1. Introduction
Dementia, is a syndrome characterized by acquired impairment of cognitive function. This impairment seriously affects the patient's daily life, learning, work and social interaction. In modern medical classification, dementia can be categorized into degenerative and non-degenerative diseases according to its pathogenesis. Degenerative diseases include Alzheimer's disease (AD), Parkinson disease with dementia (PDD) and so on. Non-degenerative diseases include vascular dementia (VD) and brain injury diseases caused by other factors [1]. Against the backdrop of an increasingly aging global population, the incidence of dementia is rising, causing serious health and economic burdens on families and society.
Astragaloside IV (AS-IV) (Figure 1) is one of the major components of Astragalus. As a triterpene glycoside analog, astragaloside exhibits a variety of pharmacological activities. Astragaloside possesses significant anti-oxidative stress, anti-apoptotic, and anti-inflammatory effects, and has attracted much attention especially in neuroprotection, revealing its potential in the treatment of cognitive disorders [2].
In this review, we will discuss the progress of mechanism research on astragaloside in dementia treatment from both degenerative and non-degenerative disease perspectives, and we are committed to providing a scientific basis for the treatment of cognitive disorders diseases with Chinese medicine.
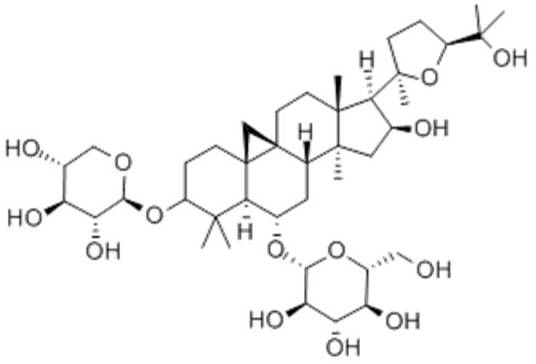
Figure 1. Astragaloside IV
2. Degenerative diseases
2.1. Alzheimer's disease
Alzheimer's disease (AD), often referred to as “dementia”, is the most common degenerative dementia, accounting for 50% to 70% of dementia cases, with cognitive and memory deficits being the main features of this neurodegenerative disease [3]. Amyloid precursor protein (APP)-derived β-amyloid (Aβ) aggregation is an important pathological hallmark. Aβ induces neuronal apoptosis and necrosis, activates microglia leading to neuroinflammation and accelerates lesions [4]. In addition, mitochondrial dysfunction and oxidative stress are also key factors in AD, both of which further contribute to neuronal damage through reactive oxygen species (ROS) release [5].
Aβ-induced microglia activation and neuroinflammation play a key role in the pathogenesis of AD. Therefore, anti-neuroinflammatory response has become an important strategy in the treatment of AD.AS-IV alleviates neuroinflammation by reducing the release of inflammatory factors such as TNF-α, IL-1β, and IL-6 and attenuating microglia activation [6]. It also attenuates oxidative stress in hippocampal tissues and ameliorates cognitive impairment and neuronal damage due to Aβ by inhibiting microglia activation and decreasing the expression of reduced coenzyme II (NADPH) oxidase protein [7].
Studies have shown that Aβ-induced apoptosis and necrosis can be mitigated by AS-IV by modulating peroxisome proliferator-activated receptor γ (PPARγ) and β-amyloid precursor protein cleavage enzyme 1 (BACE1), which reduces APP cleavage and Aβ production [8,9].AS-IV also provides neuroprotection by inhibiting the opening of the mitochondrial permeability transition pore (mPTP). This process is significantly affected by ROS [10]. A study [11] showed that pretreatment with astragaloside under the influence of Aβ1-42 enhanced neuronal cell viability, reduced apoptosis, and inhibited ROS production and mitochondrial superoxide, providing a new perspective for its application in AD prevention and treatment. Lin [12] et al. investigated the effects of astragaloside on the proliferation and differentiation of transplanted neural stem cells (NSCs) and investigated the improvement of cognitive deficits induced by intracerebral injection of Aβ protein in a rat model of Alzheimer's disease, and found that high doses of astragaloside were able to down-regulate the structural domains of Notch intracellular domains, while low doses of astragaloside were able to elevate the expression of Notch-1 and NICD, suggesting that stem cell therapy may be a therapeutic strategy. Decreased brain-derived neurotrophic factor (BDNF) is a key pathological feature of AD, AS-IV enhances BDNF expression and promotes neuronal survival and functional recovery by regulating PPARγ, and also reduces abnormal phosphorylation of tau protein and improves hippocampal synaptic deficits, a mechanism that contributes to the improvement of learning and memory abilities in AD patients [13].
In summary, astragaloside exerts anti-inflammatory, antioxidant and neuroprotective effects through multi-target and multi-pathway collaboration to improve cognitive function and quality of life in AD patients. These effects provide firm support for the further development of astragaloside as an AD therapeutic agent.
2.2. Parkinson's disease
Parkinson's disease (PD) is a neurodegenerative disorder with progressive lesions of dopaminergic neurons in the substantia nigra and is second only to Alzheimer's disease in terms of commonness [14]. Current studies point out that oxidative stress, mitochondrial dysfunction, neuroinflammation and apoptosis play important roles in the pathogenesis of PD [15,16].
