1. Introduction
The intestinal barrier (IB) is an important part of defense for the maintenance of homeostasis of the intestinal microenvironment, consisting of physical, chemical, immune, microbial and intestinal vascular barriers [1]. IB is broadly divided into three interacting layers: the lumen layer of commensal microorganisms, the mucus layer of static water, glycocalyx and mucus, and the epithelial layer [2]. The intestinal barrier responds to and interacts with different intestinal stimuli and microbiomes to protect human health while absorbing nutrients. The passage of pathogenic bacteria and endotoxins through the intestinal wall into the bloodstream may trigger a range of systemic hazards [3]. Disruption of the intestinal barrier provides conditions for the proliferation of gram-negative bacteria in the intestinal lumen [4], and the systemic inflammatory response further triggered by the endotoxins they produce can activate the release of various inflammatory factors (e.g., leukotrienes, prostaglandins), leading to subacute and chronic inflammatory responses, which can lead to severe complications such as multiple organ dysfunction syndrome (MODS), as well as sepsis, shock, etc. [5] . In addition, endotoxin damages the liver, heart and kidneys, inducing metabolic disorders, liver damage and organ failure [6]. The intestinal barrier can prevent pathogenic bacteria and endotoxins, etc. from penetrating the intestinal wall and entering human tissues, organs and microcirculation, etc. by maintaining or increasing the favorable flora colonising microorganisms in the intestine [7-8]. Under normal conditions, the intestinal barrier regulates the digestion and absorption of nutrients and prevents the invasion of pathogens and the displacement of harmful substances from the intestine to other tissues and organs through the blood circulation. The intestinal barrier also induces immunoglobulin A (IgA) production and regulates the expression of anti-inflammatory factors, which promotes adaptive immunity in the gut by providing low levels of immune stimulation[9-10], preventing damage to the body from pathogenic antigens. However, damage to the intestinal barrier triggers strong inflammatory reactions, metabolic disorders, autoimmune diseases and even exacerbates organic pathologies.
Inflammatory factors, a group of biologically active molecules produced and released during an inflammatory response [11]. Inflammatory factors play an important role in regulating the immune response, promoting tissue repair and fighting infection [12]. They are involved in the development of inflammation by regulating the activity of immune cells and promoting the recruitment and activation of inflammatory cells [13]. There are various types of inflammatory factors, mainly including cytokines and chemokines. Cytokines are small molecular polypeptides with broad biological activity synthesised and secreted by immune cells and non-immune cells of the organism, which carry out information transfer between organisms and cells, participate in the physiological and pathological processes of the organism, and play an important regulatory role in the immune system [14]. According to their role in the inflammatory response, cytokines can be divided into pro-inflammatory factors and anti-inflammatory factors. Pro-inflammatory factors include interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-alpha) etc. [15-16]. And the anti-inflammatory factors include interleukin-4 (IL-4), interleukin-10 (IL-10) and transforming growth factor-β (TFG-β), etc. [17]. Cytokines, as an important part of the body's natural immune response, play a considerable role in the immune defence process [18]. Chemokines are able to attract immune cells to migrate towards the site of inflammation and mainly include members of the CXC and CC families [19]. They enhance the local immune response by binding to receptors on the surface of immune cells and directing them towards areas of inflammation [20].
Intestinal inflammation (e.g. inflammatory bowel disease IBD) is often accompanied by local and systemic inflammatory responses [21]. Numerous studies have shown that IBD patients have increased levels of pro-inflammatory cytokines and decreased levels of anti-inflammatory cytokines, and the imbalance between the two is the main cause of inflammation [22]. The interaction between cytokines leads to a cascading amplification effect of the inflammatory response, also known as the waterfall response. Cytokines such as TNF-α and IL-6 are at the central of the inflammatory f storm response and are known as pro-inflammatory cytokines (Pro-inflammatory cytokines), which are increased in expression and release during the occurrence of inflammatory responses, interacting with the intestinal immune cells and the epithelial cells, leading to impairment of barrier function [23-24]. Decrease in anti-inflammatory cytokines such as IL-10 and IL-4 leads to dysregulation of immune responses in the gut, resulting in an overactive immune system that is unable to effectively suppress inflammatory responses, leading to persistence and exacerbation of chronic inflammation [25]. Therefore, reducing the expression and activity of pro-inflammatory cytokines, such as TNF-α and IL-6, while promoting the production of anti-inflammatory factors, such as IL-10 and IL-4, is considered to be an effective protective mechanism(Figure 1). The reduction of pro-inflammatory cytokines facilitates the reduction of inflammation, while the enhancement of anti-inflammatory cytokines maintains the immune homeostasis in the intestinal tract, and the synergistic effect of the two helps to restore the metabolism of the intestinal barrier and further aids in the maintenance of the integrity of the intestinal barrier.
The inflammatory response is a physiological protective mechanism for the body's response to external injury, infection or autoimmune reaction, but when it is excessive or out of control, it may lead to chronic disease or tissue damage [26]. The inflammatory pathway is a series of response processes in which cytokines and signaling molecules transmit inflammatory signals inside and outside the cell, in which pro-inflammatory cytokines, such as TNF-α and IL-6, play a central role in the process. TNF-α activates the NF-κB and c-Jun pathways by binding to its receptor TNFR1, triggering the expression of a variety of pro-inflammatory cytokines such as IL-1, IL-6, GM-CSF(Granulocyte-macrophage Colony Stimulating Factor), etc., which further exacerbate the inflammatory respons [27]. CSF, etc., which further exacerbate the inflammatory response and trigger metabolic changes through multiple pathways [28]. Specifically, TNF-α and IL-6 can stimulate the upregulation of CD36(cluster of differentiation 36) expression in tissues such as the liver and kidney, increase fatty acid uptake, and lead to the accumulation of lipids in the liver, which may trigger non-alcoholic fatty liver disease (NAFLD) [29]. In addition, inflammatory factors affect insulin signaling through activation of the JNK/NF-κB(nuclear factor kappa-B) signaling pathway, leading to insulin resistance, which is particularly critical in metabolic syndrome [30]. Chronic low-intensity metabolic inflammation, induced by metabolic disorders caused by nutrient and energy excess, is closely associated with metabolic diseases such as intestinal inflammation, atherosclerosis, type 2 diabetes mellitus, obesity, and others [31]. Therefore, inflammatory factors not only play an amplifying role in the inflammatory response, but also exacerbate the occurrence and development of metabolic diseases by promoting metabolic disorders to form a vicious circle.
This review elaborates the role of modulating pro-inflammatory factors IL-6 and TNF-α as well as anti-inflammatory factors IL-4 and IL-10 in promoting intestinal barrier restoration and treating intestinal diseases, and summarises the effects of modulating inflammatory factors on cell signaling pathways and intestinal microbiota. The limitations of modulating inflammatory factors in different intestinal barrier injury diseases and the challenges faced in clinical application are analysed in depth, providing technical references for modulating inflammatory factors to restore the intestinal barrier.
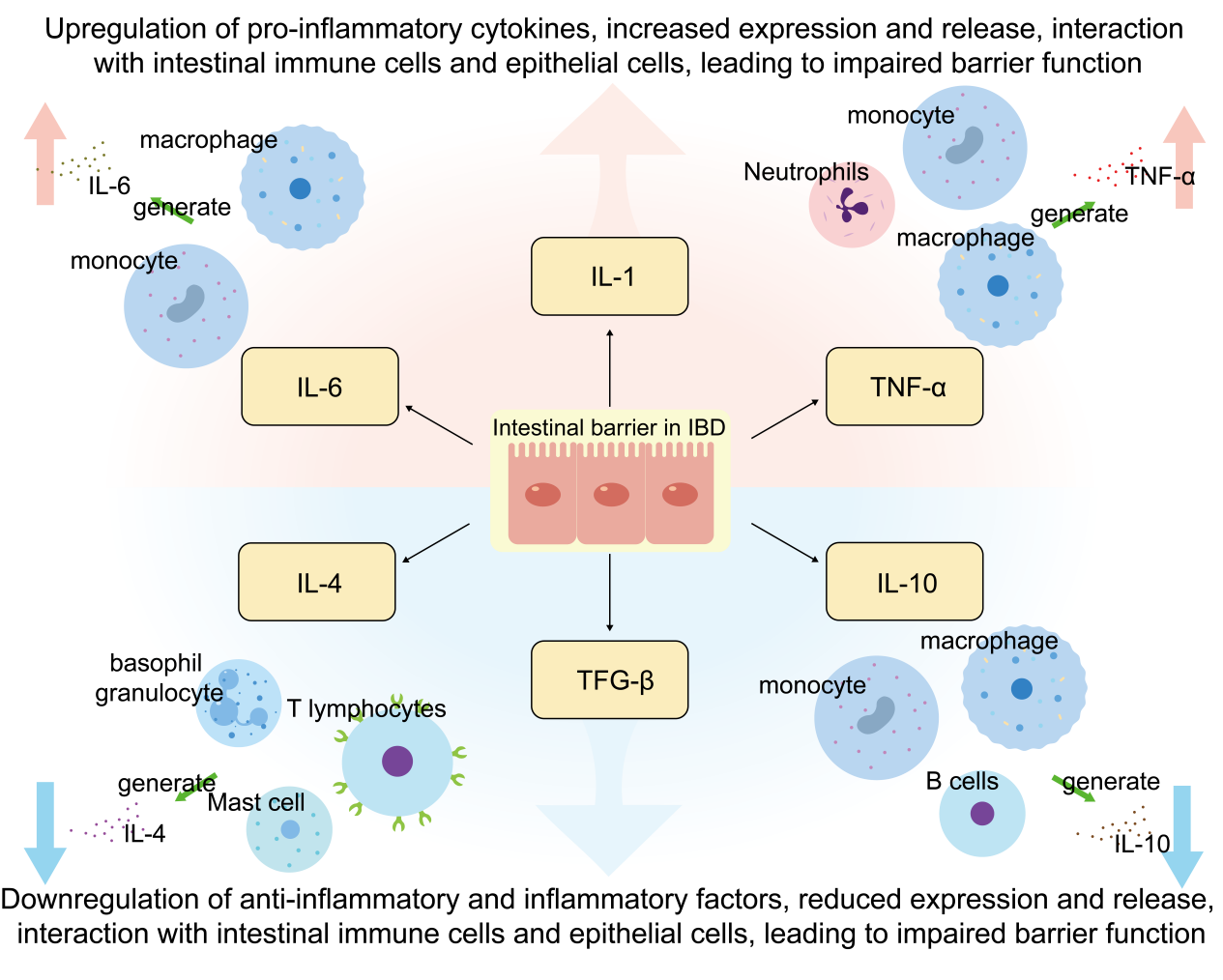
2. Mechanisms of action of inflammatory factors
2.1. Tumour Necrosis Factor (TNF-α)
TNF-α is a multifunctional pro-inflammatory cytokine and a key inflammatory factor in the pathogenesis of IBD, which up-regulates the expression of the tight junction protein claudin-2, leading to the formation of cation-selective channels and increased cellular interstitial permeability, which is associated with barrier dysfunction [32]. TNF-α overproduction is a major cause of tissue damage and is produced by activated macrophages and monocytes (Figure 2). It not only triggers a cascade response at the nuclear and subcellular levels, inducing upregulation of the expression of a variety of inflammatory factors, but can also be sufficient to activate neutrophils and lymphocytes and act on endothelial cells, enhancing the permeability of vascular endothelial cells and facilitating the exudation of inflammatory cells from the vasculature into the tissues, leading to localised ischaemia and thrombosis [33]. As a key regulator of intestinal epithelial cell proliferation and apoptosis, TNF-α is involved in the initiation and persistence of intestinal inflammation and influences the function of the intestinal barrier. In IBD, TNF-α acts locally in the intestinal mucosa mainly through paracrine and autocrine modes, triggering chemotaxis of neutrophils and monocytes and increasing the expression of intercellular adhesion molecules, thus exacerbating the inflammatory response [34]. In addition, TNF-α induces inflammatory responses, tissue cell survival and proliferation, and immune defence against pathogens by activating NF-κB and MAPKs signaling pathways [35]. Meanwhile, TNF-α activates inflammatory cells and upregulates adhesion factors, nitric oxide (NO) and oxygen free radicals, which further damage tissues and may cause sepsis and multiple organ dysfunction syndrome (MODS).
