ultraflexible PEDOT:PSS/IrOx-modified electrodes: Applications in behavioral modulation and neural signal recording in mice. Micromachines, 15(4), 447. https://www.mdpi.com/2072-666X/15/4/447
[8]. Hao, Y., Han, L., Jin, Y., et al. (2024). A 64-channel fully implantable brain-computer microsystem with custom microelectrode array. IEEE Circuits and Systems. https://ieeexplore.ieee.org/abstract/document/10798410/
[9]. Wang, T., Chen, Y., Wang, Y., & Lee, S. H. (2024). Advanced neural probe sensors toward multi-modal sensing and modulation: Design, integration, and applications. Advanced Sensor Research. https://advanced.onlinelibrary.wiley.com/doi/abs/10.1002/adsr.202400142
[10]. Xu, M., Zhao, Y., Xu, G., Zhang, Y., et al. (2022). Recent development of neural microelectrodes with dual-mode detection. Biosensors, 13(1), 59. https://www.mdpi.com/2079-6374/13/1/59
[11]. Chae, U., Shin, H., Choi, N., et al. (2021). Bimodal neural probe for highly co-located chemical and electrical monitoring of neural activities in vivo. Biosensors and Bioelectronics. https://www.sciencedirect.com/science/article/pii/S0956566321005108
[12]. Dalrymple, A. N., Huynh, M., & Nayagam, B. A. (2020). Electrochemical and biological characterization of thin-film platinum-iridium alloy electrode coatings: A chronic in vivo study. Journal of Neural Engineering. https://iopscience.iop.org/article/10.1088/1741-2552/ab933d/meta
[13]. Drakopoulou, S., Varkevisser, F., Sohail, L., et al. (2023). Hybrid neuroelectronics: towards a solution-centric way of thinking about complex problems in neurostimulation tools. Frontiers in Electronics. https://www.frontiersin.org/articles/10.3389/felec.2023.1250655/full
[14]. Cui, X. T., Siwakoti, U., Jones, S. A., & Kumbhare, D. (2025). Recent progress in flexible microelectrode arrays for combined electrophysiological and electrochemical sensing. Preprints. https://www.preprints.org/frontend/manuscript/08e841067cfadf1d6c555fe14f87ac7c/download_pub
[15]. Parashiva, P. K., Gangadaran, S., & Vinod, A. P. (2025). Subject specific deep learning model for motor imagery direction decoding. arXiv preprint arXiv:2501.01725. https://arxiv.org/pdf/2501.01725
[16]. Duvan, F. T., Cunquero, M., Masvidal-Codina, E., et al. (2024). Graphene-based microelectrodes with bidirectional functionality for next-generation retinal electronic interfaces. Nanoscale Horizons. https://pubs.rsc.org/en/content/articlehtml/2024/nh/d4nh00282b
[17]. Wang, L., Suo, Y., Wang, J., et al. (2024). High-density implantable neural electrodes and chips for massive neural recordings. Brain-X. https://onlinelibrary.wiley.com/doi/abs/10.1002/brx2.65
[18]. Sahin, K., Korkusuz, A. K., Sahin, E., & Orhan, C. (2024). The effect of water-soluble Alpinia Galanga extract on sleep and the activation of the GABAAergic/serotonergic pathway in mice. Pharmaceuticals, 17(12), 1649. https://www.mdpi.com/1424-8247/17/12/1649
[19]. Shin, H., Lee, H. J., Choi, N., et al. (2015). Neural probes with multi-drug delivery capability. Lab on a Chip. https://pubs.rsc.org/en/content/articlehtml/2015/lc/c5lc00582e
[20]. Sahasrabudhe, A. (2024). Multifunctional wireless gut-brain neurotechnology. Massachusetts Institute of Technology. https://dspace.mit.edu/handle/1721.1/157069
[21]. Billa, S. (2024). Advancing electrochemical microsensors for multiplexed neurotransmitters and environmental toxins detection. Louisiana Tech University. https://digitalcommons.latech.edu/dissertations/1022/
[22]. Celshia, S., Selvamani, M., & Suresh, V. (2024). Synthesis and characterization of Mn₂O₃ and its electrochemical properties in relation to dopamine. Cureus. https://pmc.ncbi.nlm.nih.gov/articles/PMC11426951/
[23]. Xu, S., Liu, Y., Yang, Y., Zhang, K., Liang, W., Xu, Z., & Wu, Y. (2023). Recent progress and perspectives on neural chip platforms integrating PDMS-based microfluidic devices and microelectrode arrays. Micromachines, 14(4), 709. https://www.mdpi.com/2072-666X/14/4/709
[24]. Mathieu, F., Marty, F. H., & Blatche, M. C. (2024). Real-time and high-resolution monitoring of neuronal electrical activity and pH variations based on the co-integration of nanoelectrodes and Chem-FinFETs. Small. https://onlinelibrary.wiley.com/doi/abs/10.1002/smll.202309055
[25]. Jonsson, A., Inal, S., & Uguz, I. (2016). Bioelectronic neural pixel: Chemical stimulation and electrical sensing at the same site. Proceedings of the National Academy of Sciences, 113(34), 9440-9445. https://www.pnas.org/doi/abs/10.1073/pnas.1604231113
[26]. Takeuchi, S., Ziegler, D., Yoshida, Y., Mabuchi, K., & Suzuki, T. (2005). Parylene flexible neural probes integrated with microfluidic channels. Lab on a Chip, 5(5), 519-523. https://pubs.rsc.org/en/content/articlehtml/2005/lc/b417497f