The development of PD is closely related to the inflammatory response in brain tissue. Neuroglia in the brains of patients are often in an over-activated state, leading to overexpression of inflammatory factors such as TNF-α, IL-6 and IL-1β, which accelerate apoptosis of dopaminergic neurons [17]. Astragaloside reduces the expression of inflammatory factors by inhibiting the activation of astrocytes and microglia, thereby alleviating neuroinflammation. This may be achieved by inhibiting nuclear factor kappa-B (NF-κB) and NLRP3 inflammatory signaling pathways [16]. In addition, astragaloside enhances cell viability and inhibits inflammatory responses by activating the JAK2/STAT3 signaling pathway [18].
Apoptosis of neurons is the main mechanism by which PD leads to the death of dopaminergic neurons, so inhibition of apoptosis has become one of the keys to the treatment of PD. Studies have shown that astragaloside significantly inhibits neuronal apoptosis, which is achieved by decreasing the expression of proteins such as BCL-2-associated X protein (BaX) and cystatinase-3 (caspase-3), while increasing the expression of B lymphocytoma-2 (Bcl-2) [19]. In addition, it inhibits neuronal apoptosis and ameliorates cognitive deficits in PD model mice by activating the PI3K/Akt signaling pathway [20]. Some studies have shown that astragaloside treatment improves cognitive impairment in PD mice, inhibits the expression of long-stranded noncoding RNA-p21 (lincRNA-p21), reduces the expression of C/EBP homologous protein (CHOP), and decreases endoplasmic reticulum stress to achieve anti-neuroapoptotic effects [21].
The occurrence of PD is often associated with autophagy dysfunction. Autophagy removes abnormal proteins and damaged organelles and transports them to lysosomes for degradation, preventing cell damage and reducing the accumulation of harmful components. When autophagy is impaired, the clearance level of lysosomes is hindered, leading to the accumulation of α-synuclein protein (α-Syn) and toxicity to neurons [22]. Astragaloside can increase the level of cellular autophagy to remove damaged neurons, and the PI3K/Akt/mammalian target of rapamycin (mTOR) signaling pathway is one of the important signaling pathways that regulate autophagy [16]. Astragaloside accelerates the occurrence of autophagy in mitochondria, reduces the production of ROS by damaged mitochondria, inhibits astrocyte senescence, and prevents dopaminergic neurons from degenerating in PD [23].
Studies have shown that by activating the SIRT1-FOXO1, JAK2/STAT3, and nuclear factor (erythroid-derived 2)-related factor 2 (Nrf2) signaling pathways, astragaloside enhances the activity of antioxidant enzymes (e.g., SOD, CAT, and GSH) and reduces the generation of ROS, which can alleviate the neurological damage brought about by oxidative stress [24,25].
In conclusion, astragaloside exerts its neuroprotective effects through multiple mechanisms, including inhibition of oxidative stress, alleviation of inflammation, reduction of neuronal apoptosis and promotion of autophagy. These effects provide theoretical support and broad prospects for its use as a therapeutic agent for Parkinson's disease, which is worthy of further research and development.
3. Non-degenerative diseases
3.1. Vascular dementia
Vascular dementia (VD) is a clinical syndrome of intellectual and cognitive dysfunction that develops on the basis of multiple episodes of cerebrovascular disease [26]. Astragaloside exhibits complex and multiple mechanisms of action in the treatment of vascular dementia.
Normal brain function requires a stable supply of energy, and in particular, the need for ATP is critical.AST-IV demonstrated a significant protective effect in improving energy metabolism in brain tissues after cerebral ischemia and reperfusion. Studies have shown [27] that AST-IV can act by promoting the activation of AMP-dependent protein kinase (AMPK) α1/2 and enhancing the expression of glucose transporter protein 3 (GLUT3), which enhances glucose uptake and utilization to improve energy metabolism after cerebral ischemia-reperfusion injury. In addition, AST-IV may interact with G protein-coupled receptors to promote cAMP production by membrane-associated adenylate cyclase and activate the PKA/CREB pathway to further increase ATP production [28].
Oxidative stress exacerbates brain damage and consequent cognitive impairment. AST-IV can ameliorate oxidative damage in astrocytes by modulating the Nrf2/JNK signaling pathway, enhancing total antioxidant capacity, and alleviating secondary damage and cognitive impairment [29].
Inflammation is a major contributor to cognitive impairment after stroke. The anti-inflammatory mechanism of AST-IV involves inhibition of the NF-κB and NLRP3 inflammatory vesicle signaling pathways, and decreases the production of endothelial cell adhesion molecules and inflammatory factors such as IL-1β and IL-18 [30].
In terms of apoptosis, studies have shown that astragaloside can counteract stroke-induced apoptosis by decreasing the activities of caspase-3 and caspase-8, balancing the ratio of Bcl-2 and Bax expression, and decreasing the release of mitochondrial pro-apoptotic factors [31].
Astragaloside exhibits a unique mechanism in the treatment of stroke-induced dementia by protecting the blood-brain barrier (BBB). Stroke leads to the disruption of the tight junctions of the BBB and increases permeability, which triggers brain edema and neurological damage. Astragaloside inhibits the overexpression of matrix metalloproteinase-9 (MMP-9) and aquaporin 4 (AQP-4) and maintains the tight junction complex [32]. Together, these effects maintain the integrity of the BBB and alleviate tissue damage and cognitive impairment after stroke.