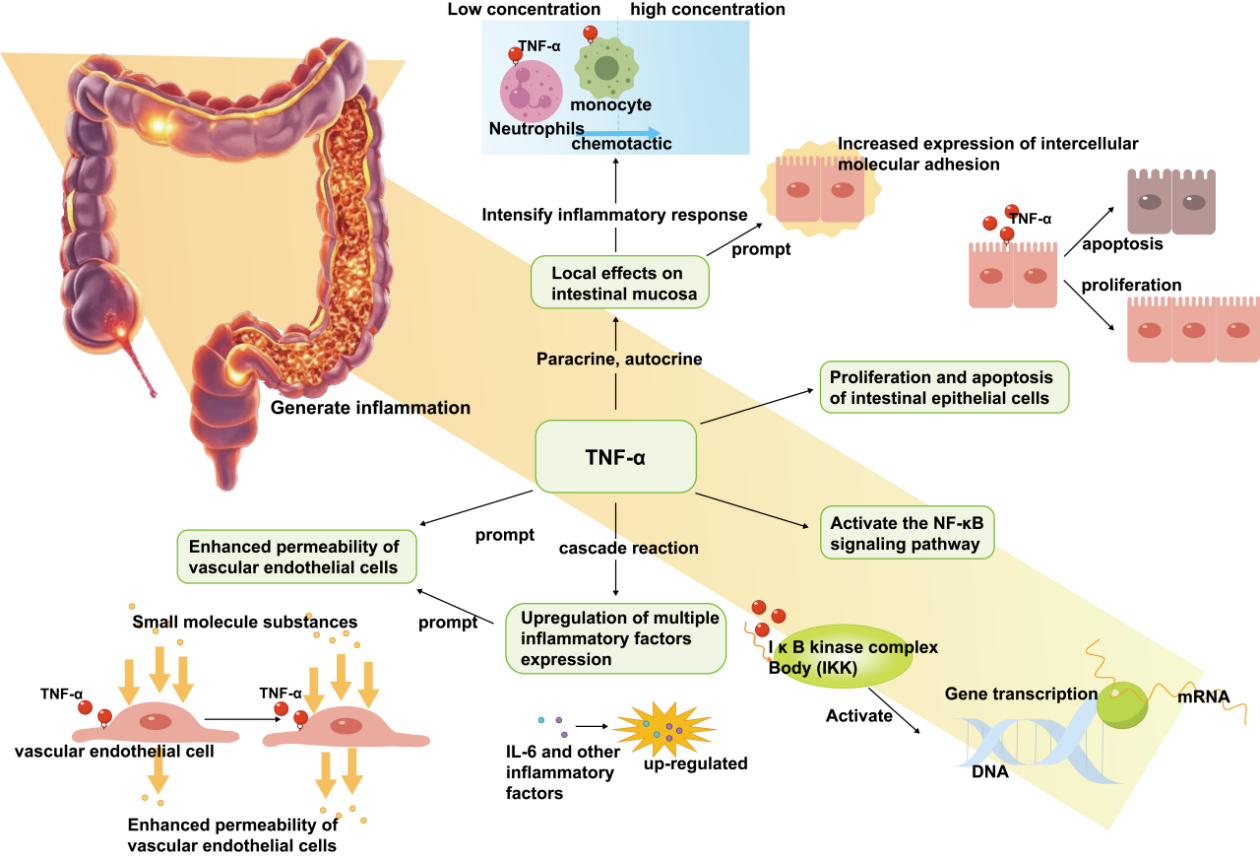
2.2. Interleukin-6 (IL-6)
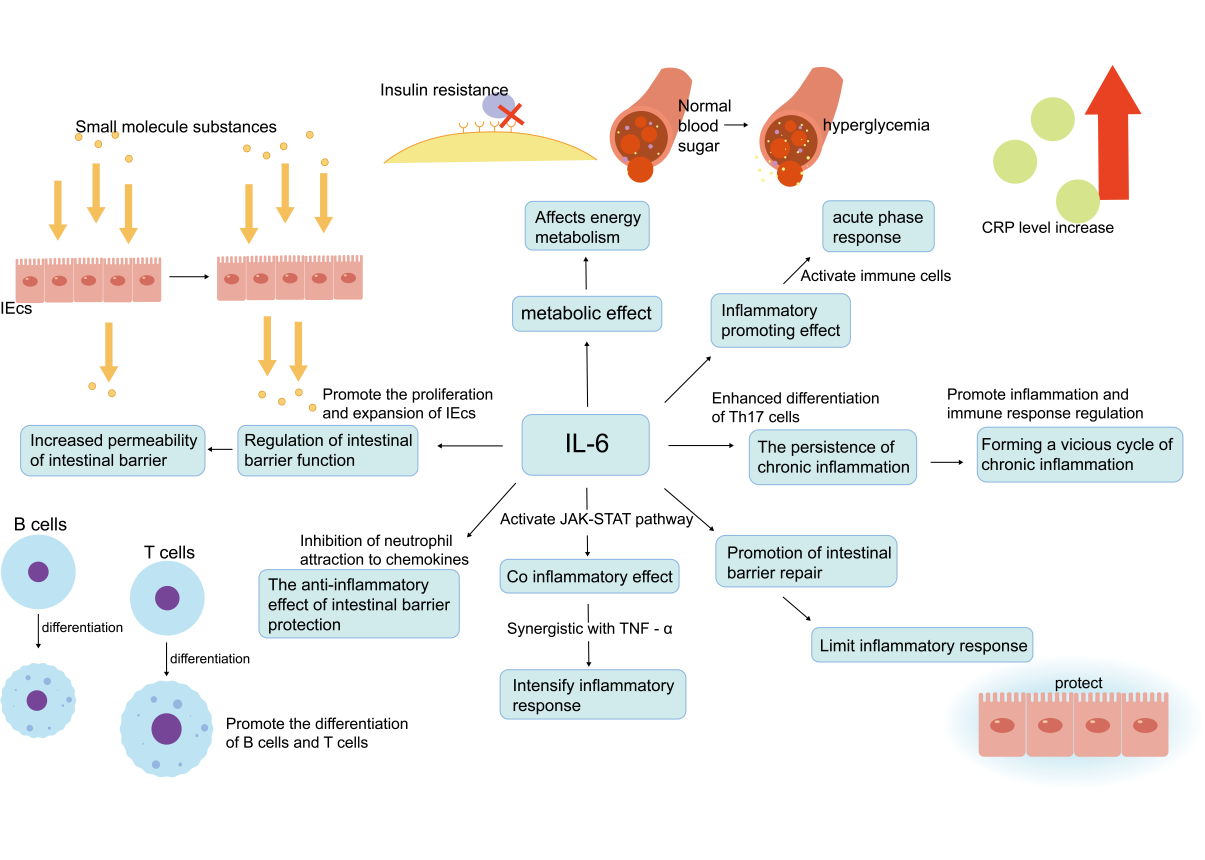
IL-6 plays a crucial role in intestinal barrier function and inflammatory bowel disease (IBD).IL-6 is released by immune cells (e.g., macrophages) along with TNF-α and is involved in both acute and chronic inflammatory responses.IL-6 activates immune cells by increasing the synthesis of acute-phase proteins (e.g., the C-reactive protein CRP) [36], exacerbates inflammatory responses and contributes to chronic inflammatory responses by activating Th17 cell differentiation and regulation of T-cell function, which, together with TNF-α, promotes the persistence of chronic inflammation [37]. IL-6 also affects energy metabolism by influencing glucose metabolism and fatty acid metabolism leading to insulin resistance and hyperglycaemia (Figure 3).
In addition, IL-6 plays a dual role in regulating intestinal barrier function; on the one hand, it may exacerbate the increased permeability of the intestinal barrier by affecting the function of intestinal epithelial cells (IECs), leading to the translocation of intestinal contents and bacterial products and further activating inflammatory responses. On the other hand, it plays an important role in host defence by regulating the immune and inflammatory response, promoting the proliferation and expansion of IECs and maintaining the homeostatic function of the intestinal barrier [37]. IL-6 may exacerbate tissue damage in the early stages of inflammation, but ultimately promotes the abatement of inflammation and the initiation of tissue repair, regulating the production of macrophage cell types, particularly M2-type macrophages, which are involved in tissue repair [38]. IL-6 signaling is essential for intestinal epithelial repair and regeneration, helping to restore the integrity of the intestinal barrier by promoting the proliferation and differentiation of IECs [39]. In addition, IL-6 protects the intestinal barrier from further damage by inhibiting neutrophil attraction of chemokines, promoting monocyte-directed chemokine production, and T-cell and B-cell differentiation(Figure 2), targeting the immune response to specific exogenous antigens, and limiting the inflammatory response [40].
