1. Introduction
Supercapacitors—also known as electrochemical capacitors or electric double-layer capacitors (EDLCs)—are energy storage devices positioned between conventional capacitors and batteries in terms of performance characteristics [1]. Combining the advantages of high power density, rapid charge-discharge capability, long cycle life, and operational safety, supercapacitors have found broad application in electronic products, memory backup systems, and various types of equipment. They are also employed in energy recovery systems during braking processes and serve as auxiliary power sources in the powertrain systems of urban buses and waste collection vehicles, thereby mitigating the impact of frequent start-stop operations on vehicle mileage. The following sections provide a detailed overview of the core characteristics and application domains of supercapacitors.
2. Energy storage mechanism
The energy storage mechanism of supercapacitors primarily relies on three approaches [2].
Electric Double-Layer Capacitance (EDLC):Traditional capacitors exhibit low energy density, primarily due to limitations in charge storage area and the distance between the two electrodes. Electric double-layer supercapacitors possess a significantly larger effective specific surface area and a much thinner dielectric layer (determined by the thickness of the electric double layer), resulting in a substantial enhancement in charge storage capacity.The working principle of the double electric layer is shown in Figure 1.
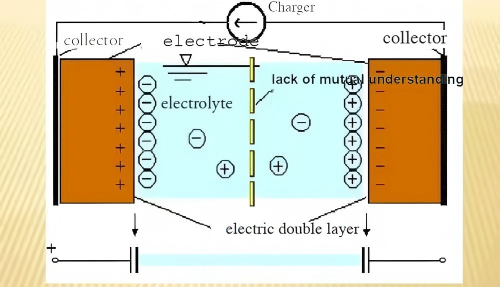
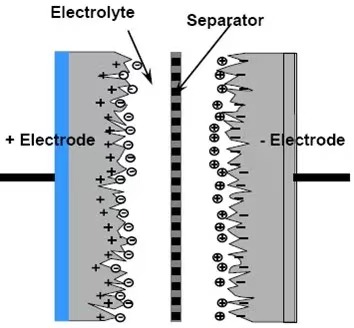
Traditional electric double-layer capacitors rely on the potential difference generated by surface charges, in which the charges are stored electrostatically at the electrode–electrolyte interface (i.e., in a non-Faradaic manner). Pseudocapacitive electrode materials (such as RuO₂, MnO₂, etc.) exhibit electrochemical behavior similar to that of EDLCs, with a linear relationship between stored charge and the voltage window. However, the origin of the charge is fundamentally different, stemming from distinct storage mechanisms. Pseudocapacitance arises from certain electro-adsorption processes and redox reactions occurring at the electrode surface or within oxide films (such as RuO₂, IrO₂, Cr₃O₄). In principle, this constitutes a Faradaic process involving charge transfer across the electric double layer.The working principle of the dummy capacitor is shown in Figure 2.