Astragaloside ameliorates stroke-induced cognitive impairment by promoting cell proliferation through multiple mechanisms. Its effects include upregulating the expression of BDNF, vascular endothelial growth factor (VEGF) and its receptor VEGFR2, which in turn promotes the proliferation and differentiation of neural stem cells (NSCs), enhances neovascularization, and inhibits neural cell apoptosis [33,34]. In addition, astragaloside supports the transformation of bone marrow MSCs to neural cells by regulating the Notch-1 signaling pathway [35]. Astragaloside also increases the proliferation rate and neural regeneration capacity of NSCs by regulating the EGFR/MAPK signaling pathway [36]. It also reduces axonal damage by inhibiting the RhoA signaling pathway and enhances synaptic plasticity and neuronal maturation by activating BDNF-TrkB signaling [37,38].
Iron death in cerebral ischemia-reperfusion injury (CIRI) exacerbates the onset of ischemic stroke and is one of the important pathological mechanisms of CIRI. Studies have shown that AST IV effectively inhibited iron death in human neuroblastoma cell line (SH-SY5Y) cells and a rat model, resulting in improved behavioral function. The mechanism of action of AST IV involves activation of the P62/Keap1/Nrf2 pathway, which enhances Nrf2 activation to inhibit iron death and ultimately attenuates neurological damage [39]. This mechanism provides a new perspective for improving the treatment of cognitive impairment.
3.2. Brain-damaging diseases caused by other factors
Radiation exposure can lead to DNA damage, neuronal structural changes and related gene dysfunction, which in turn trigger oxidative stress and inflammatory responses. These damages contribute to brain cell apoptosis and neuronal growth arrest, and ultimately lead to cognitive deficits [40,41].AS-IV provides neuroprotection through activation of the BDNF-TrkB pathway, which alleviates radiation-induced cognitive impairment [42]. In addition, AS-IV inhibits radiation-induced cell proliferation and reduces neuronal arrest by modulating the extracellular regulatory protein kinase (ERK) signaling pathway, which has a positive effect on delaying the development of cognitive impairment. The mechanism includes decreasing the expression of p-ERK and cell cycle protein-dependent kinase 2 (CDK2), as well as up-regulating cell cycle protein-dependent kinase inhibitor (p21) and RB to reduce the number of cells positive for markers of cellular aging [43].AS-IV also reduces apoptosis and neuronal structural damage by regulating JNK-p38 phosphorylation, thereby alleviating the radiation-induced adverse effects of radiation [44].
In type II diabetes-induced cognitive impairment, astragaloside has also shown significant improvement. It was pointed out that astragaloside reduced oxidative stress and neuroinflammation by regulating the Nrf2/Keap1/HO1/NQO1 pathway, thereby protecting brain function in diabetic mice [45]. The results showed that astragaloside increased superoxide dismutase (SOD) activity, decreased malondialdehyde (MDA) levels, reduced oxidative damage, and improved insulin resistance. These effects were associated with astragaloside elevating growth hormone-releasing peptide levels in the brain, which ultimately promotes the repair of neurological damage and delays the progression of cognitive impairment [46].
For traumatic brain injury (TBI), studies have shown that inflammatory factors such as IL-6, IL-1β and TNF-α are increased, microglia are activated, and endoplasmic reticulum (ER) stress-related proteins (p-PERK, p-eIF2a, ATF4, ATF6, and p-IRE1a) levels were also significantly elevated.AS-IV treatment inhibited the expression of these inflammatory factors and related proteins, while altering the polarization status of microglia/macrophages.These results suggest that AS-IV attenuates neuroinflammation and brain damage after TBI through the PERK pathway, and neurological dysfunction is improved in TBI mice [47].
Central nervous system dysfunction due to heat stroke is often difficult to treat. However, AS-IV attenuated heatstroke-induced neuroinflammation and brain damage by activating the PI3K/AKT pathway and promoting M2 microglia polarization. This mechanism provides a new therapeutic avenue for heatstroke-associated neurological injury [48].
In addition, AS-IV effectively attenuates lead poisoning-induced oxidative stress and consequent cognitive impairment by targeting Nrf2. Experiments showed that AS-IV was able to attenuate lead-induced inhibition of neurite growth, which further demonstrated its potential role in protecting neurite growth and cognitive function [49-51].
4. Conclusion and outlook
In this study, a literature review revealed that astragaloside demonstrated effective neuroprotective effects in multiple mechanisms of degenerative and non-degenerative diseases, such as Alzheimer's disease, Parkinson's disease, vascular dementia, and other factor-induced brain injuries.
It should be noted, however, that most of the current studies have focused on animal and cellular experiments, and there are still relatively few examples of astragaloside being used as a Chinese medicine monomer in clinical studies. The complexity of compound preparations limits, to some extent, the clear understanding of the mechanism of action of single components and the precise assessment of the potency of action. In the future, clinical studies on astragaloside monomer should be strengthened, clinical trial design should be enhanced, pharmacokinetic studies and clinical safety and efficacy evaluations of different doses should be promoted, so as to fully explore its potential and unique advantages in the treatment of dementia. With further in-depth studies, it is expected to promote greater breakthroughs in the treatment of dementia with astragaloside. This will not only set a new benchmark for the application of TCM monomers in modern medicine, but also provide more diversified options and strategies for the treatment of dementia patients in an aging society.
References
[1]. National Medical Journal of China. (2018). 2018 Chinese guidelines for diagnosis and treatment of dementia and cognitive impairment (I): Dementia and its diagnostic criteria for classification. National Medical Journal of China, 98(13), 965-970.