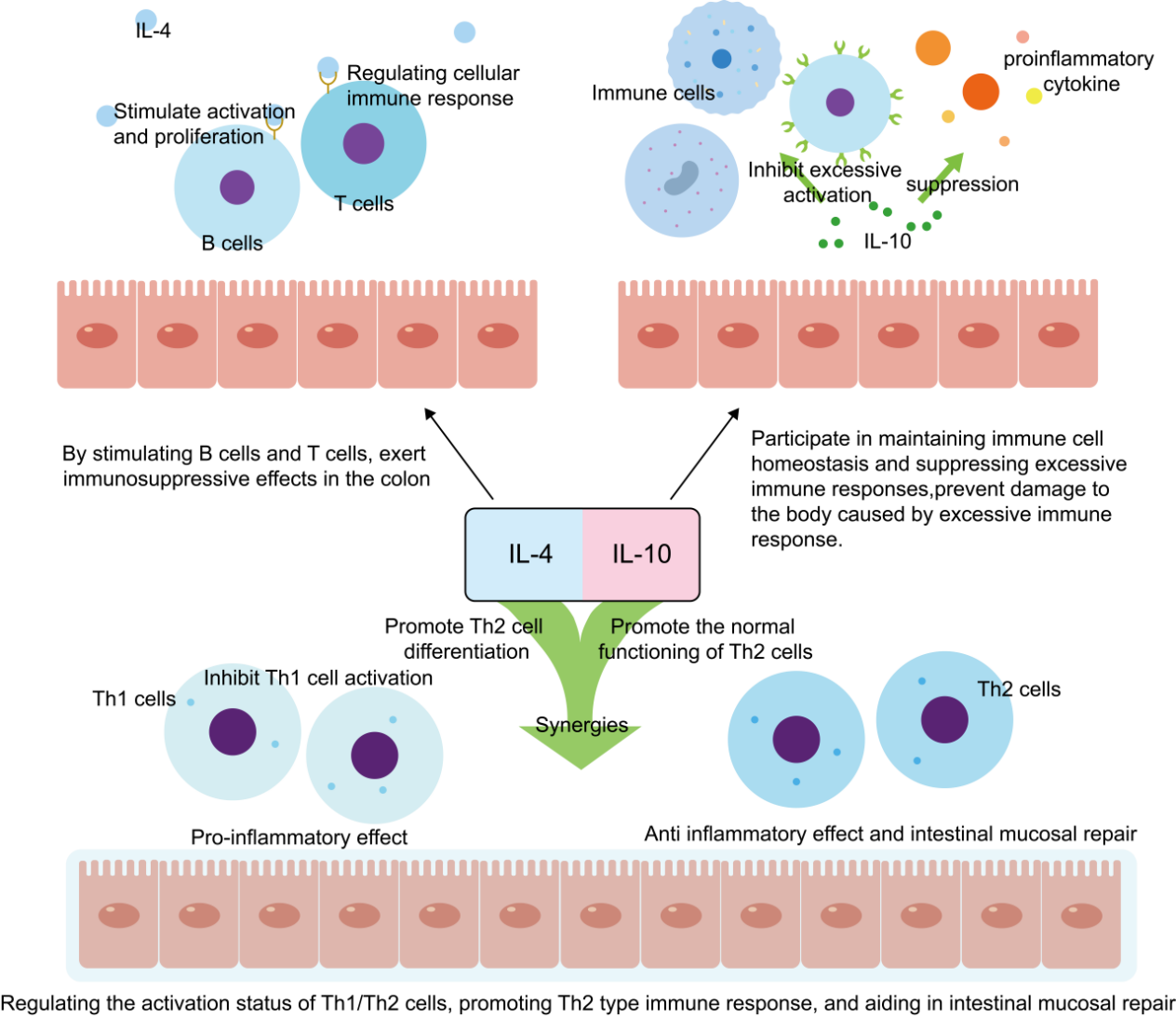
2.3. IL-4 and IL-10
IL-4 is an anti-inflammatory factor produced by activated T-lymphocytes and mast cells, as well as basophils, which exerts an immunosuppressive effect in the colon by stimulating B- and T-cells [41]. IL-10 is a multifunctional negative regulator produced by Th2, monocytes, B-cells, macrophages, etc., which participates in the maintenance of immune cell homeostasis, the suppression of over-immune responses, the It prevents damage to the organism due to excessive immune response. At the same time, it plays an important role in regulating cell growth and differentiation, and participating in inflammatory and immune responses(Figure 4).
IL-4 acts synergistically with IL-10 to regulate the activation state of Th1/Th2 cells, promoting Th2-type immune responses and contributing to intestinal mucosal repair [42], while in the acute inflammatory phase of lung injury, IL-4 expression is increased, showing an important role in inflammation and fibrosis [43], decreasing T-cell infiltration and inflammatory factor production but in the later stages of fibrosis, the IL-4 and IL-13 may promote the fibrotic process, showing their complex roles at different stages.
3. Intestinal barrier repair and inflammation elimination
3.1. Pharmacological interventions
Studies have shown that down-regulating the expression of inflammatory factors by targeting the site where inflammation occurs promotes the termination of the inflammatory state as well as the stabilisation of the normal state of the gut. A common mechanism for down-regulating inflammatory factors is through the use of anti-inflammatory drugs or natural compounds, e.g., glucocorticosteroids inhibit the inflammatory response by modulating the production of the anti-inflammatory metabolite, itaconic acid [44], and non-steroidal anti-inflammatory drugs decrease prostaglandin synthesis by inhibiting the activity of cyclooxygenase [45], which results in a reduction of pain and inflammation. This reduces intestinal inflammation and improves the intestinal barrier. In addition, certain natural anti-inflammatory compounds, such as soybean saponin Ab through the Nrf2 / HO-1 / NQO1 signaling pathway; tea polyphenols modulate e.g. nuclear factor-κB, activator protein 1, signal transduction and transcriptional activator-related signaling pathways; and curcumin inhibits IL-2, IL-6, IL-8, IL-12, TNF-α, macrophage inhibitory protein (MIP) and monocyte chemotaxis protein-1 (MCP-1) [46]. Pro-inflammatory cytokine and chemokine production, etc, which promotes the transition from inflammatory to normal homeostatic metabolism at the intestinal site and restores intestinal barrier function. Many small molecule drugs have been developed to regulate the restoration of the intestinal barrier, such as dichloromethane [47], allyl isothiocyanate [48], heptaphyllum saponin, etc. They can reduce oxidative stress and enhance the protection of intestinal epithelial cells by activating the Nrf2-mediated NQO1 pathway [49]. In addition, small molecule drugs targeting gut-specific inflammatory pathways, such as specific anti-IL-1β monoclonal antibodies, have also shown potential to promote intestinal barrier restoration in clinical trials [50]. In addition, immunomodulators, such as anti-TNF-α antibodies (e.g. infliximab) and IL-12/IL-23 inhibitors, are also used clinically for the treatment of enterocolitis, and are effective in reducing the levels of inflammatory factors and improving intestinal barrier function [51]. Among them, IL-12/IL-23 blockers show potential in the treatment of IBD, and they protect the intestinal barrier function by inhibiting the differentiation and activity of Th1 and Th17 cells and reducing the production of inflammatory factors [52].
3.2. Nutritional interventions
Proper nutritional therapy can improve the nutritional status of the patient and reduce the inflammatory response. For example, enteral nutrition(EEN) can induce remission of Crohn's disease (CD), allowing the inflammatory response of the intestinal mucosa to subside [53]. Probiotics promote nutrient absorption and reduce intestinal inflammation by restoring intestinal microecological balance [54]. Certain specific probiotic strains (e.g., Bifidobacterium, Lactobacillus,etc) have been found to enhance the tight junctions of intestinal epithelial cells, thereby improving the function of the intestinal barrier [55]. Prebiotics, as one of the nutritional sources of probiotics, can also influence immune stimulation, gut barrier enhancement and alteration of gastrointestinal flora, and these effects seem to depend on bacterial composition and altered short-chain fatty acid (SCFA) production [56].
In addition, the intake of specific nutrients is also beneficial in reducing inflammation levels, such as Omega-3 fatty acids [57-58] (EPA and DHA) which promote the restoration of the intestinal barrier by inhibiting the synthesis of inflammatory mediators and slowing down the chronic inflammatory state [59]. Vitamin D also plays an important role in regulating immune responsesand inflammation [60,61]. Studies have shown that vitamin D modulates the intestinal immune response, reduces the release of inflammatory factors, promotes the repair of epithelial cells, maintains lower levels of chronic inflammation [62], and enhances the body's anti-inflammatory capacity. In addition, nutrients such as zinc and L-glutamine help regenerate epithelial cells and maintain the integrity of the intestinal barrier.
3.3. Lifestyle changes
As an important line of defence for human health, the intestinal barrier plays a key role in maintaining the stability of the intestinal internal environment and preventing the invasion of harmful substances [63]. The occurrence of inflammation often affects the structure and function of the intestinal barrier, and immune cells also play an important role in it [64]. In addition to the use of medications and other nutrients to help repair the intestinal barrier, changes in daily lifestyle can be made to reduce inflammation or improve immunity as a way to promote the restoration of the intestinal barrier function.
Strategies such as dietary changes, moderate exercise and stress reduction are all beneficial in reducing inflammation, which in turn aids in the repair and maintenance of the intestinal barrier. Changes to the daily diet that are rich in fruits, vegetables, nuts and fish, especially whole grains, can effectively reduce inflammatory water. Regular physical activity not only improves fitness, but also helps regulate the immune system and lower inflammation levels. Meanwhile, managing stress is an important strategy for down-regulating inflammation, and some studies have shown that in inflammatory bowel disease (IBD), psychological stress is associated with increased disease flare-ups [65].
4. Experimental study of the treatment of intestinal inflammation by restoring the intestinal barrier
4.1. Experimental research
4.1.1. Results of animal experiments
In recent years, studies have shown that inhibition of intestinal inflammatory factors plays an important role in intestinal barrier repair. Several animal experiments have found that certain natural compounds and drugs significantly improve intestinal barrier function by down-regulating inflammatory factors [66,67]. For example, isochaetene (ISO) attenuated Crohn's disease-like colitis induced by trinitrobenzene sulfonic acid (TNBS) in mice. It enhances intestinal barrier integrity by decreasing the expression of pro-inflammatory factors such as TNF-α, IFN-γ, IL-1β, and IL-6 and enhancing the expression of intestinal tight junction proteins such as ZO-1 and claudin-1 [68]. In addition, quercetin attenuates LPS-induced inflammatory responses and modulates the intestinal microbiota by inhibiting ERK, c-Jun amino-terminal kinase (JNK) and p38 phosphorylation [69]. The anti-inflammatory effect was further confirmed by improving gut health through down-regulation of inflammatory factors. In addition, infliximab (Infliximab), which inhibits the production of inflammatory factors by administering it to mice, also demonstrated a favourable therapeutic effect on IBD [70]. The results showed that the drug was able to significantly down-regulate the level of inflammatory factors in the intestine, while promoting the proliferation and differentiation of intestinal epithelial cells and accelerating the repair process of the intestinal barrier.
In addition, natural substances such as jujube polysaccharides and lycium barbarum polysaccharides reduce the synthesis and release of inflammatory factors by inhibiting the activation of the NF-κB signaling pathway, thereby protecting the intestinal barrier [71]. Similarly, thujaplicins reduce inflammatory responses and promote intestinal barrier repair by inhibiting the NF-κB signaling pathway and decreasing the levels of TNF-α, IL-6 and IL-1β. Dihydroquercetin (DHQ), on the other hand, improves intestinal dysbiosis and attenuates colitis by modulating the PI3K-Akt signaling pathway [72], further illustrating the potential for modulating the intestinal barrier by inhibiting inflammatory signaling pathways.
In addition to this, probiotics, a common intervention, have been shown to have positive effects on gut health [73]. For example, by feeding mice a diet containing Lactobacillus rhamnosus (Lactobacillus rhamnosus), a study found that these probiotics were able to significantly down-regulate the expression levels of inflammatory factors, such as IL-1β, IL-6, and TNF-α, in the gut. The down-regulation of inflammatory factors not only reduced intestinal inflammation, but also promoted the repair of the intestinal barrier. By inhibiting the growth of pathogenic bacteria and increasing the abundance of beneficial bacteria, probiotics not only improve the intestinal microecological environment, but also enhance the intestinal barrier function by increasing the expression of tight junction proteins and reducing intestinal permeability. Studies have shown that probiotics such as Bifidobacterium bifidum and Lactobacillus rhamnosus can significantly reduce intestinal inflammation [74] and improve the integrity of the intestinal barrier.