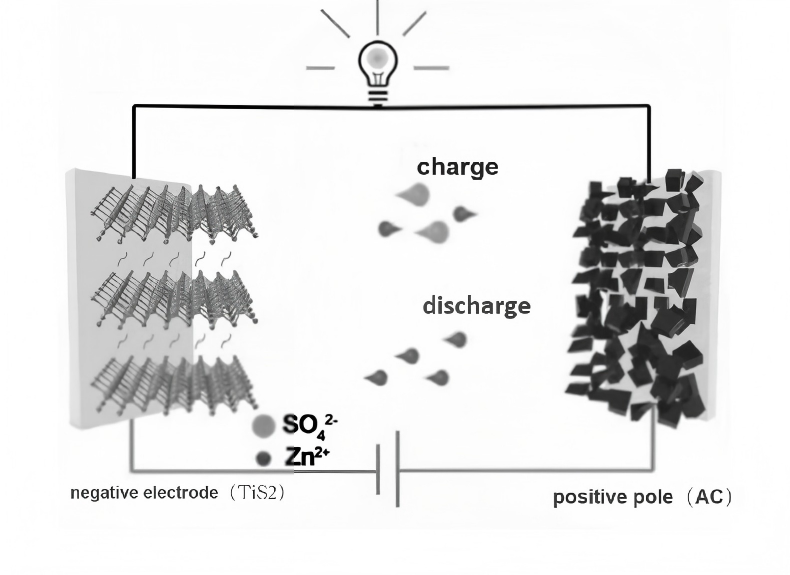
Hybrid supercapacitors are energy storage devices that combine the characteristics of both batteries and supercapacitors. By integrating two distinct energy storage mechanisms—electrostatic adsorption and Faradaic redox reactions—they achieve a balance between high energy density and high power density, thereby enhancing overall performance. The positive electrode typically employs battery-type materials, such as lithium-ion or metal-ion compounds, which store charge through Faradaic reactions. The negative electrode uses carbon-based materials, such as graphene, titanium carbide, or activated carbon, to store energy via electrostatic double-layer adsorption.The working principle of hybrid ultracapacitors is shown in Figure 3.
3. Electrode materials
The performance of electric double-layer supercapacitors (EDLCs) primarily depends on the specific surface area of the materials, pore structure, and the wettability of the material surface with the electrolyte. Due to their high specific surface area and pore volume, stable performance, low cost, and environmental friendliness, carbon-based materials have become the most widely used electrode materials. Currently, the carbon materials employed in supercapacitors include activated carbon, carbon aerogels, activated carbon fibers, templated carbon, carbon nanotubes, and graphene.
Pseudocapacitive electrode materials are typically transition metal oxides (such as RuO₂, MnO₂) and conductive polymers. Owing to their chemically and structurally reversible redox reactions, these materials undergo highly reversible chemical adsorption, desorption, or redox processes on the electrode surface or within two-dimensional or quasi-two-dimensional spaces of the bulk phase. These processes generate capacitance that is directly related to the electrode charging potential.
Among the electrode materials used in battery-type supercapacitors, metal oxide systems have consistently played a crucial role. As a representative battery-type electrode material, nickel oxide (NiO) offers excellent thermal stability, low cost, and high capacitance (up to 3,750 F·g⁻¹), making it highly promising for widespread application. Cobalt oxide (Co₃O₄) exhibits strong redox activity and a high theoretical specific capacitance of up to 3,560 F·g⁻¹. However, Co₃O₄ suffers from significant capacity fading, often requiring modification treatments to enhance its cycling stability. Additionally, copper oxide (CuO) is non-toxic, abundantly available in nature, easily synthesized into various nanostructures, and exhibits excellent electrochemical performance, making it another viable candidate for supercapacitor electrode materials.
4. Electrolytes
4.1. Aqueous electrolytes: high conductivity, safe, but narrow voltage window (≤1.2 V)
Aqueous electrolytes possess several advantages, including high ionic conductivity, high concentration, and small solvated ion size, which contribute to low internal resistance and are beneficial for achieving higher power density in EDLCs [3]. Moreover, aqueous electrolytes are low-cost and environmentally friendly. During device assembly, they eliminate the need for an anhydrous and oxygen-free environment, significantly simplifying the manufacturing process and reducing production costs. Compared to non-aqueous electrolytes, aqueous electrolytes enable higher specific capacitance in EDLCs. However, a major challenge facing aqueous electrolytes is their narrow electrochemical voltage window. Water undergoes hydrogen evolution at 0 V vs. SHE and oxygen evolution at 1.23 V vs. SHE, theoretically limiting the operational voltage window to 1.23 V. In practice, the operating voltage of most devices is around 1.0 V. Exceeding this range results in water decomposition and gas evolution, which can lead to device failure. This narrow voltage window significantly constrains the further improvement of energy density.