[2]. Yao, M., Zhang, L., & Wang, L. (2023). Astragaloside IV: A promising natural neuroprotective agent for neurological disorders. Biomedicine & Pharmacotherapy, 159, 114229.
[3]. Hugo, J., & Ganguli, M. (2014). Dementia and cognitive impairment. Clinics in Geriatric Medicine, 30(3), 421-442.
[4]. Lane, C. A., Hardy, J., & Schott, J. M. (2018). Alzheimer’s disease. European Journal of Neurology, 25(1), 59-70.
[5]. Li, E. L., & Li, Z. G. (2024). Research progress on the correlation between mitochondrial dysfunction and Alzheimer's disease. World Science and Technology - Modernization of Traditional Chinese Medicine, 12, 3025-3033.
[6]. Bao, M., Bade, R., Liu, H. (2023). Astragaloside IV against Alzheimer’s disease via microglia-mediated neuroinflammation using network pharmacology and experimental validation. European Journal of Pharmacology, 957, 175992.
[7]. F, C., D, Y., XY, C. (2021). Astragaloside IV ameliorates cognitive impairment and neuroinflammation in an oligomeric Aβ induced Alzheimer’s disease mouse model via inhibition of microglial activation and NADPH oxidase expression. Biological & Pharmaceutical Bulletin, 44(11).
[8]. Wang, X., Wang, Y., Hu, J.P., Yu, S., Li, B.K., Cui, Y., Ren, L., Zhang, L.D. (2017). Astragaloside IV, a natural PPARγ agonist, reduces Aβ production in Alzheimer’s disease through inhibition of BACE1. Molecular Neurobiology, 54(4):2939-2949. doi: 10.1007/s12035-016-9874-6
[9]. Li, K. L., Zhou, Y. T., Li, Y. R. (2023). Research progress on the mechanism of action of Astragaloside in treating central nervous system diseases. Traditional Chinese Medicine, 45(8), 2634-2641.
[10]. Costa, I. M., Lima, F. O. V., Fernandes, L. C. B. (2019). Astragaloside IV supplementation promotes a neuroprotective effect in experimental models of neurological disorders: A systematic review. Current Neuropharmacology, 17(7), 648-665.
[11]. Sun, Q., Jia, N., Wang, W. (2014). Protective effects of Astragaloside IV against amyloid Beta1-42 neurotoxicity by inhibiting the mitochondrial permeability transition pore opening. PLOS ONE, 9(6).
[12]. Hayashi, Y. (2020). Effects of neural stem cell transplantation in Alzheimer’s disease models. Journal of Biomedical Science, 27(1), 29.
[13]. Wang, X., Gao, F., Xu, W. (2023). Deciphering the effects of Astragaloside IV on AD-like phenotypes: A systematic and experimental investigation. Oxidative Medicine and Cellular Longevity, 2021(1):1020614. doi: 10.1155/2021/1020614
[14]. Kumar, S., Goyal, L., & Singh, S. (2022). Tremor and rigidity in patients with Parkinson’s disease: Emphasis on epidemiology, pathophysiology and contributing factors. CNS & Neurological Disorders Drug Targets, 21(7), 596-609.
[15]. Cui, X. M., Wang, Y. L., Han, B. (2014). Excitotoxicity in Parkinson's disease and hepatocyte growth factor. Chinese Journal of Practical Neurological Diseases, 17(4), 102-103.
[16]. Zhang, C. Y., Zhu, X. Y., Zhai, T. (2024). Research progress on the mechanism of Astragalus in the treatment of Parkinson's disease. West China Journal of Pharmaceutical Sciences, 39(5), 611-616.
[17]. Tansey, M. G., Wallings, R. L., Houser, M. C. (2022). Inflammation and immune dysfunction in Parkinson disease. Nature Reviews Immunology, 22(11), 657-673.
[18]. Xu, Z., Yang, D., Huang, X. (2021). Astragaloside IV protects 6-hydroxydopamine-induced SH-SY5Y cell model of Parkinson’s disease via activating the JAK2/STAT3 pathway. Frontiers in Neuroscience, 15, 631501.
[19]. Xia, L., Guo, D., & Chen, B. (2017). Neuroprotective effects of astragaloside IV on Parkinson disease models of mice and primary astrocytes. Experimental and Therapeutic Medicine, 14(6), 5569-5575.
[20]. Zhang, T. Q., Li, C. C., Zhang, T. F. (2021). [Mechanism of astragaloside Ⅳ alleviating PC12 cell injury by activating PI3K/AKT signaling pathway: Based on network pharmacology and in vitro experiments]. Zhongguo Zhong Yao Za Zhi, 46(24), 6465-6473.
[21]. Ge, B., Li, S. L., & Li, F. R. (2020). Astragaloside-IV regulates endoplasmic reticulum stress-mediated neuronal apoptosis in a murine model of Parkinson’s disease via the lincRNA-p21/CHOP pathway. Experimental and Molecular Pathology, 115, 104478. https://doi.org/10.1016/j.yexmp.2020.104478
[22]. Dehay, B., Martinez-Vicente, M., Caldwell, G. A. (2013). Lysosomal impairment in Parkinson’s disease. Movement Disorders, 28(6), 725-732.
[23]. Xia, M. L., Xie, X. H., Ding, J. H. (2020). Astragaloside IV inhibits astrocyte senescence: Implication in Parkinson’s disease. Journal of Neuroinflammation, 17, 105.