In addition, other experimental studies have revealed the role of specific molecules and nutrients in intestinal barrier repair, e.g., glutamine-fortified enteral nutrients enhance the expression of intestinal epithelial occludin proteins and promote intestinal barrier recovery [75]. In contrast, the Dclk1 gene in intestinal Tuft cells plays a key role in intestinal barrier repair and inflammatory regulation. It was found that mice knocked out of this gene showed inhibition of intestinal epithelial cell proliferation, delayed mucosal repair and increased permeability [76], further emphasising the importance of this gene in intestinal barrier function.
The results of these animal experiments not only confirm the promotive effect of reducing inflammatory factor levels on the restoration of intestinal barrier function, but also provide key insights for a deeper understanding of the association between intestinal inflammation and intestinal barrier function. These studies provide a scientific basis for the development of therapeutic strategies for diseases associated with intestinal inflammation (e.g., inflammatory bowel disease, Crohn's disease, etc.).
4.1.2. Clinical trial data
Although animal experiments have yielded remarkable results, the application of strategies to down-regulate inflammatory factors in the treatment of human intestinal diseases requires further clinical trial validation [77]. Several clinical trials have already begun to explore the potential of downregulating inflammatory factors in the treatment of intestinal diseases. For example, a clinical trial in patients with ulcerative colitis (UC) found that administration of a specific probiotic preparation to patients significantly down-regulated inflammatory factor levels in the intestines and reduced intestinal inflammatory symptoms. It was also observed that the intestinal barrier function of the patients was restored to a certain extent, with a decrease in intestinal permeability and an increase in the expression of tight junction proteins.
Hypoxia inducible factor (FIH and PHD1-3) are cellular oxygen sensors that confer hypoxia sensitivity to the hypoxia inducible factors HIF-1α and HIF-2α. Microenvironmental hypoxia strongly affects epithelial and immune cell function through HIF-dependent gene expression, thereby influencing the disease progression process in ulcerative colitis (UC). It was found that in a mouse model of DSS-induced colitis, treatment with HIF-1α stabilisers (e.g. DMOG) resulted in a significant reduction of inflammation and restoration of the intestinal barrier function, suggesting that HIF-1α has a protective effect on the intestinal tract. In contrast, reduced inflammation and restoration of barrier function were also observed after treatment of the same mouse model with a HIF-2α inhibitor (e.g., PT2385), further confirming that HIF-2α disrupts the intestinal barrier function and promotes the disease process in IBD [78]. In addition, a number of clinical trials in patients with Crohn's disease (CD) have found that down-regulation of inflammatory factor levels by medication or dietary modification has resulted in improvement of intestinal symptoms and restoration of intestinal barrier function after treatment. The key finding was that in non-responders, the TNFRSF1B gene was significantly down-regulated in both inflamed and non-inflamed colonic tissues, whereas the FCGR3A and interleukin 1B genes were significantly up-regulated in inflamed colonic regions. In addition, in vitro studies further revealed that anti-TNF drugs significantly reduced the expression of TNFRSF1B and FCGR3A genes in non-responders. These results suggest that the expression levels of TNFRSF1B, FCGR3A, and interleukin 1B genes may serve as important predictors of response to anti-TNF therapy in patients with Crohn's disease (CD) [79]. These clinical trial data further support the important role of down-regulating inflammatory factors in the treatment of intestinal diseases. However, it is important to note that the exact effects and mechanisms of down-regulated inflammatory factors in the treatment of intestinal diseases still need to be further investigated and validated at this time due to the complexity and individual differences of clinical trials. In the future, with more high-quality clinical trials, we are expected to gain a deeper understanding of the potential and mechanisms of down-regulated inflammatory factors in the treatment of intestinal diseases.
4.2. Dual mechanisms of intestinal barrier regulation
4.2.1. Effects of inflammatory signaling pathways
Intestinal barrier function is closely related to inflammatory signaling pathways. Inflammatory signaling pathways (e.g. NF-κB, MAPK, etc.) directly affect the integrity of the intestinal barrier and the inflammatory state by regulating the function, structure and immune response of intestinal epithelial cells. The most typical is the NF-κB signaling pathway, which acts as a major inflammatory regulator of cells and promotes the release of a variety of inflammatory mediators [80], such as tumour necrosis factor (TNF), interleukins (IL-1, IL-6, etc.), which further exacerbate local inflammatory responses through stimulation of receptors, such as Toll-like receptor (TLR), in intestinal epithelial cells [81]. It has been shown that persistent activation of NF-κB is closely associated with the loss of intestinal barrier function, as NF-κB is able to up-regulate the expression of proteins associated with cellular junctions (e.g., adhesion molecules, tight junction proteins), leading to disruption of cellular junctions and reduction of barrier function.
In addition, MAPK signaling pathways (e.g. ERK, JNK and p38 pathways) also play an important role in intestinal inflammation. Activation of these pathways can lead to the upregulation of pro-inflammatory factors and affect the intestinal barrier repair process by altering apoptosis, proliferation and migration behaviour [82]. Especially in acute enteritis or chronic inflammatory states, hyperactivation of the MAPK pathway is often associated with dysfunction of the intestinal barrier and persistence of inflammation [83].
Down-regulation of these pathways became an important strategy to restore the intestinal barrier function. Inhibition of NF-κB, MAPK, and other pathways can reduce the excessive release of inflammatory factors, thereby attenuating the damage to intestinal epithelial cells. For example, the use of NF-κB inhibitors (e.g., BAY 11-7082) or MAPK inhibitors (e.g., SP600125) has shown significant improvement in gut barrier function in experimental animals [84]. By these methods, the restoration of tight junction proteins (e.g., occludin, ZO-1) can be promoted, thereby enhancing the barrier integrity of the intestinal epithelium, attenuating intestinal permeability, and avoiding the invasion of harmful substances. The interactions and regulatory mechanisms between these signaling pathways still need to be further studied and explored.
4.2.2. Regulation of the intestinal microbiota
The gut microbiota, an important component of gut health, also plays an important role in the restoration of the intestinal barrier. Down-regulation of inflammatory factors may contribute to the restoration of the intestinal barrier by modulating the composition and structure of the gut microbiota [85]. On the one hand, down-regulation of inflammatory factors may reduce negative effects on the gut microbiota, such as reduced inhibition of beneficial bacteria and proliferation of harmful bacteria [86]. This effect helps to restore the balance and diversity of the intestinal microbiota and improves intestinal immunity and resistance. On the other hand, down-regulation of inflammatory factors may also promote the production of beneficial metabolites by the gut microbiota, such as short-chain fatty acids (SCFAs) [87]. These metabolites can improve the intestinal environment, promote the proliferation and differentiation of intestinal epithelial cells, and strengthen the function of the intestinal barrier. At the same time, they can inhibit the growth and reproduction of harmful bacteria, further maintaining intestinal health.
In summary, the promotional effect of down-regulated inflammatory factors on intestinal barrier recovery may involve multiple mechanisms, including the regulation of cellular signaling pathways and the improvement of the composition and structure of the intestinal microbiota. In the future, with deeper research and technological advances, we are expected to gain a more comprehensive understanding of these mechanisms and provide new ideas and approaches for the treatment of intestinal diseases. At the same time, it is also necessary to note the impact of factors such as individual differences and complexity on the results of the study in order to develop more personalised and precise treatment plans.
5. Summary
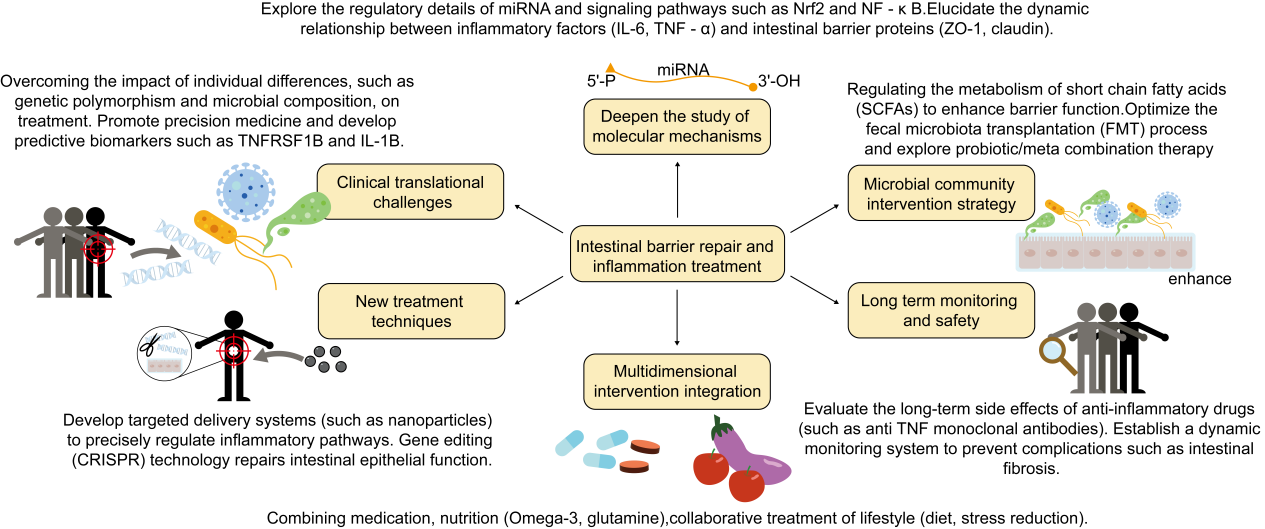
This curren review presents the role of down-regulation of inflammatory factors in promoting intestinal barrier recovery and treating intestinal diseases, discusses their effects on cell signaling pathways and gut microbiota, and analyses the limitations and challenges of the clinical application of this approach in different intestinal barrier-injury diseases. It was shown that by modulating inflammatory factors, intestinal inflammation can be reduced and intestinal barrier function can be enhanced, providing a new perspective for the treatment of IBD and other intestinal diseases. Maintaining the integrity of the intestinal barrier is essential for the prevention of inflammatory bowel disease, allergic reactions, metabolic syndrome, and other health problems. Therefore, inhibiting the expression and activity of these inflammatory factors or enhancing the expression and activity of anti-inflammatory factors is an effective strategy to protect and restore intestinal barrier integrity, reducing intestinal inflammation and enhancing barrier function. However, current research on the intestinal barrier and inflammatory factors is faced with a lack of model diversity, challenges in clinical applications, and limited research perspectives. To overcome these challenges, future research needs to expand disease models, deepen the understanding of the regulatory mechanisms of molecules such as miRNAs, and explore the interactions of gut microbial communities and their effects on the host, as well as develop new therapeutic approaches such as fecal transplants to optimise and personalise therapeutic strategies(Figure 5). This will provide more precise and effective strategies for the treatment of intestinal diseases.