4.2. Organic electrolytes: wide voltage window (2.5–3.5 V), high energy density, but flammable and costly
Organic electrolytes have become the most commonly used electrolyte system in commercial EDLCs due to their relatively wide and stable operating voltage window [4]. Generally, an organic electrolyte consists of a solvent and an electrolyte salt. Common solvents include acetonitrile (AN) and propylene carbonate (PC). Compared to PC, AN possesses a higher dielectric constant and lower viscosity; consequently, AN-based electrolytes exhibit higher ionic conductivity and lower viscosity, which translates into AN-based EDLCs having higher power density and better low-temperature performance. However, the stable operating voltage window for both AN- and PC-based organic electrolytes is limited to approximately 2.5–2.8 V. Increasing the operating voltage beyond this range results in rapid device failure. Therefore, further development of organic solvents with improved high-voltage stability is required to meet market demands.
4.3. Ionic liquids: wide voltage window (>3 V), high thermal stability, and represent a future development direction
Ionic liquids (also known as low-temperature or room-temperature molten salts) are typically defined as salts composed of ions with melting points below 100 °C. They generally consist of large, asymmetric organic cations paired with inorganic or organic anions [5]. Ionic liquids possess several potential advantages, including high thermal stability, chemical stability, electrochemical stability, negligible volatility, and non-flammability. The diverse combinations of cations and anions allow for tunable physical and chemical properties, enabling ionic liquids to meet various performance requirements for EDLCs, such as high operating voltage and wide operating temperature range. Based on their composition, ionic liquids can be categorized into aprotic, protic, and zwitterionic types. Common ionic liquids used in EDLCs include cations such as imidazolium, pyrrolidinium, ammonium, sulfonium, and phosphonium, paired with anions such as tetrafluoroborate (BF₄⁻), hexafluorophosphate (PF₆⁻), bis(trifluoromethanesulfonyl)imide (TFSI⁻), bis(fluorosulfonyl)imide (FSI⁻), and dicyanamide (DCA⁻).
5. Separator materials
The separator is a critical component of supercapacitors, and its performance directly influences the overall performance of the device. The primary function of the separator is to physically separate the positive and negative electrodes to prevent internal short circuits caused by contact between the active materials of the two electrodes. At the same time, it must allow the passage of electrolyte ions to ensure efficient charge-discharge processes. Currently, commonly used supercapacitor separators include separator paper, polypropylene-based membranes, nonwoven fabrics, bio-based membranes, and high ion-permeable membranes.
5.1. Requirements for separator materials
High Ionic Permeability: The separator must have a high porosity with uniform pore distribution to ensure rapid transport of electrolyte ions and low internal resistance, while maintaining smooth electrolyte circulation.
Electrical Insulation: It must completely block electron transfer to prevent direct contact between the positive and negative electrodes, thereby avoiding short circuits.
Mechanical Strength: The separator should possess sufficient puncture resistance and tensile strength to withstand mechanical stresses during battery assembly and cycling.
Electrolyte Absorption and Retention: It must efficiently absorb and retain the electrolyte to ensure thorough contact between the electrode and electrolyte, thereby enhancing ionic conductivity.
Chemical and Electrochemical Stability: The separator should maintain structural stability and corrosion resistance in the operating environment of the supercapacitor (such as in various electrolyte systems or wide voltage windows) without participating in electrochemical reactions.
5.2. Common materials
Biomass-Based Separators: These include microfibrillated cellulose (MFC), lyocell fibers, and regenerated cellulose. They are fabricated through processes such as fibrillation or wet forming, exhibiting high porosity and excellent electrolyte absorption capacity.
Synthetic Polymer-Based Separators: Examples include polypropylene (PP), polytetrafluoroethylene (PTFE), and polyvinylidene fluoride (PVDF). These materials offer high mechanical strength and chemical stability but often require enhancements such as plasma treatment or inorganic material composites to improve thermal stability and wettability.