[24]. Liu, Y., Chong, L., Li, X. (2017). Astragaloside IV rescues MPP+-induced mitochondrial dysfunction through upregulation of methionine sulfoxide reductase A. Experimental and Therapeutic Medicine, 14(3), 2650-2656.
[25]. Yang, C., Mo, Y., Xu, E. (2019). Astragaloside IV ameliorates motor deficits and dopaminergic neuron degeneration via inhibiting neuroinflammation and oxidative stress in a Parkinson’s disease mouse model. International Immunopharmacology, 75, 105651.
[26]. Ma, Q., Tang, M. K., & Sun, W. Y. (2018). Modern research summary on the pathogenesis of vascular dementia from the perspective of traditional Chinese medicine. Chinese Journal of Traditional Chinese Medicine and Pharmacy, 33(1), 212-215.
[27]. Huang, X. P., Ding, H., Wang, B. (2015). Effects of the main active components combinations of Astragalus and Panax notoginseng on energy metabolism in brain tissues after cerebral ischemia-reperfusion in mice. Pharmacognosy Magazine, 11(44), 732.
[28]. Xue, B., Huang, J., Ma, B. (2019). Astragaloside IV protects primary cerebral cortical neurons from oxygen and glucose deprivation/reoxygenation by activating the PKA/CREB pathway. Neuroscience, 404, 326-337.
[29]. Yang, J., Shao, C., Li, W., Wan, H., He, Y., Yang, J. (2021). Protective effects of Astragaloside IV against oxidative injury and apoptosis in cultured astrocytes by regulating Nrf2/JNK signaling. Experimental Brain Research, 239(6):1827-1840. doi: 10.1007/s00221-021-06096-7
[30]. Tang, B., Tang, W. J., Tang, Y. H. (2019). Astragaloside alleviates cerebral ischemia-reperfusion injury in rats and inhibits NF-κB phosphorylation and NLRP3 inflammasome activation. Acta Physiologica Sinica, 71(3), 424-430.
[31]. Yin, F., Zhou, H., Fang, Y. (2020). Astragaloside IV alleviates ischemia reperfusion-induced apoptosis by inhibiting the activation of key factors in death receptor pathway and mitochondrial pathway. Journal of Ethnopharmacology, 248, 112319.
[32]. Li, M., Ma, R.N., Li, L.H., Qu, Y.Z., Gao, G.D. (2013). Astragaloside IV reduces cerebral edema post-ischemia/reperfusion correlating the suppression of MMP-9 and AQP4. European Journal of Pharmacology, 715(1-3):189-95. doi: 10.1016/j.ejphar.2013.05.022
[33]. Li, L., Gan, H., Jin, H. (2021). Astragaloside IV promotes microglia/macrophages M2 polarization and enhances neurogenesis and angiogenesis through PPARγ pathway after cerebral ischemia/reperfusion injury in rats. International Immunopharmacology, 92, 107335.
[34]. Sun, L., Wang, L., Li, Y. (2014). Protective effects and mechanisms of Astragaloside on cerebral ischemia reperfusion injury in rats. Chinese Journal of Clinical Neuroscience, 22(1), 43-49.
[35]. Li, P. T., Gao, Z. H., Duan, X. L. (2018). Experimental study on differentiation of SAMR1 mouse bone marrow mesenchymal stem cells into neurons induced by Astragaloside IV. Journal of Hebei Medical University, 39(11), 1338-1343.
[36]. Chen, X., Wu, H., Chen, H., Wang, Q., Xie, X.J., Shen, J. (2019). Astragaloside VI promotes neural stem cell proliferation and enhances neurological function recovery in transient cerebral ischemic injury via activating EGFR/MAPK signaling cascades. Molecular Neurobiology, 56(4):3053-3067. doi: 10.1007/s12035-018-1294-3
[37]. Ni, G. X., Liang, C., Wang, J. (2020). Astragaloside IV improves neurobehavior and promotes hippocampal neurogenesis in MCAO rats though BDNF-TrkB signaling pathway. Biomedicine & Pharmacotherapy, 130, 110353.
[38]. Gan, H. X. (2022). Mechanism study on Astragaloside-mediated RhoA pathway promotion of neurite outgrowth [Doctoral dissertation, Hubei University for Nationalities].
[39]. Wang, L., Liu, C., Wang, L., Tang, B. (2023). Astragaloside IV mitigates cerebral ischaemia-reperfusion injury via inhibition of P62/Keap1/Nrf2 pathway-mediated ferroptosis. European Journal of Pharmacology, 944, 175516.
[40]. Oyefeso, F. A., Goldberg, G., Opoku, N. Y. P. S. (2023). Effects of acute low-moderate dose ionizing radiation to human brain organoids. PLOS ONE, 18(5), e0282958.
[41]. Kempf, S. J., Azimzadeh, O., Atkinson, M. J. (2013). Long-term effects of ionising radiation on the brain: Cause for concern? Radiation and Environmental Biophysics. 52(2013), 5-16.
[42]. Liu, X., Ding, Y., Jiang, C. (2024). Astragaloside IV mediates radiation-induced neuronal damage through activation of BDNF-TrkB signaling. Phytomedicine, 132, 155803.
[43]. Ding, Y., Jiang, C., Chen, L. (2025). Astragaloside IV confers neuroprotection against radiation-induced neuronal senescence via the ERK pathway. Experimental Neurology, 386, 115135.