References
[1]. Holmberg, F. E. O., & et al. (2018). Intestinal barrier integrity and inflammatory bowel disease: Stem cell-based approaches to regenerate the barrier.Journal of Tissue Engineering and Regenerative Medicine,12(4), 923–935.doi:10.1002/term.2506
[2]. Odenwald, M. A., & Turner, J. R. (2017). The intestinal epithelial barrier: a therapeutic target? Nature Reviews.Gastroenterology & Hepatology, 14(1), 9–21. doi:10.1002/term.2506
[3]. Dey, P. (2020). Targeting gut barrier dysfunction with phytotherapies: Effective strategy against chronic diseases.Pharmacological Research,161, 105135. doi: 10.1016/j.phrs.2020.105135
[4]. Li, L., & et al. (2007). Development of sorbent therapy for multiple organ dysfunction syndrome (MODS).Biomedical Materials (Bristol, England), 2(2), R12–R16. doi:10.1088/1748-6041/2/2/R02
[5]. Maier, R. V. (2000). Pathogenesis of multiple organ dysfunction syndrome--endotoxin, inflammatory cells, and their mediators: cytokines and reactive oxygen species. Surgical Infections, 1(3), 197–205; discussion 204–5. https: //doi.org/10.1089/109629600750018123
[6]. Wang, Y.-H. (2021). Current progress of research on intestinal bacterial translocation. Microbial Pathogenesis, 152, 104652. https: //doi.org/10.1016/j.micpath.2020.104652
[7]. Gou, H.-Z., & et al. (2022). How do intestinal probiotics restore the intestinal barrier?Frontiers in Microbiology,13, 929346. https: //doi.org/10.3389/fmicb.2022.929346
[8]. König, J., & et al. (2016). Human Intestinal Barrier Function in Health and Disease. Clinical and Translational Gastroenterology,7(10), e196. https: //doi.org/10.1038/ctg.2016.54
[9]. Chopyk, D. M., & Grakoui, A. (2020). Contribution of the Intestinal Microbiome and Gut Barrier to Hepatic Disorders.Gastroenterology,159(3), 849–863. https: //doi.org/10.1053/j.gastro.2020.04.077
[10]. An, J., & et al. (2022). The Role of Intestinal Mucosal Barrier in Autoimmune Disease: a Potential Target.Frontiers in Immunology,13, 871713. https: //doi.org/10.3389/fimmu.2022.871713
[11]. Kany, S., & et al. (2019). Cytokines in Inflammatory Disease.International Journal of Molecular Sciences,20(23), 6008. https: //doi.org/10.3390/ijms20236008
[12]. Oliver, A. J., & et al. (2024). Single-cell integration reveals metaplasia in inflammatory gut diseases.Nature, 635(8039), 699–707. https: //doi.org/10.1038/s41586-024-07571-1
[13]. Serhan, C. N., & et al. (2008). Anti-inflammatory and proresolving lipid mediators.Annual Review of Pathology,3, 279–312. https: //doi.org/10.1146/annurev.pathmechdis.3.121806.151409
[14]. Zheng, X., & et al. (2022). The use of supercytokines, immunocytokines, engager cytokines, and other synthetic cytokines in immunotherapy.Cellular & Molecular Immunology,19(2), 192–209. https: //doi.org/10.1038/s41423-021-00786-6
[15]. Wang, T., & He, C. (2018). Pro-inflammatory cytokines: the link between obesity and osteoarthritis.Cytokine & Growth Factor Reviews,44, 38–50. https: //doi.org/10.1016/j.cytogfr.2018.10.002
[16]. Jung, H. C., & et al. (1995). A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion.The Journal of Clinical Investigation,95(1), 55–65. https: //doi.org/10.1172/JCI117676
[17]. Jin, Y., & et al. (2014). Regulation of anti-inflammatory cytokines IL-10 and TGF-β in mouse dendritic cells through treatment with Clonorchis sinensis crude antigen.Experimental & Molecular Medicine,46(1), e74. https: //doi.org/10.1038/emm.2013.144
[18]. Chang, S. H., & Dong, C. (2011). Signaling of interleukin-17 family cytokines in immunity and inflammation.Cellular Signaling, 23(7), 1069–1075. https: //doi.org/10.1016/j.cellsig.2010.11.022
[19]. Zhou, C., & et al. (2023). The role of CXCL family members in different diseases.Cell Death Discovery,9(1), 212. https: //doi.org/10.1038/s41420-023-01524-9
[20]. Chen, K., & et al. (2018). Chemokines in homeostasis and diseases.Cellular & Molecular Immunology,15(4), 324–334. https: //doi.org/10.1038/cmi.2017.134
[21]. Neuman, Manuela G. (2007) "Immune dysfunction in inflammatory bowel disease."Translational research: the journal of laboratory and clinical medicine.149,(4), 173-86. doi: 10.1016/j.trsl.2006.11.009
[22]. Park, J. H., et al. (2017). IBD immunopathogenesis: A comprehensive review of inflammatory molecules.Autoimmunity Reviews, 16(4), 416–426. https: //doi.org/10.1016/j.autrev.2017.02.013
[23]. Matsukawa, A., et al. (1997). Analysis of the inflammatory cytokine network among TNF alpha, IL-1 beta, IL-1 receptor antagonist, and IL-8 in LPS-induced rabbit arthritis. Laboratory Investigation;a Journal of Technical Methods and Pathology,76(5), 629–638.
[24]. Utsunomiya, I., et al. (1998). Generation of inflammatory cytokines in zymosan-induced pleurisy in rats: TNF induces IL-6 and cytokine-induced neutrophil chemoattractant (CINC) in vivo.Cytokine,10(12), 956–963. https: //doi.org/10.1006/cyto.1998.0376
[25]. York, A. G., et al. (2024). IL-10 constrains sphingolipid metabolism to limit inflammation.Nature,627(8004), 628–635. https: //doi.org/10.1038/s41586-024-07098-5
[26]. Li, R., et al. (2020). Pro-Inflammatory Cytokines in the Formation of the Pre-Metastatic Niche.Cancers,12(12), 3752. https: //doi.org/10.3390/cancers12123752
[27]. Medzhitov, R. (2021). The spectrum of inflammatory responses.Science, 374(6571), 1070–1075. https: //doi.org/10.1126/science.abi5200
[28]. Liu, T., et al. (2017). NF-κB signaling in inflammation.Signal Transduction and Targeted Therapy,2, 17023. https: //doi.org/10.1038/sigtrans.2017.23
[29]. Schuster, S., et al. (2018). Triggering and resolution of inflammation in NASH. Nature Reviews.Gastroenterology & Hepatology, 15(6), 349–364. https: //doi.org/10.1038/s41575-018-0009-6
[30]. Xi, M., et al. (2023). Galactooligosaccharide Mediates NF-κB Pathway to Improve Intestinal Barrier Function and Intestinal Microbiota.Molecules, 28(22), 7611. https: //doi.org/10.3390/molecules28227611
[31]. Kawai, T., et al. (2021). Adipose tissue inflammation and metabolic dysfunction in obesity. American Journal of Physiology.Cell Physiology,320(3), C375–C391. https: //doi.org/10.1152/ajpcell.00379.2020
[32]. Zhang, C., et al. (2017). Inhibition of Autophagic Degradation Process Contributes to Claudin-2 Expression Increase and Epithelial Tight Junction Dysfunction in TNF-α Treated Cell Monolayers.International Journal of Molecular Sciences,18(1), 157. https: //doi.org/10.3390/ijms18010157
[33]. Van Loo, G., & Bertrand, M. J. M. (2023). Death by TNF: a road to inflammation. Nature Reviews.Immunology, 23(5), 289–303. https: //doi.org/10.1038/s41577-022-00792-3
[34]. Danese, S., et al. (2006). TNF-alpha blockade down-regulates the CD40/CD40L pathway in the mucosal microcirculation: a novel anti-inflammatory mechanism of infliximab in Crohn's disease.Journal of Immunology,176(4), 2617–2624. https: //doi.org/10.4049/jimmunol.176.4.2617
[35]. Swetha, K., et al. (2025). Selenium Mitigates Caerulein and LPS-induced Severe Acute Pancreatitis by Inhibiting MAPK, NF-κB, and STAT3 Signaling via the Nrf2/HO-1 Pathway. Biological Trace Element Research.Advance online publication. https: //doi.org/10.1007/s12011-025-04531-2
[36]. Heath, D. I., et al. (1993). Role of interleukin-6 in mediating the acute phase protein response and potential as an early means of severity assessment in acute pancreatitis.Gut,34(1), 41–45. https: //doi.org/10.1136/gut.34.1.41
[37]. Korn, T., et al. (2008). IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells.Proceedings of the National Academy of Sciences of the United States of America,105(47), 18460–18465. https: //doi.org/10.1073/pnas.0809850105
[38]. Mauer, J., et al. (2014). Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin.Nature Immunology, 15(5), 423–430. https: //doi.org/10.1038/ni.2865
[39]. Tanaka, T., & Kishimoto, T. (2014). The biology and medical implications of interleukin-6.Cancer Immunology Research, 2(4), 288–294. https: //doi.org/10.1158/2326-6066.CIR-14-0022
[40]. Perez-Penco, M., et al. (2025). The antitumor activity of TGFβ-specific T cells is dependent on IL-6 signaling.Cellular & Molecular Immunology, 22(1), 111–126. https: //doi.org/10.1038/s41423-024-01238-7
[41]. Yamanishi, Y., & Karasuyama, H. (2016). Basophil-derived IL-4 plays versatile roles in immunity.Seminars in Immunopathology, 38(5), 615–622. https: //doi.org/10.1007/s00281-016-0568-y
[42]. Inoue, S et al. (1999) "Characterization of cytokine expression in the rectal mucosa of ulcerative colitis: correlation with disease activity. "The American journal of gastroenterology.94(9), 2441-6. doi: 10.1111/j.1572-0241.1999. 01372.x
[43]. Huaux, F., Liu, T., & Phan, S. H. (2003). Dual roles of IL-4 in lung injury and fibrosis.Journal of Immunology (Baltimore, Md.: 1950), 170(4), 2083–2092. https: //doi.org/10.4049/jimmunol.170.4.2083
[44]. Auger, J.-P., Zhang, Y., Wang, X., Li, M., Zhao, Z., Yang, W., ... & Zhou, B. (2024). Metabolic rewiring promotes anti-inflammatory effects of glucocorticoids.Nature,629(8010), 184–192. https: //doi.org/10.1038/s41586-024-07282-7
[45]. Dahl, J. B., & Kehlet, H. (1991). Non-steroidal anti-inflammatory drugs: Rationale for use in severe postoperative pain.British Journal of Anaesthesia,66(6), 703–712. https: //doi.org/10.1093/bja/66.6.703
[46]. Abe, Y., Sagara, I., & Ito, Y. (1999). Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages.Pharmacological Research, 39(1), 41–47. https: //doi.org/10.1006/phrs.1998.0404
[47]. Zhang, Q., Zhang, L., Zhang, Y., Chen, D., & Wang, Y. (2017). Ethanol extract and its dichloromethane fraction of Alpinia oxyphylla Miquel exhibited hepatoprotective effects against CCl4-induced oxidative damage in vitro and in vivo with the involvement of Nrf2.Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 91, 812–822. https: //doi.org/10.1016/j.biopha.2017.04.131