Inorganic Composite Separators: These typically involve cellulose substrates coated with porous ceramic layers or the incorporation of metal oxides (e.g., zinc oxide) to reinforce mechanical strength and thermal stability. Physical Properties Analysis of Common Separators is shown in Table 1.
|
Lack of mutual understanding |
Thickness um |
The puncture intensity gf |
Porosity % |
Breath ability second/100cc |
TD Kgf/cm2 |
MD Kgf/cm2 |
|
Celgard2400 |
25 |
450 |
41 |
520 |
140 |
1420 |
|
MPF04Q-15 |
13 |
150 |
66.1 |
40 |
120 |
|
|
pvdfXB-30 |
30 |
200 |
60 |
8 |
150 |
200 |
|
CO2 |
50 |
750 |
750 |
220 |
90 |
130 |
6. System integration and application cases
6.1. New energy vehicles: used for regenerative braking energy recovery
6.1.1. Pure electric drive mode
This mode primarily relies on a supercapacitor-powered motor. During vehicle operation, the motor drives the car, while during braking, it acts as a generator to recover energy. Vehicles operating under this mode are driven solely by electricity, making their principle relatively simple and enabling zero emissions. However, since these vehicles depend on charging stations and require a relatively long time to complete charging, their driving range is limited. The distance achievable on a full charge is comparatively short, making them more suitable as urban commuter vehicles.
6.1.2. Hybrid drive mode
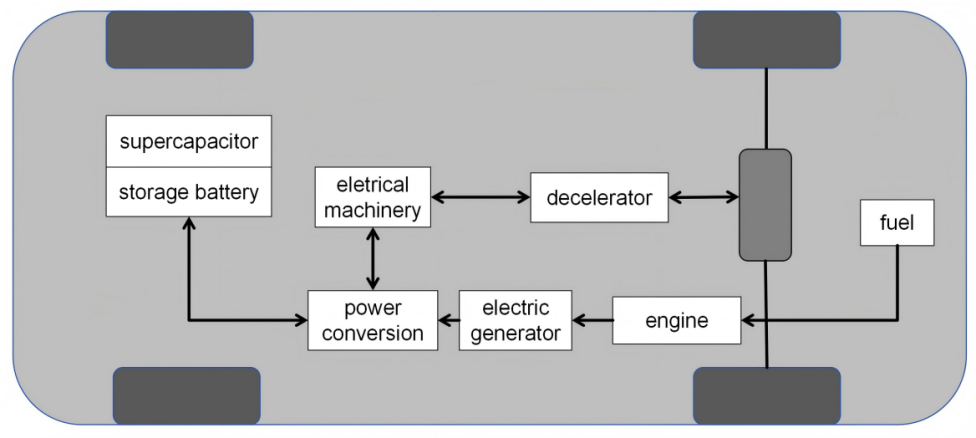
In this mode, the supercapacitor serves an auxiliary role within the engine system. When the vehicle starts, the engine converts kinetic energy into electrical energy through a generator, which is then converted by an inverter into DC power supplied to the electric motor. If the vehicle speed does not reach the preset threshold after startup, the motor drives the vehicle. When the vehicle encounters rough road conditions during operation, the supercapacitor can be activated to supply power to the motor. Once the vehicle speed reaches the preset value, the motor stops, and the vehicle is powered by the engine. During this process, any excess power is used to charge the supercapacitor. After the vehicle stops, the supercapacitor can automatically discharge to the battery.Workflow of Hybrid Supercapacitor is shown in Figure 4.
6.2. Grid frequency regulation: combined with batteries to form a hybrid energy storage system, responding to grid fluctuations
Lithium-ion batteries possess advantages such as fast response, strong short-term power throughput, and flexible regulation, enabling full power output within milliseconds to seconds. Utilizing lithium-ion battery energy storage systems to assist thermal power units in frequency regulation can effectively enhance the frequency regulation capability of these units and alleviate the grid’s frequency regulation assessment pressure. However, when supporting thermal power units in frequency regulation, lithium-ion battery systems must frequently switch between charging and discharging states, which rapidly consumes the battery’s lifespan. Additionally, to assist thermal units, lithium-ion battery cells are often grouped in large packs. During operation, inconsistencies among cells can cause some cells to age prematurely, leading to overcharge and over-discharge issues in these cells. Consequently, this results in rapid capacity degradation, shortened service life, and increased safety risks for the lithium-ion battery energy storage system.