[44]. Liu, X., Chu, W., Shang, S. (2020). Preliminary study on the anti-apoptotic mechanism of Astragaloside IV on radiation-induced brain cells. International Journal of Immunopathology and Pharmacology, 34, 2058738420954594.
[45]. Zhang, Y., Yuan, Y., Zhang, J. , Fu, J. (2022). Astragaloside IV supplementation attenuates cognitive impairment by inhibiting neuroinflammation and oxidative stress in type 2 diabetic mice. Frontiers in Aging Neuroscience, 14:1004557. doi: 10.3389/fnagi.2022.1004557
[46]. Zhang, R., Cao, S., Shi, Y. (2023). Astragaloside IV-mediated inhibition of oxidative stress by upregulation of ghrelin in type 2 diabetes–induced cognitive impairment. Naunyn-Schmiedeberg's Archives of Pharmacology, 396(10), 2637-2650.
[47]. Zhao, J., Zhao, G., Lang, J. (2024). Astragaloside IV ameliorated neuroinflammation and improved neurological functions in mice exposed to traumatic brain injury by modulating the PERK-eIF2α-ATF4 signaling pathway. Journal of Investigative Medicine, 72(7), 747-762.
[48]. Wang, Z., Luo, Z., Tan, Y., He, G., Li, P., Liu, X., Shen, T., Liu, Y., Yang, X., Luo, X. (2024). Astragaloside IV alleviates heatstroke brain injury and neuroinflammation in male mice by regulating microglial polarization via the PI3K/Akt signaling pathway. Biomedicine & Pharmacotherapy, 180: 117545. doi: 10.1016/j.biopha.2024.117545
[49]. Yu, C., Pan, S., Dong, M. (2017). Astragaloside IV attenuates lead acetate-induced inhibition of neurite outgrowth through activation of Akt-dependent Nrf2 pathway in vitro. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1863(6), 1195-1203.
[50]. Liang, Y., Zou, Y., Niu, C., Niu, Y. (2019). Astragaloside IV and ferulic acid synergistically promote neurite outgrowth through Nrf2 activation. Mechanisms of Ageing and Development, 180: 70-81. doi: 10.1016/j.mad.2019.04.002.
[51]. Yu, C., Zhang, J., Li, X. (2021). Astragaloside IV-induced Nrf2 nuclear translocation ameliorates lead-related cognitive impairments in mice. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 1868(1), 118853.
Cite this article
Chen,Z.;Baranenko,D.;Lu,W. (2025). Progress in the treatment of dementia with astragaloside. Journal of Clinical Technology and Theory,3(1),39-44.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Journal:Journal of Clinical Technology and Theory
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. National Medical Journal of China. (2018). 2018 Chinese guidelines for diagnosis and treatment of dementia and cognitive impairment (I): Dementia and its diagnostic criteria for classification. National Medical Journal of China, 98(13), 965-970.
[2]. Yao, M., Zhang, L., & Wang, L. (2023). Astragaloside IV: A promising natural neuroprotective agent for neurological disorders. Biomedicine & Pharmacotherapy, 159, 114229.
[3]. Hugo, J., & Ganguli, M. (2014). Dementia and cognitive impairment. Clinics in Geriatric Medicine, 30(3), 421-442.
[4]. Lane, C. A., Hardy, J., & Schott, J. M. (2018). Alzheimer’s disease. European Journal of Neurology, 25(1), 59-70.
[5]. Li, E. L., & Li, Z. G. (2024). Research progress on the correlation between mitochondrial dysfunction and Alzheimer's disease. World Science and Technology - Modernization of Traditional Chinese Medicine, 12, 3025-3033.
[6]. Bao, M., Bade, R., Liu, H. (2023). Astragaloside IV against Alzheimer’s disease via microglia-mediated neuroinflammation using network pharmacology and experimental validation. European Journal of Pharmacology, 957, 175992.
[7]. F, C., D, Y., XY, C. (2021). Astragaloside IV ameliorates cognitive impairment and neuroinflammation in an oligomeric Aβ induced Alzheimer’s disease mouse model via inhibition of microglial activation and NADPH oxidase expression. Biological & Pharmaceutical Bulletin, 44(11).
[8]. Wang, X., Wang, Y., Hu, J.P., Yu, S., Li, B.K., Cui, Y., Ren, L., Zhang, L.D. (2017). Astragaloside IV, a natural PPARγ agonist, reduces Aβ production in Alzheimer’s disease through inhibition of BACE1. Molecular Neurobiology, 54(4):2939-2949. doi: 10.1007/s12035-016-9874-6
[9]. Li, K. L., Zhou, Y. T., Li, Y. R. (2023). Research progress on the mechanism of action of Astragaloside in treating central nervous system diseases. Traditional Chinese Medicine, 45(8), 2634-2641.
[10]. Costa, I. M., Lima, F. O. V., Fernandes, L. C. B. (2019). Astragaloside IV supplementation promotes a neuroprotective effect in experimental models of neurological disorders: A systematic review. Current Neuropharmacology, 17(7), 648-665.
[11]. Sun, Q., Jia, N., Wang, W. (2014). Protective effects of Astragaloside IV against amyloid Beta1-42 neurotoxicity by inhibiting the mitochondrial permeability transition pore opening. PLOS ONE, 9(6).