[48]. Zhu, W.-T., Zhang, Y., Liu, Y., & Sun, Y. (2023). Effect of allyl isothiocyanate on oxidative stress in COPD via the AhR / CYP1A1 and Nrf2 / NQO1 pathways and the underlying mechanism.Phytomedicine: International Journal of Phytotherapy and Phytopharmacology,114, 154774. https: //doi.org/10.1016/j.phymed.2023.154774
[49]. Han, M. H., Park, G., Lee, H. J., Kim, G. Y., Cheong, J., Hong, S. H., ... & Choi, Y. H. (2017). Cytoprotective effects of esculetin against oxidative stress are associated with the upregulation of Nrf2-mediated NQO1 expression via the activation of the ERK pathway.International Journal of Molecular Medicine, 39(2), 380–386. https: //doi.org/10.3892/ijmm.2016.2834
[50]. Turner, J. R. (2009). Intestinal mucosal barrier function in health and disease. Nature Reviews.Immunology,9(11), 799–809. https: //doi.org/10.1038/nri2653
[51]. Luzentales-Simpson, M., Zhang, D., & Plevy, S. E. (2021). Vedolizumab: Potential Mechanisms of Action for Reducing Pathological Inflammation in Inflammatory Bowel Diseases.Frontiers in Cell and Developmental Biology,9, 612830. https: //doi.org/10.3389/fcell.2021.612830
[52]. Meng, F., Xu, L., Zhang, X., Tian, X., Guo, Y., Li, Y., ... & Zheng, L. (2023). Tumor necrosis factor-like cytokine 1A plays a role in inflammatory bowel disease pathogenesis.Proceedings of the National Academy of Sciences of the United States of America, 120(34), e2120771120. https: //doi.org/10.1073/pnas.2120771120
[53]. Cohen-Dolev, N., Hyams, J. S., Lerer, T., Mack, D. R., Griffiths, A. M., Markowitz, J., ... & Rufo, P. A. (2018). Differences in Outcomes Over Time with Exclusive Enteral Nutrition Compared with Steroids in Children with Mild to Moderate Crohn's Disease: Results from the GROWTH CD Study.Journal of Crohn's & Colitis,12(3), 306–312. https: //doi.org/10.1093/ecco-jcc/jjx150
[54]. Li, D., Wang, C., Wang, S., Ding, Z., Zhang, W., & Gao, C. (2016). The gut microbiota: A treasure for human health. Biotechnology Advances, 34(7), 1210–1224. https: //doi.org/10.1016/j.biotechadv.2016.08.003
[55]. Zuo, T., & Ng, S. C. (2018). The Gut Microbiota in the Pathogenesis and Therapeutics of Inflammatory Bowel Disease.Frontiers in Microbiology,9, 2247. https: //doi.org/10.3389/fmicb.2018.02247
[56]. Gao, J., Yin, Y., Zhang, Y., Liu, H., & Wu, T. (2020). Impact of Prebiotics on Enteric Diseases and Oxidative Stress.Current Pharmaceutical Design,26(22), 2630–2641. https: //doi.org/10.2174/1381612826666200211121916
[57]. Singh, J. E. (2020). Dietary Sources of Omega-3 Fatty Acids Versus Omega-3 Fatty Acid Supplementation Effects on Cognition and Inflammation.Current Nutrition Reports,9(3), 264–277. https: //doi.org/10.1007/s13668-020-00329-x
[58]. Hacquebard, M., Caron, M., Mitchell, P. L., & Blache, D. (2009). The metabolic syndrome of omega3-depleted rats. IV. Intestinal phospholipid omega3 fatty acids.International Journal of Molecular Medicine,24(6), 859–865. https: //doi.org/10.3892/ijmm_00000304
[59]. Singh, Jessica E. (2020) "Dietary Sources of Omega-3 Fatty Acids Versus Omega-3 Fatty Acid Supplementation Effects on Cognition and Inflammation."Current nutrition reports. 9(3), 264-277. doi: 10.1007/s13668-020-00329-x
[60]. Nielsen, O. H., Seidelin, J. B., & Vainer, B. (2019). Managing vitamin D deficiency in inflammatory bowel disease. Frontline Gastroenterology, 10(4), 394–400. https: //doi.org/10.1136/flgastro-2018-101055
[61]. Minton, K. (2022). Vitamin D shuts down T cell-mediated inflammation. Nature Reviews.Immunology,22(1), 1. https: //doi.org/10.1038/s41577-021-00663-3
[62]. Limketkai, B. N., Shah, P. M., & Bechtold, M. L. (2017). Role of Vitamin D in Inflammatory Bowel Disease.Nutrition in Clinical Practice: Official Publication of the American Society for Parenteral and Enteral Nutrition, 32(3), 337–345. https: //doi.org/10.1177/0884533616674492
[63]. Martini, Eva et al. (2017) "Mend Your Fences: The Epithelial Barrier and its Relationship with Mucosal Immunity in Inflammatory Bowel Disease. "Cellular and molecular gastroenterology and hepatology.4(1), 33-46. 23, doi: 10.1016/j.jcmgh.2017.03.007
[64]. Li, C., et al. (2024). Comprehensive modulatory effects of whole grain consumption on immune-mediated inflammation in middle-aged and elderly community residents: A real-world randomized controlled trial.Redox Biology,76, 103337. https: //doi.org/10.1016/j.redox.2024.103337
[65]. Schneider, K. M., et al. (2023). The enteric nervous system relays psychological stress to intestinal inflammation.Cell,186(13), 2823–2838.e20. https: //doi.org/10.1016/j.cell.2023.05.001
[66]. Duan, L., et al. (2021). Natural Anti-Inflammatory Compounds as Drug Candidates for Inflammatory Bowel Disease.Frontiers in Pharmacology,12, 684486. https: //doi.org/10.3389/fphar.2021.684486
[67]. Chen, M., et al. (2024). Wuwei Kushen Changrong capsule alleviates DSS-induced colitis in mice via inhibition of NLRP3 inflammasome and STAT3 pathway.Frontiers in Pharmacology,15, 1423012. https: //doi.org/10.3389/fphar.2024.1423012
[68]. Duan, T., et al. (2024). Isongifolene Improves Crohn's Disease-Like Colitis in Mice by Reducing Apoptosis of Intestinal Epithelial Cells. Sichuan Da Xue Xue Bao. Yi Xue Ban = Journal of Sichuan University.Medical Science Edition,55(5), 1175–1185. https: //doi.org/10.12182/20240960204
[69]. Lyu, Y.-L., et al. (2022). Biological Activities Underlying the Therapeutic Effect of Quercetin on Inflammatory Bowel Disease.Mediators of Inflammation, 2022, 5665778. https: //doi.org/10.1155/2022/5665778
[70]. Ben-Horin, S., et al. (2014). Optimising anti-TNF treatments in inflammatory bowel disease. Autoimmunity Reviews, 13(1), 24–30. https: //doi.org/10.1016/j.autrev.2013.06.002
[71]. Yue, Y., et al. (2015). Wild jujube polysaccharides protect against experimental inflammatory bowel disease by enabling enhanced intestinal barrier function.Food & Function,6(8), 2568–2577. https: //doi.org/10.1039/c5fo00378d
[72]. Liu, T., et al. (2024). Dietary Dihydroquercetin Alleviated Colitis via the Short-Chain Fatty Acids/miR-10a-5p/PI3K-Akt Signaling Pathway.Journal of Agricultural and Food Chemistry,72(42), 23211–23223. https: //doi.org/10.1021/acs.jafc.4c03278
[73]. Zheng, Y., et al. (2023). Probiotics fortify intestinal barrier function: A systematic review and meta-analysis of randomized trials.Frontiers in Immunology,14, 1143548. https: //doi.org/10.3389/fimmu.2023.1143548
[74]. Di Vito, R., et al. (2022). A Multi-Strain Probiotic Formulation Improves Intestinal Barrier Function by the Modulation of Tight and Adherent Junction Proteins.Cells,11(16), 2617. https: //doi.org/10.3390/cells11162617
[75]. L'Huillier, C., et al. (2019). Glutamine, but not Branched-Chain Amino Acids, Restores Intestinal Barrier Function during Activity-Based Anorexia.Nutrients,11(6), 1348. https: //doi.org/10.3390/nu11061348
[76]. Yi, J., et al. (2019). Dclk1 in tuft cells promotes inflammation-driven epithelial restitution and mitigates chronic colitis.Cell Death and Differentiation,26(9), 1656–1669. https: //doi.org/10.1038/s41418-018-0237-x
[77]. Shah, B., & Solanki, N. (2024). Aegeline attenuates TNBS-induced colitis by suppressing the NFƙB-mediated NLRP3 inflammasome pathway in mice. Inflammopharmacology, 32(4), 2589–2599. https: //doi.org/10.1007/s10787-024-01493-0
[78]. Brown, E., et al. (2020). Mucosal inflammation downregulates PHD1 expression promoting a barrier-protective HIF-1α response in ulcerative colitis patients. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 34(3), 3732–3742. https: //doi.org/10.1096/fj.201902103R
[79]. Lykowska-Szuber, L., et al. (2021). Effect of Anti-TNF Therapy on Mucosal Apoptosis Genes Expression in Crohn's Disease. Frontiers in Immunology, 12, 615539. https: //doi.org/10.3389/fimmu.2021.615539
[80]. Zhou, X., et al. (2022). The nociceptin receptor promotes autophagy through NF-kB signaling and is transcriptionally regulated by E2F1 in HCC. Cell Death Discovery, 8(1), 165. https: //doi.org/10.1038/s41420-022-00978-7
[81]. Akhtar, M., et al. (2020). Upregulated-gene expression of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) via TLRs following NF-κB and MAPKs in bovine mastitis. Acta Tropica, 207, 105458. https: //doi.org/10.1016/j.actatropica.2020.105458
[82]. Yang, Y., et al. (2022). Alpha-Lipoic Acid Promotes Intestinal Epithelial Injury Repair by Regulating MAPK Signaling Pathways.Mediators of Inflammation, 2022, 1894379. https: //doi.org/10.1155/2022/1894379
[83]. Múnera-Rodríguez, A. M., et al. (2024). Sulforaphane-mediated immune regulation through inhibition of NF-kB and MAPK signaling pathways in human dendritic cells.Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 177, 117056. https: //doi.org/10.1016/j.biopha.2024.117056
[84]. Yang, M., et al. (2025). Adipose-derived stem cells promote the recovery of intestinal barrier function by inhibiting the p38 MAPK signaling pathway.European Journal of Histochemistry: EJH,69(1), 4158. https: //doi.org/10.4081/ejh.2025.4158
[85]. Mendes, V., et al. (2019). Mechanisms by Which the Gut Microbiota Influences Cytokine Production and Modulates Host Inflammatory Responses.Journal of Interferon & Cytokine Research: The Official Journal of the International Society for Interferon and Cytokine Research,39(7), 393–409. https: //doi.org/10.1089/jir.2019.0011
[86]. Di, Y., et al. (2024). Chicoric Acid Alleviates Colitis via Targeting the Gut Microbiota Accompanied by Maintaining Intestinal Barrier Integrity and Inhibiting Inflammatory Responses.Journal of Agricultural and Food Chemistry, 72(12), 6276–6288. https: //doi.org/10.1021/acs.jafc.3c08363
[87]. Sun, M., et al. (2017). Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases.Journal of Gastroenterology,52(1), 1–8. https: //doi.org/10.1007/s00535-016-1242-9
Cite this article
Cheng,X.;Lu,X.;Tan,Y.;Shen,D.;Zhang,J. (2025). Protecting the intestinal barrier by modulating inflammatory factors to treat intestinal inflammation. Journal of Clinical Technology and Theory,3(2),82-94.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Journal:Journal of Clinical Technology and Theory