Supercapacitors possess advantages such as high power density, long cycle life, and rapid response, making them suitable for assisting renewable energy plants and thermal power units in grid frequency regulation. Under small-scale frequency regulation commands from the grid, using supercapacitor energy storage systems to support thermal power units can reduce the frequent cycling of lithium-ion batteries, thereby significantly extending their service life.An Example of Hybrid Energy Storage Assisting Frequency Regulation is shown in Figure 5.
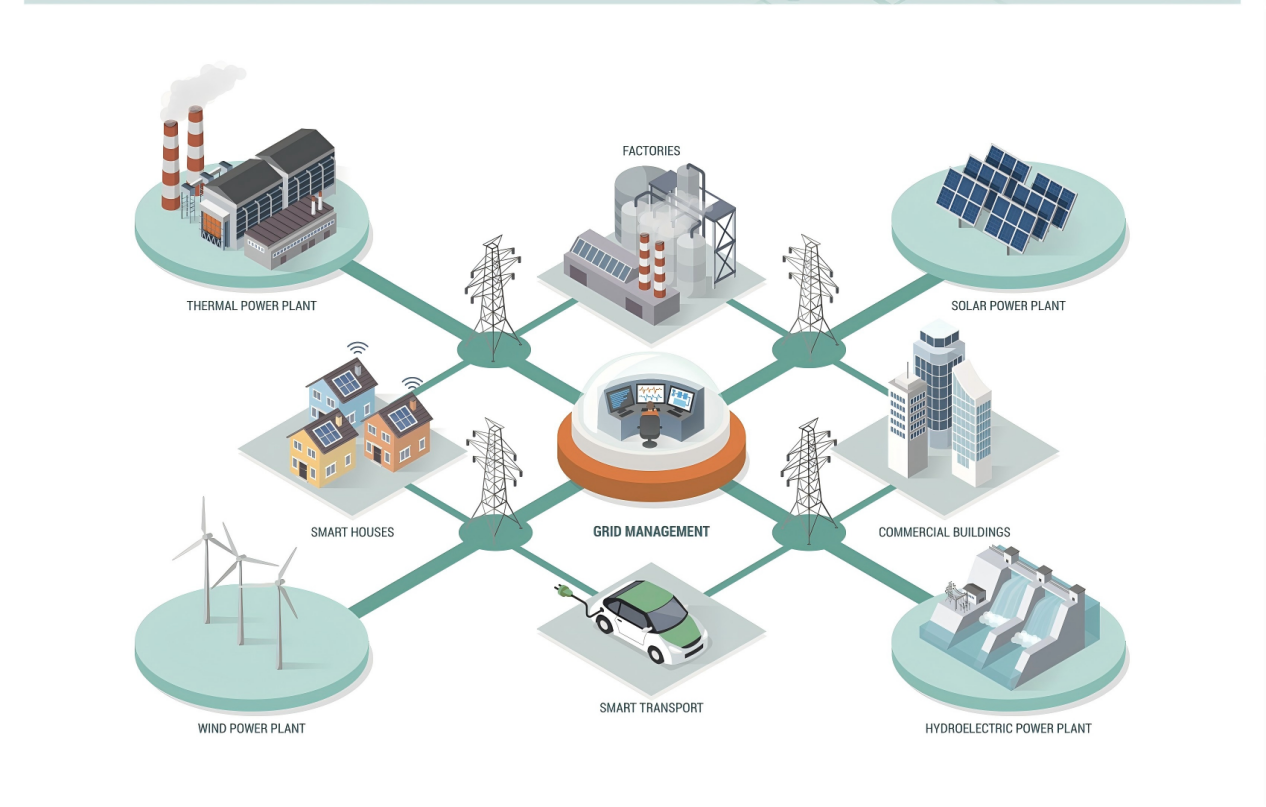
Combining lithium-ion batteries and supercapacitors in a hybrid energy storage system at a certain power-to-capacity ratio leverages the respective advantages of both storage types. This approach holds significant development potential for supporting thermal power units in frequency regulation.