[12]. Hayashi, Y. (2020). Effects of neural stem cell transplantation in Alzheimer’s disease models. Journal of Biomedical Science, 27(1), 29.
[13]. Wang, X., Gao, F., Xu, W. (2023). Deciphering the effects of Astragaloside IV on AD-like phenotypes: A systematic and experimental investigation. Oxidative Medicine and Cellular Longevity, 2021(1):1020614. doi: 10.1155/2021/1020614
[14]. Kumar, S., Goyal, L., & Singh, S. (2022). Tremor and rigidity in patients with Parkinson’s disease: Emphasis on epidemiology, pathophysiology and contributing factors. CNS & Neurological Disorders Drug Targets, 21(7), 596-609.
[15]. Cui, X. M., Wang, Y. L., Han, B. (2014). Excitotoxicity in Parkinson's disease and hepatocyte growth factor. Chinese Journal of Practical Neurological Diseases, 17(4), 102-103.
[16]. Zhang, C. Y., Zhu, X. Y., Zhai, T. (2024). Research progress on the mechanism of Astragalus in the treatment of Parkinson's disease. West China Journal of Pharmaceutical Sciences, 39(5), 611-616.
[17]. Tansey, M. G., Wallings, R. L., Houser, M. C. (2022). Inflammation and immune dysfunction in Parkinson disease. Nature Reviews Immunology, 22(11), 657-673.
[18]. Xu, Z., Yang, D., Huang, X. (2021). Astragaloside IV protects 6-hydroxydopamine-induced SH-SY5Y cell model of Parkinson’s disease via activating the JAK2/STAT3 pathway. Frontiers in Neuroscience, 15, 631501.
[19]. Xia, L., Guo, D., & Chen, B. (2017). Neuroprotective effects of astragaloside IV on Parkinson disease models of mice and primary astrocytes. Experimental and Therapeutic Medicine, 14(6), 5569-5575.
[20]. Zhang, T. Q., Li, C. C., Zhang, T. F. (2021). [Mechanism of astragaloside Ⅳ alleviating PC12 cell injury by activating PI3K/AKT signaling pathway: Based on network pharmacology and in vitro experiments]. Zhongguo Zhong Yao Za Zhi, 46(24), 6465-6473.
[21]. Ge, B., Li, S. L., & Li, F. R. (2020). Astragaloside-IV regulates endoplasmic reticulum stress-mediated neuronal apoptosis in a murine model of Parkinson’s disease via the lincRNA-p21/CHOP pathway. Experimental and Molecular Pathology, 115, 104478. https://doi.org/10.1016/j.yexmp.2020.104478
[22]. Dehay, B., Martinez-Vicente, M., Caldwell, G. A. (2013). Lysosomal impairment in Parkinson’s disease. Movement Disorders, 28(6), 725-732.
[23]. Xia, M. L., Xie, X. H., Ding, J. H. (2020). Astragaloside IV inhibits astrocyte senescence: Implication in Parkinson’s disease. Journal of Neuroinflammation, 17, 105.
[24]. Liu, Y., Chong, L., Li, X. (2017). Astragaloside IV rescues MPP+-induced mitochondrial dysfunction through upregulation of methionine sulfoxide reductase A. Experimental and Therapeutic Medicine, 14(3), 2650-2656.
[25]. Yang, C., Mo, Y., Xu, E. (2019). Astragaloside IV ameliorates motor deficits and dopaminergic neuron degeneration via inhibiting neuroinflammation and oxidative stress in a Parkinson’s disease mouse model. International Immunopharmacology, 75, 105651.
[26]. Ma, Q., Tang, M. K., & Sun, W. Y. (2018). Modern research summary on the pathogenesis of vascular dementia from the perspective of traditional Chinese medicine. Chinese Journal of Traditional Chinese Medicine and Pharmacy, 33(1), 212-215.
[27]. Huang, X. P., Ding, H., Wang, B. (2015). Effects of the main active components combinations of Astragalus and Panax notoginseng on energy metabolism in brain tissues after cerebral ischemia-reperfusion in mice. Pharmacognosy Magazine, 11(44), 732.
[28]. Xue, B., Huang, J., Ma, B. (2019). Astragaloside IV protects primary cerebral cortical neurons from oxygen and glucose deprivation/reoxygenation by activating the PKA/CREB pathway. Neuroscience, 404, 326-337.
[29]. Yang, J., Shao, C., Li, W., Wan, H., He, Y., Yang, J. (2021). Protective effects of Astragaloside IV against oxidative injury and apoptosis in cultured astrocytes by regulating Nrf2/JNK signaling. Experimental Brain Research, 239(6):1827-1840. doi: 10.1007/s00221-021-06096-7
[30]. Tang, B., Tang, W. J., Tang, Y. H. (2019). Astragaloside alleviates cerebral ischemia-reperfusion injury in rats and inhibits NF-κB phosphorylation and NLRP3 inflammasome activation. Acta Physiologica Sinica, 71(3), 424-430.
[31]. Yin, F., Zhou, H., Fang, Y. (2020). Astragaloside IV alleviates ischemia reperfusion-induced apoptosis by inhibiting the activation of key factors in death receptor pathway and mitochondrial pathway. Journal of Ethnopharmacology, 248, 112319.