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Holmberg, F. E. O., & et al. (2018). Intestinal barrier integrity and inflammatory bowel disease: Stem cell-based approaches to regenerate the barrier.Journal of Tissue Engineering and Regenerative Medicine,12(4), 923–935.doi:10.1002/term.2506
[2]. Odenwald, M. A., & Turner, J. R. (2017). The intestinal epithelial barrier: a therapeutic target? Nature Reviews.Gastroenterology & Hepatology, 14(1), 9–21. doi:10.1002/term.2506
[3]. Dey, P. (2020). Targeting gut barrier dysfunction with phytotherapies: Effective strategy against chronic diseases.Pharmacological Research,161, 105135. doi: 10.1016/j.phrs.2020.105135
[4]. Li, L., & et al. (2007). Development of sorbent therapy for multiple organ dysfunction syndrome (MODS).Biomedical Materials (Bristol, England), 2(2), R12–R16. doi:10.1088/1748-6041/2/2/R02
[5]. Maier, R. V. (2000). Pathogenesis of multiple organ dysfunction syndrome--endotoxin, inflammatory cells, and their mediators: cytokines and reactive oxygen species. Surgical Infections, 1(3), 197–205; discussion 204–5. https: //doi.org/10.1089/109629600750018123
[6]. Wang, Y.-H. (2021). Current progress of research on intestinal bacterial translocation. Microbial Pathogenesis, 152, 104652. https: //doi.org/10.1016/j.micpath.2020.104652
[7]. Gou, H.-Z., & et al. (2022). How do intestinal probiotics restore the intestinal barrier?Frontiers in Microbiology,13, 929346. https: //doi.org/10.3389/fmicb.2022.929346
[8]. König, J., & et al. (2016). Human Intestinal Barrier Function in Health and Disease. Clinical and Translational Gastroenterology,7(10), e196. https: //doi.org/10.1038/ctg.2016.54
[9]. Chopyk, D. M., & Grakoui, A. (2020). Contribution of the Intestinal Microbiome and Gut Barrier to Hepatic Disorders.Gastroenterology,159(3), 849–863. https: //doi.org/10.1053/j.gastro.2020.04.077
[10]. An, J., & et al. (2022). The Role of Intestinal Mucosal Barrier in Autoimmune Disease: a Potential Target.Frontiers in Immunology,13, 871713. https: //doi.org/10.3389/fimmu.2022.871713
[11]. Kany, S., & et al. (2019). Cytokines in Inflammatory Disease.International Journal of Molecular Sciences,20(23), 6008. https: //doi.org/10.3390/ijms20236008
[12]. Oliver, A. J., & et al. (2024). Single-cell integration reveals metaplasia in inflammatory gut diseases.Nature, 635(8039), 699–707. https: //doi.org/10.1038/s41586-024-07571-1
[13]. Serhan, C. N., & et al. (2008). Anti-inflammatory and proresolving lipid mediators.Annual Review of Pathology,3, 279–312. https: //doi.org/10.1146/annurev.pathmechdis.3.121806.151409
[14]. Zheng, X., & et al. (2022). The use of supercytokines, immunocytokines, engager cytokines, and other synthetic cytokines in immunotherapy.Cellular & Molecular Immunology,19(2), 192–209. https: //doi.org/10.1038/s41423-021-00786-6
[15]. Wang, T., & He, C. (2018). Pro-inflammatory cytokines: the link between obesity and osteoarthritis.Cytokine & Growth Factor Reviews,44, 38–50. https: //doi.org/10.1016/j.cytogfr.2018.10.002
[16]. Jung, H. C., & et al. (1995). A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion.The Journal of Clinical Investigation,95(1), 55–65. https: //doi.org/10.1172/JCI117676
[17]. Jin, Y., & et al. (2014). Regulation of anti-inflammatory cytokines IL-10 and TGF-β in mouse dendritic cells through treatment with Clonorchis sinensis crude antigen.Experimental & Molecular Medicine,46(1), e74. https: //doi.org/10.1038/emm.2013.144
[18]. Chang, S. H., & Dong, C. (2011). Signaling of interleukin-17 family cytokines in immunity and inflammation.Cellular Signaling, 23(7), 1069–1075. https: //doi.org/10.1016/j.cellsig.2010.11.022
[19]. Zhou, C., & et al. (2023). The role of CXCL family members in different diseases.Cell Death Discovery,9(1), 212. https: //doi.org/10.1038/s41420-023-01524-9
[20]. Chen, K., & et al. (2018). Chemokines in homeostasis and diseases.Cellular & Molecular Immunology,15(4), 324–334. https: //doi.org/10.1038/cmi.2017.134
[21]. Neuman, Manuela G. (2007) "Immune dysfunction in inflammatory bowel disease."Translational research: the journal of laboratory and clinical medicine.149,(4), 173-86. doi: 10.1016/j.trsl.2006.11.009
[22]. Park, J. H., et al. (2017). IBD immunopathogenesis: A comprehensive review of inflammatory molecules.Autoimmunity Reviews, 16(4), 416–426. https: //doi.org/10.1016/j.autrev.2017.02.013
[23]. Matsukawa, A., et al. (1997). Analysis of the inflammatory cytokine network among TNF alpha, IL-1 beta, IL-1 receptor antagonist, and IL-8 in LPS-induced rabbit arthritis. Laboratory Investigation;a Journal of Technical Methods and Pathology,76(5), 629–638.
[24]. Utsunomiya, I., et al. (1998). Generation of inflammatory cytokines in zymosan-induced pleurisy in rats: TNF induces IL-6 and cytokine-induced neutrophil chemoattractant (CINC) in vivo.Cytokine,10(12), 956–963. https: //doi.org/10.1006/cyto.1998.0376
[25]. York, A. G., et al. (2024). IL-10 constrains sphingolipid metabolism to limit inflammation.Nature,627(8004), 628–635. https: //doi.org/10.1038/s41586-024-07098-5
[26]. Li, R., et al. (2020). Pro-Inflammatory Cytokines in the Formation of the Pre-Metastatic Niche.Cancers,12(12), 3752. https: //doi.org/10.3390/cancers12123752
[27]. Medzhitov, R. (2021). The spectrum of inflammatory responses.Science, 374(6571), 1070–1075. https: //doi.org/10.1126/science.abi5200
[28]. Liu, T., et al. (2017). NF-κB signaling in inflammation.Signal Transduction and Targeted Therapy,2, 17023. https: //doi.org/10.1038/sigtrans.2017.23
[29]. Schuster, S., et al. (2018). Triggering and resolution of inflammation in NASH. Nature Reviews.Gastroenterology & Hepatology, 15(6), 349–364. https: //doi.org/10.1038/s41575-018-0009-6
[30]. Xi, M., et al. (2023). Galactooligosaccharide Mediates NF-κB Pathway to Improve Intestinal Barrier Function and Intestinal Microbiota.Molecules, 28(22), 7611. https: //doi.org/10.3390/molecules28227611
[31]. Kawai, T., et al. (2021). Adipose tissue inflammation and metabolic dysfunction in obesity. American Journal of Physiology.Cell Physiology,320(3), C375–C391. https: //doi.org/10.1152/ajpcell.00379.2020
[32]. Zhang, C., et al. (2017). Inhibition of Autophagic Degradation Process Contributes to Claudin-2 Expression Increase and Epithelial Tight Junction Dysfunction in TNF-α Treated Cell Monolayers.International Journal of Molecular Sciences,18(1), 157. https: //doi.org/10.3390/ijms18010157
[33]. Van Loo, G., & Bertrand, M. J. M. (2023). Death by TNF: a road to inflammation. Nature Reviews.Immunology, 23(5), 289–303. https: //doi.org/10.1038/s41577-022-00792-3
[34]. Danese, S., et al. (2006). TNF-alpha blockade down-regulates the CD40/CD40L pathway in the mucosal microcirculation: a novel anti-inflammatory mechanism of infliximab in Crohn's disease.Journal of Immunology,176(4), 2617–2624. https: //doi.org/10.4049/jimmunol.176.4.2617
[35]. Swetha, K., et al. (2025). Selenium Mitigates Caerulein and LPS-induced Severe Acute Pancreatitis by Inhibiting MAPK, NF-κB, and STAT3 Signaling via the Nrf2/HO-1 Pathway. Biological Trace Element Research.Advance online publication. https: //doi.org/10.1007/s12011-025-04531-2
[36]. Heath, D. I., et al. (1993). Role of interleukin-6 in mediating the acute phase protein response and potential as an early means of severity assessment in acute pancreatitis.Gut,34(1), 41–45. https: //doi.org/10.1136/gut.34.1.41
[37]. Korn, T., et al. (2008). IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells.Proceedings of the National Academy of Sciences of the United States of America,105(47), 18460–18465. https: //doi.org/10.1073/pnas.0809850105
[38]. Mauer, J., et al. (2014). Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin.Nature Immunology, 15(5), 423–430. https: //doi.org/10.1038/ni.2865
[39]. Tanaka, T., & Kishimoto, T. (2014). The biology and medical implications of interleukin-6.Cancer Immunology Research, 2(4), 288–294. https: //doi.org/10.1158/2326-6066.CIR-14-0022
[40]. Perez-Penco, M., et al. (2025). The antitumor activity of TGFβ-specific T cells is dependent on IL-6 signaling.Cellular & Molecular Immunology, 22(1), 111–126. https: //doi.org/10.1038/s41423-024-01238-7
[41]. Yamanishi, Y., & Karasuyama, H. (2016). Basophil-derived IL-4 plays versatile roles in immunity.Seminars in Immunopathology, 38(5), 615–622. https: //doi.org/10.1007/s00281-016-0568-y
[42]. Inoue, S et al. (1999) "Characterization of cytokine expression in the rectal mucosa of ulcerative colitis: correlation with disease activity. "The American journal of gastroenterology.94(9), 2441-6. doi: 10.1111/j.1572-0241.1999. 01372.x
[43]. Huaux, F., Liu, T., & Phan, S. H. (2003). Dual roles of IL-4 in lung injury and fibrosis.Journal of Immunology (Baltimore, Md.: 1950), 170(4), 2083–2092. https: //doi.org/10.4049/jimmunol.170.4.2083
[44]. Auger, J.-P., Zhang, Y., Wang, X., Li, M., Zhao, Z., Yang, W., ... & Zhou, B. (2024). Metabolic rewiring promotes anti-inflammatory effects of glucocorticoids.Nature,629(8010), 184–192. https: //doi.org/10.1038/s41586-024-07282-7
[45]. Dahl, J. B., & Kehlet, H. (1991). Non-steroidal anti-inflammatory drugs: Rationale for use in severe postoperative pain.British Journal of Anaesthesia,66(6), 703–712. https: //doi.org/10.1093/bja/66.6.703
[46]. Abe, Y., Sagara, I., & Ito, Y. (1999). Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages.Pharmacological Research, 39(1), 41–47. https: //doi.org/10.1006/phrs.1998.0404
[47]. Zhang, Q., Zhang, L., Zhang, Y., Chen, D., & Wang, Y. (2017). Ethanol extract and its dichloromethane fraction of Alpinia oxyphylla Miquel exhibited hepatoprotective effects against CCl4-induced oxidative damage in vitro and in vivo with the involvement of Nrf2.Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 91, 812–822. https: //doi.org/10.1016/j.biopha.2017.04.131