6.3. Consumer electronics: huawei smartphones equipped with graphene-based supercapacitors achieve fast charging
Graphene-based supercapacitors, owing to their high specific surface area and excellent electrical conductivity, theoretically enable charge and discharge within seconds. Huawei has incorporated graphene materials into their batteries to achieve efficient heat dissipation. Combined with high-temperature decomposition-resistant electrolyte additives and modified large single-crystal ternary cathode materials, this significantly enhances the battery’s thermal stability under high-temperature conditions, thereby supporting fast charging.Huawei phone with graphene battery is shown in Figure 6.
7. Challenges and prospects
Currently, supercapacitors have been widely applied in various industries, including electric transportation (such as railways, trams, trucks, and buses), electronic devices (such as wearable electronics and sensors), industrial equipment, and the medical field (such as sweatbands and implantable medical devices). However, due to inherent material properties, supercapacitors still face limitations in energy density and cost. Addressing these challenges primarily involves two aspects.
7.1. Electrode materials
As the primary active component of supercapacitors, electrode materials function based on their specific surface area, pore structure, and wettability with the electrolyte. Besides these factors, specific capacitance is also a crucial property of electrodes. Specific capacitance is influenced not only by surface area but also by parameters such as pore size distribution, pore shape, and dimension of the electrode materials. Electrode materials are generally classified into carbon-based materials, metal oxides, and conductive polymers. Carbon-based materials, characterized by high surface area, high porosity, low cost, environmental friendliness, and facile synthesis methods, are the main electrode materials for electric double-layer capacitors. Their thermal stability, chemical and electrochemical stability, high conductivity, symmetrical constant-current charge–discharge curves, and well-shaped rectangular cyclic voltammograms all indicate strong application potential. At the nanoscale, tuning and controlling defects and surface interfaces can further enhance capacitance. Although energy density has improved, metal oxide materials face practical limitations due to low conductivity, uncontrollable volume expansion, and slow ion diffusion within the bulk phase. Conductive polymers exhibit one notable drawback: slow ion diffusion inside the material, which results in relatively low power density or charge–discharge rates.
7.2. Electrolytes
Electrolytes can be classified into liquid, solid, and redox electrolytes. Pure ionic liquid electrolytes have relatively low melting points; at their melting temperatures, ionic mobility decreases, which adversely affects the performance of supercapacitors. Solid polymer electrolytes are divided into three types: solid polymer electrolytes, gel polymer electrolytes, and polyelectrolytes. Based on the type of plasticizer used, gel polymer electrolytes are further categorized into hydrogels, organic gels, and ion gels. In solid-state electrolytes, ionic liquids can be incorporated into polymers to form gel polymers, and electrolytes can also be synthesized via polymerization reactions to produce polyelectrolytes. Polyelectrolytes are polymers whose repeating units contain both a polymer backbone and ionic liquid components, thereby combining the properties of both. In redox electrolytes, ionic liquids serve not only as solvents dissolving redox-active species but also function as redox electrolytes themselves.
7.3. Prospects
As a novel energy storage device, supercapacitors still face several challenges in practical applications. For example, optimizing electrode materials and developing materials with higher energy density, higher power density, and longer cycle life remain critical tasks. The three types of electrode materials introduced above each have their own advantages and disadvantages, and various modification strategies can be employed to enhance the energy density, power density, and cycle life of supercapacitors.