[32]. Li, M., Ma, R.N., Li, L.H., Qu, Y.Z., Gao, G.D. (2013). Astragaloside IV reduces cerebral edema post-ischemia/reperfusion correlating the suppression of MMP-9 and AQP4. European Journal of Pharmacology, 715(1-3):189-95. doi: 10.1016/j.ejphar.2013.05.022
[33]. Li, L., Gan, H., Jin, H. (2021). Astragaloside IV promotes microglia/macrophages M2 polarization and enhances neurogenesis and angiogenesis through PPARγ pathway after cerebral ischemia/reperfusion injury in rats. International Immunopharmacology, 92, 107335.
[34]. Sun, L., Wang, L., Li, Y. (2014). Protective effects and mechanisms of Astragaloside on cerebral ischemia reperfusion injury in rats. Chinese Journal of Clinical Neuroscience, 22(1), 43-49.
[35]. Li, P. T., Gao, Z. H., Duan, X. L. (2018). Experimental study on differentiation of SAMR1 mouse bone marrow mesenchymal stem cells into neurons induced by Astragaloside IV. Journal of Hebei Medical University, 39(11), 1338-1343.
[36]. Chen, X., Wu, H., Chen, H., Wang, Q., Xie, X.J., Shen, J. (2019). Astragaloside VI promotes neural stem cell proliferation and enhances neurological function recovery in transient cerebral ischemic injury via activating EGFR/MAPK signaling cascades. Molecular Neurobiology, 56(4):3053-3067. doi: 10.1007/s12035-018-1294-3
[37]. Ni, G. X., Liang, C., Wang, J. (2020). Astragaloside IV improves neurobehavior and promotes hippocampal neurogenesis in MCAO rats though BDNF-TrkB signaling pathway. Biomedicine & Pharmacotherapy, 130, 110353.
[38]. Gan, H. X. (2022). Mechanism study on Astragaloside-mediated RhoA pathway promotion of neurite outgrowth [Doctoral dissertation, Hubei University for Nationalities].
[39]. Wang, L., Liu, C., Wang, L., Tang, B. (2023). Astragaloside IV mitigates cerebral ischaemia-reperfusion injury via inhibition of P62/Keap1/Nrf2 pathway-mediated ferroptosis. European Journal of Pharmacology, 944, 175516.
[40]. Oyefeso, F. A., Goldberg, G., Opoku, N. Y. P. S. (2023). Effects of acute low-moderate dose ionizing radiation to human brain organoids. PLOS ONE, 18(5), e0282958.
[41]. Kempf, S. J., Azimzadeh, O., Atkinson, M. J. (2013). Long-term effects of ionising radiation on the brain: Cause for concern? Radiation and Environmental Biophysics. 52(2013), 5-16.
[42]. Liu, X., Ding, Y., Jiang, C. (2024). Astragaloside IV mediates radiation-induced neuronal damage through activation of BDNF-TrkB signaling. Phytomedicine, 132, 155803.
[43]. Ding, Y., Jiang, C., Chen, L. (2025). Astragaloside IV confers neuroprotection against radiation-induced neuronal senescence via the ERK pathway. Experimental Neurology, 386, 115135.
[44]. Liu, X., Chu, W., Shang, S. (2020). Preliminary study on the anti-apoptotic mechanism of Astragaloside IV on radiation-induced brain cells. International Journal of Immunopathology and Pharmacology, 34, 2058738420954594.
[45]. Zhang, Y., Yuan, Y., Zhang, J. , Fu, J. (2022). Astragaloside IV supplementation attenuates cognitive impairment by inhibiting neuroinflammation and oxidative stress in type 2 diabetic mice. Frontiers in Aging Neuroscience, 14:1004557. doi: 10.3389/fnagi.2022.1004557
[46]. Zhang, R., Cao, S., Shi, Y. (2023). Astragaloside IV-mediated inhibition of oxidative stress by upregulation of ghrelin in type 2 diabetes–induced cognitive impairment. Naunyn-Schmiedeberg's Archives of Pharmacology, 396(10), 2637-2650.
[47]. Zhao, J., Zhao, G., Lang, J. (2024). Astragaloside IV ameliorated neuroinflammation and improved neurological functions in mice exposed to traumatic brain injury by modulating the PERK-eIF2α-ATF4 signaling pathway. Journal of Investigative Medicine, 72(7), 747-762.
[48]. Wang, Z., Luo, Z., Tan, Y., He, G., Li, P., Liu, X., Shen, T., Liu, Y., Yang, X., Luo, X. (2024). Astragaloside IV alleviates heatstroke brain injury and neuroinflammation in male mice by regulating microglial polarization via the PI3K/Akt signaling pathway. Biomedicine & Pharmacotherapy, 180: 117545. doi: 10.1016/j.biopha.2024.117545
[49]. Yu, C., Pan, S., Dong, M. (2017). Astragaloside IV attenuates lead acetate-induced inhibition of neurite outgrowth through activation of Akt-dependent Nrf2 pathway in vitro. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1863(6), 1195-1203.
[50]. Liang, Y., Zou, Y., Niu, C., Niu, Y. (2019). Astragaloside IV and ferulic acid synergistically promote neurite outgrowth through Nrf2 activation. Mechanisms of Ageing and Development, 180: 70-81. doi: 10.1016/j.mad.2019.04.002.
[51]. Yu, C., Zhang, J., Li, X. (2021). Astragaloside IV-induced Nrf2 nuclear translocation ameliorates lead-related cognitive impairments in mice. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 1868(1), 118853.