[48]. Zhu, W.-T., Zhang, Y., Liu, Y., & Sun, Y. (2023). Effect of allyl isothiocyanate on oxidative stress in COPD via the AhR / CYP1A1 and Nrf2 / NQO1 pathways and the underlying mechanism.Phytomedicine: International Journal of Phytotherapy and Phytopharmacology,114, 154774. https: //doi.org/10.1016/j.phymed.2023.154774
[49]. Han, M. H., Park, G., Lee, H. J., Kim, G. Y., Cheong, J., Hong, S. H., ... & Choi, Y. H. (2017). Cytoprotective effects of esculetin against oxidative stress are associated with the upregulation of Nrf2-mediated NQO1 expression via the activation of the ERK pathway.International Journal of Molecular Medicine, 39(2), 380–386. https: //doi.org/10.3892/ijmm.2016.2834
[50]. Turner, J. R. (2009). Intestinal mucosal barrier function in health and disease. Nature Reviews.Immunology,9(11), 799–809. https: //doi.org/10.1038/nri2653
[51]. Luzentales-Simpson, M., Zhang, D., & Plevy, S. E. (2021). Vedolizumab: Potential Mechanisms of Action for Reducing Pathological Inflammation in Inflammatory Bowel Diseases.Frontiers in Cell and Developmental Biology,9, 612830. https: //doi.org/10.3389/fcell.2021.612830
[52]. Meng, F., Xu, L., Zhang, X., Tian, X., Guo, Y., Li, Y., ... & Zheng, L. (2023). Tumor necrosis factor-like cytokine 1A plays a role in inflammatory bowel disease pathogenesis.Proceedings of the National Academy of Sciences of the United States of America, 120(34), e2120771120. https: //doi.org/10.1073/pnas.2120771120
[53]. Cohen-Dolev, N., Hyams, J. S., Lerer, T., Mack, D. R., Griffiths, A. M., Markowitz, J., ... & Rufo, P. A. (2018). Differences in Outcomes Over Time with Exclusive Enteral Nutrition Compared with Steroids in Children with Mild to Moderate Crohn's Disease: Results from the GROWTH CD Study.Journal of Crohn's & Colitis,12(3), 306–312. https: //doi.org/10.1093/ecco-jcc/jjx150
[54]. Li, D., Wang, C., Wang, S., Ding, Z., Zhang, W., & Gao, C. (2016). The gut microbiota: A treasure for human health. Biotechnology Advances, 34(7), 1210–1224. https: //doi.org/10.1016/j.biotechadv.2016.08.003
[55]. Zuo, T., & Ng, S. C. (2018). The Gut Microbiota in the Pathogenesis and Therapeutics of Inflammatory Bowel Disease.Frontiers in Microbiology,9, 2247. https: //doi.org/10.3389/fmicb.2018.02247
[56]. Gao, J., Yin, Y., Zhang, Y., Liu, H., & Wu, T. (2020). Impact of Prebiotics on Enteric Diseases and Oxidative Stress.Current Pharmaceutical Design,26(22), 2630–2641. https: //doi.org/10.2174/1381612826666200211121916
[57]. Singh, J. E. (2020). Dietary Sources of Omega-3 Fatty Acids Versus Omega-3 Fatty Acid Supplementation Effects on Cognition and Inflammation.Current Nutrition Reports,9(3), 264–277. https: //doi.org/10.1007/s13668-020-00329-x
[58]. Hacquebard, M., Caron, M., Mitchell, P. L., & Blache, D. (2009). The metabolic syndrome of omega3-depleted rats. IV. Intestinal phospholipid omega3 fatty acids.International Journal of Molecular Medicine,24(6), 859–865. https: //doi.org/10.3892/ijmm_00000304
[59]. Singh, Jessica E. (2020) "Dietary Sources of Omega-3 Fatty Acids Versus Omega-3 Fatty Acid Supplementation Effects on Cognition and Inflammation."Current nutrition reports. 9(3), 264-277. doi: 10.1007/s13668-020-00329-x
[60]. Nielsen, O. H., Seidelin, J. B., & Vainer, B. (2019). Managing vitamin D deficiency in inflammatory bowel disease. Frontline Gastroenterology, 10(4), 394–400. https: //doi.org/10.1136/flgastro-2018-101055
[61]. Minton, K. (2022). Vitamin D shuts down T cell-mediated inflammation. Nature Reviews.Immunology,22(1), 1. https: //doi.org/10.1038/s41577-021-00663-3
[62]. Limketkai, B. N., Shah, P. M., & Bechtold, M. L. (2017). Role of Vitamin D in Inflammatory Bowel Disease.Nutrition in Clinical Practice: Official Publication of the American Society for Parenteral and Enteral Nutrition, 32(3), 337–345. https: //doi.org/10.1177/0884533616674492
[63]. Martini, Eva et al. (2017) "Mend Your Fences: The Epithelial Barrier and its Relationship with Mucosal Immunity in Inflammatory Bowel Disease. "Cellular and molecular gastroenterology and hepatology.4(1), 33-46. 23, doi: 10.1016/j.jcmgh.2017.03.007
[64]. Li, C., et al. (2024). Comprehensive modulatory effects of whole grain consumption on immune-mediated inflammation in middle-aged and elderly community residents: A real-world randomized controlled trial.Redox Biology,76, 103337. https: //doi.org/10.1016/j.redox.2024.103337
[65]. Schneider, K. M., et al. (2023). The enteric nervous system relays psychological stress to intestinal inflammation.Cell,186(13), 2823–2838.e20. https: //doi.org/10.1016/j.cell.2023.05.001
[66]. Duan, L., et al. (2021). Natural Anti-Inflammatory Compounds as Drug Candidates for Inflammatory Bowel Disease.Frontiers in Pharmacology,12, 684486. https: //doi.org/10.3389/fphar.2021.684486
[67]. Chen, M., et al. (2024). Wuwei Kushen Changrong capsule alleviates DSS-induced colitis in mice via inhibition of NLRP3 inflammasome and STAT3 pathway.Frontiers in Pharmacology,15, 1423012. https: //doi.org/10.3389/fphar.2024.1423012
[68]. Duan, T., et al. (2024). Isongifolene Improves Crohn's Disease-Like Colitis in Mice by Reducing Apoptosis of Intestinal Epithelial Cells. Sichuan Da Xue Xue Bao. Yi Xue Ban = Journal of Sichuan University.Medical Science Edition,55(5), 1175–1185. https: //doi.org/10.12182/20240960204
[69]. Lyu, Y.-L., et al. (2022). Biological Activities Underlying the Therapeutic Effect of Quercetin on Inflammatory Bowel Disease.Mediators of Inflammation, 2022, 5665778. https: //doi.org/10.1155/2022/5665778
[70]. Ben-Horin, S., et al. (2014). Optimising anti-TNF treatments in inflammatory bowel disease. Autoimmunity Reviews, 13(1), 24–30. https: //doi.org/10.1016/j.autrev.2013.06.002
[71]. Yue, Y., et al. (2015). Wild jujube polysaccharides protect against experimental inflammatory bowel disease by enabling enhanced intestinal barrier function.Food & Function,6(8), 2568–2577. https: //doi.org/10.1039/c5fo00378d
[72]. Liu, T., et al. (2024). Dietary Dihydroquercetin Alleviated Colitis via the Short-Chain Fatty Acids/miR-10a-5p/PI3K-Akt Signaling Pathway.Journal of Agricultural and Food Chemistry,72(42), 23211–23223. https: //doi.org/10.1021/acs.jafc.4c03278
[73]. Zheng, Y., et al. (2023). Probiotics fortify intestinal barrier function: A systematic review and meta-analysis of randomized trials.Frontiers in Immunology,14, 1143548. https: //doi.org/10.3389/fimmu.2023.1143548
[74]. Di Vito, R., et al. (2022). A Multi-Strain Probiotic Formulation Improves Intestinal Barrier Function by the Modulation of Tight and Adherent Junction Proteins.Cells,11(16), 2617. https: //doi.org/10.3390/cells11162617
[75]. L'Huillier, C., et al. (2019). Glutamine, but not Branched-Chain Amino Acids, Restores Intestinal Barrier Function during Activity-Based Anorexia.Nutrients,11(6), 1348. https: //doi.org/10.3390/nu11061348
[76]. Yi, J., et al. (2019). Dclk1 in tuft cells promotes inflammation-driven epithelial restitution and mitigates chronic colitis.Cell Death and Differentiation,26(9), 1656–1669. https: //doi.org/10.1038/s41418-018-0237-x
[77]. Shah, B., & Solanki, N. (2024). Aegeline attenuates TNBS-induced colitis by suppressing the NFƙB-mediated NLRP3 inflammasome pathway in mice. Inflammopharmacology, 32(4), 2589–2599. https: //doi.org/10.1007/s10787-024-01493-0
[78]. Brown, E., et al. (2020). Mucosal inflammation downregulates PHD1 expression promoting a barrier-protective HIF-1α response in ulcerative colitis patients. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 34(3), 3732–3742. https: //doi.org/10.1096/fj.201902103R
[79]. Lykowska-Szuber, L., et al. (2021). Effect of Anti-TNF Therapy on Mucosal Apoptosis Genes Expression in Crohn's Disease. Frontiers in Immunology, 12, 615539. https: //doi.org/10.3389/fimmu.2021.615539
[80]. Zhou, X., et al. (2022). The nociceptin receptor promotes autophagy through NF-kB signaling and is transcriptionally regulated by E2F1 in HCC. Cell Death Discovery, 8(1), 165. https: //doi.org/10.1038/s41420-022-00978-7
[81]. Akhtar, M., et al. (2020). Upregulated-gene expression of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) via TLRs following NF-κB and MAPKs in bovine mastitis. Acta Tropica, 207, 105458. https: //doi.org/10.1016/j.actatropica.2020.105458
[82]. Yang, Y., et al. (2022). Alpha-Lipoic Acid Promotes Intestinal Epithelial Injury Repair by Regulating MAPK Signaling Pathways.Mediators of Inflammation, 2022, 1894379. https: //doi.org/10.1155/2022/1894379
[83]. Múnera-Rodríguez, A. M., et al. (2024). Sulforaphane-mediated immune regulation through inhibition of NF-kB and MAPK signaling pathways in human dendritic cells.Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 177, 117056. https: //doi.org/10.1016/j.biopha.2024.117056
[84]. Yang, M., et al. (2025). Adipose-derived stem cells promote the recovery of intestinal barrier function by inhibiting the p38 MAPK signaling pathway.European Journal of Histochemistry: EJH,69(1), 4158. https: //doi.org/10.4081/ejh.2025.4158
[85]. Mendes, V., et al. (2019). Mechanisms by Which the Gut Microbiota Influences Cytokine Production and Modulates Host Inflammatory Responses.Journal of Interferon & Cytokine Research: The Official Journal of the International Society for Interferon and Cytokine Research,39(7), 393–409. https: //doi.org/10.1089/jir.2019.0011
[86]. Di, Y., et al. (2024). Chicoric Acid Alleviates Colitis via Targeting the Gut Microbiota Accompanied by Maintaining Intestinal Barrier Integrity and Inhibiting Inflammatory Responses.Journal of Agricultural and Food Chemistry, 72(12), 6276–6288. https: //doi.org/10.1021/acs.jafc.3c08363
[87]. Sun, M., et al. (2017). Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases.Journal of Gastroenterology,52(1), 1–8. https: //doi.org/10.1007/s00535-016-1242-9