[27]. Jiao, Y., Lei, M., Zhu, J., Chang, R., & Qu, X. (2023). Advances in electrode interface materials and modification technologies for brain-computer interfaces. Biomaterials Translational. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10817795/
[28]. Wen, R., Tao, C., Ji, H., & Qiu, J. (2024). Dual-modal fusion PRI-SWT model for eddy current detection of cracks, delamination, and impact damage in carbon fiber-reinforced plastic materials. Applied Sciences, 14(22), 10282. https://www.mdpi.com/2076-3417/14/22/10282
[29]. Wang, X., Han, S., Yan, P., Lin, Y., & Wang, C. (2024). A 1024-channel simultaneous electrophysiological and electrochemical neural recording system with in-pixel digitization and crosstalk compensation. IEEE Transactions on Circuits and Systems. https://ieeexplore.ieee.org/abstract/document/10680349/
[30]. Cui, X. T., Siwakoti, U., Jones, S. A., & Kumbhare, D. (2025). Recent progress in flexible microelectrode arrays for combined electrophysiological and electrochemical sensing. Preprints. https://www.preprints.org/frontend/manuscript/08e841067cfadf1d6c555fe14f87ac7c/download_pub
[31]. Cho, Y., Park, S., Lee, J., & Yu, K. J. (2021). Emerging materials and technologies with applications in flexible neural implants: A comprehensive review of current issues with neural devices. Advanced Materials, 33(9), 2005786. https://onlinelibrary.wiley.com/doi/abs/10.1002/adma.202005786
[32]. Xu, M., Zhao, Y., Xu, G., Zhang, Y., Sun, S., Sun, Y., & Wang, J. (2022). Recent development of neural microelectrodes with dual-mode detection. Biosensors, 13(1), 59. https://www.mdpi.com/2079-6374/13/1/59
[33]. He, L., Feng, H., & Li, Y. (2020). Recent development of implantable and flexible nerve electrodes. Smart Materials in Medicine, 1, 1-12. https://www.sciencedirect.com/science/article/pii/S2590183420300132
[34]. Zhang, F., Dong, S., & Zhang, S. (2024). Soft electronics for neural engineering. In Advanced Soft Electronics in Biomedical Applications (pp. 75-98). Taylor & Francis. https://www.taylorfrancis.com/chapters/edit/10.1201/9781003493631-5/soft-electronics-neural-engineering-fan-zhang-shurong-dong-shaomin-zhang
[35]. Wei, W., & Wang, X. (2021). Graphene-based electrode materials for neural activity detection. Materials, 14(20), 6170. https://doi.org/10.3390/ma14206170
[36]. Pimenta, S. F. R., Freitas, J. R. M., & Correia, J. H. (2024). Flexible neural probes: a review of the current advantages, drawbacks, and future demands. Journal of Zhejiang University. B. Science, 25(2), 153-167. https://doi.org/10.1631/jzus.B2300337
[37]. Robinson, J., Chawla, N., Patel, S., Spey, E., & McNulty, O. (2024). Neurodevelopmental abnormalities in Down syndrome: assessing structural and functional deficits. Cureus. https://pmc.ncbi.nlm.nih.gov/articles/PMC11750628/
[38]. Deshetty, U. M., & Periyasamy, P. (2023). Potential biomarkers in experimental animal models for traumatic brain injury. Journal of Clinical Medicine, 12(12), 3923. https://www.mdpi.com/2077-0383/12/12/3923
[39]. Jackson, A., & Zimmermann, J. B. (2012). Neural interfaces for the brain and spinal cord—restoring motor function. Nature Reviews Neurology, 8(12), 690-699. https://www.nature.com/articles/nrneurol.2012.219
[40]. Zhang, H., Jiao, L., Yang, S., Li, H., & Jiang, X. (2024). Brain–computer interfaces: the innovative key to unlocking neurological conditions. International Journal of Surgery. https://journals.lww.com/international-journal-of-surgery/fulltext/2024/09000/brain_computer_interfaces__the_innovative_key_to.43.aspx
[41]. Wei, K., Ping, H., Tang, X., Li, D., Zhan, S., Sun, B., & Kong, X. (2025). The effect of L-dopa and DBS on cortical oscillations in Parkinson's disease analyzed by hidden Markov model algorithm. NeuroImage. https://www.sciencedirect.com/science/article/pii/S1053811924004890
[42]. Zakaria, Z., Idris, Z., Halim, S. A., Ghani, A. R. I., & Abdullah, J. M. (2023). STN-deep brain stimulation reduces the power of mu and beta rhythms and enhances synchrony at the motor cortices in Parkinson's disease: a report of … Cureus. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10024512/
[43]. Litt, B., Esteller, R., Echauz, J., D'Alessandro, M., Shor, R., & Vachtsevanos, G. (2001). Epileptic seizures may begin hours in advance of clinical onset: a report of five patients. Neuron, 30(1), 51-64. https://www.cell.com/neuron/fulltext/S0896-6273(01)00262-8
[44]. Jiménez-Sánchez, L., Wong, T. P., & Ouro, A. (2024). Regulation of AMPA receptors in brain diseases, from the genetic to the functional level, volume II. Frontiers in Synaptic Neuroscience. https://www.frontiersin.org/journals/synaptic-neuroscience/articles/10.3389/fnsyn.2024.1470791/full