8. Conclusion
Supercapacitors are expected to play a major role in future electrochemical energy storage devices. They are irreplaceable in scenarios requiring rapid charge-discharge and high power output. However, the current limited energy density of supercapacitors restricts their widespread application in the energy storage field. The next generation of supercapacitors aims to significantly increase energy density while maintaining high power density, approaching that of lithium-ion batteries. There are three main pathways to enhance energy density: utilizing ionic liquids with wider electrochemical windows, developing new materials that combine electric double-layer capacitance and pseudocapacitance, and advancing hybrid capacitors. With the improvement of supercapacitor performance, they may replace batteries in certain fields, but in most cases, they will be used in conjunction with batteries to increase power output, extend service life, and improve reliability. As materials science progresses, the application potential of supercapacitors in new energy and smart grid sectors will be further unlocked. In the future, supercapacitors will play an important role in the energy storage field by reducing total energy consumption and minimizing the use of hydrocarbon fuels.
9. Research reflections
After several weeks of study and research, I have gained a certain understanding of supercapacitors, including their properties and practical applications. During the research process, I frequently encountered difficulties in reading and comprehending complex issues. By approaching these challenges with questions in mind, I was able to resolve most of them independently through extensive literature review and consultations with my advisor. This problem-driven approach not only enhanced my ability to search and analyze academic materials but also greatly improved my communication skills with my advisor. Through reading a large number of related research papers, I learned effective methods for academic writing and strengthened my writing skills, academic expression, and abilities in summarizing and synthesizing information. These skills and methods will provide substantial support for my future learning and research endeavors.
I feel very fortunate to have had this research experience during the intense final stage of my high school studies. It not only enabled me to grasp essential knowledge about supercapacitors but also deepened my understanding of the research mindset centered on “problem orientation, material-driven approaches, and scenario adaptation.” This mindset will serve as an important guide for my future learning and research.
References
[1]. Hou, B., Yin, L. H., Xia, J. S., Yan, R. W., & Chen, Y. (2016). Research progress on supercapacitor electrode materials.Journal of Henan University, 3, 286–306.
[2]. Wei, Z. S., Wu, L., Li, L., & Ji, Y. (2023). Flexible supercapacitors and their research progress.Progress in Textile Science and Technology, 3, 1–5.
[3]. Sun, H. X., Qi, J. L., Yu, K. P., & Yin, J. Z. (2024). Research progress on ionic liquids as electrolytes for supercapacitors.Chemical New Materials, 9, 13–17.
[4]. Na, D., Zeng, L. Y., Li, H. B., & Zhao, Y. Q. (2025). Opportunities and challenges of biochar—an emerging carbon-based material in electrochemical energy storage.Yunnan Chemical Industry, 3, 1–4.
[5]. Liu, H., & Han, W. (2015). Research on supercapacitor separators and electrolytes (Master’s thesis, Jilin University).
Cite this article
Lv,C. (2025). Current status and challenges in supercapacitor research. Advances in Engineering Innovation,16(8),5-12.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Journal:Advances in Engineering Innovation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Hou, B., Yin, L. H., Xia, J. S., Yan, R. W., & Chen, Y. (2016). Research progress on supercapacitor electrode materials.Journal of Henan University, 3, 286–306.
[2]. Wei, Z. S., Wu, L., Li, L., & Ji, Y. (2023). Flexible supercapacitors and their research progress.Progress in Textile Science and Technology, 3, 1–5.
[3]. Sun, H. X., Qi, J. L., Yu, K. P., & Yin, J. Z. (2024). Research progress on ionic liquids as electrolytes for supercapacitors.Chemical New Materials, 9, 13–17.
[4]. Na, D., Zeng, L. Y., Li, H. B., & Zhao, Y. Q. (2025). Opportunities and challenges of biochar—an emerging carbon-based material in electrochemical energy storage.Yunnan Chemical Industry, 3, 1–4.
[5]. Liu, H., & Han, W. (2015). Research on supercapacitor separators and electrolytes (Master’s thesis, Jilin University).









