1. Introduction
Rice (Oryza sativa) is one of the most important staple food crops globally, and its yield and quality are directly linked to the food security of hundreds of millions of people around the world [1]. Under the dual pressure of climate change and reduced arable land, deciphering the regulatory mechanisms of photosynthesis in rice has become a crucial research direction to break through yield bottlenecks.
The photosynthetic reaction center of rice is the chloroplast, which is a semi-autonomous photosynthetic organelle. The main components of chloroplasts include two parts: the highly specialized thylakoid membrane and the surrounding membrane that encloses the soluble stroma. Chloroplasts play a vital role in the growth and development of rice by regulating their photosynthetic capacity and the biosynthesis of carbon skeletons [2]. The thylakoid is a key site for the light reactions, and the thylakoid membrane integrates four supramolecular protein complexes—photosystem I (PSI), photosystem II (PSII), ATP synthase (ATPase), and the cytochrome b6f complex (Cyt b6f)—to construct a complete electron transport chain and photosynthetic phosphorylation system [3-7]. The thylakoid lumen is a narrow, continuous space surrounded by the thylakoid membrane, containing many proteins involved in photosynthesis. The thylakoid lumen provides space for photoprotection and helps to conserve moisture for PSII oxidation [8]. PSII exhibits dynamic distribution on the thylakoid membrane, moving to the stroma lamellae region for repair and assembly after light-induced damage [9]. The repair of PSII involves a series of complex steps, including kinase-mediated phosphorylation of the D1 protein, which promotes the dissociation of the supercomplex, dephosphorylation of the PSII core monomer, partial degradation of damaged D1 proteins, de novo synthesis and insertion of new D1 proteins, and reassembly of the full complex. These processes enable the replacement of light-damaged D1 reaction center proteins with newly synthesized copies [10-12].
OsTLP16.5 (encoded by Os06g0705100) is a soluble protein located in the thylakoid lumen of rice, with a molecular weight of 16.5 kDa. Studies have shown that the deletion of the OsTLP16.5 gene in rice leads to a decline in photosynthetic capacity in rice leaves. OsTLP16.5 may influence the metabolism of organic acids, amino acids, and vitamins in rice by participating in photosynthetic regulation [13]. The homologous protein MPH2 in Arabidopsis (encoded by At4g02530) is one of the proteins essential for Arabidopsis’ adaptation to strong and fluctuating light environments, and its deletion impairs PSII repair in Arabidopsis [14]. Currently, there are few reports on the OsTLP16.5 protein, and its specific function and involvement in PSII repair remain unclear.
The yeast two-hybrid (Y2H) system, developed in 1989, remains an important tool for studying and verifying protein-protein interactions [15]. Y2H-seq, a strategy integrating yeast two-hybrid screening with next-generation sequencing (NGS), allows for more efficient and reliable screening of cDNA libraries [16].
In this study, the rice thylakoid lumen protein OsTLP16.5 was used as the research target. The yeast two-hybrid bait vector pGBKT7-OsTLP16.5 (BD-16.5) was successfully constructed, and its interacting target proteins were screened, providing a foundation for further research into the molecular regulatory mechanisms of OsTLP16.5.
2. Materials and methods
2.1. Materials
Rice seeds of the ZH11 variety were purchased from Baige Biotechnology Co., Ltd. Total RNA was extracted from rice leaves at the tillering stage using the Trizol method, and cDNA was synthesized via reverse transcription. The yeast competent strain AH109 and the Escherichia coli competent strain DH5α were prepared and stored in our laboratory. The yeast two-hybrid vectors pGBKT7 and pGADT7 were purchased from Wuhan Miaoling Biotechnology Co., Ltd.
2.2. Methods
2.2.1. Preparation of cDNA library and bait vector
Using the pGBKT7 vector as a template, linearized vectors were obtained by inverse PCR using primers BD-F: 5’-GGATCCGTCGACCTGCAGCGG-3’ and BD-R: 5’-AATTCGGCCTCCATGGCCAT-3’. Primers containing homologous arms for OsTLP16.5 were designed: BD-16.5-F: 5`-atggccatggaggccgaattcATGGTGGTGGCCATCGCT-3`, BD-16.5-R: 5`-ccgctgcaggtcgacggatccTTAGAATCCTTTGAGCTGACCACTC-3`(the lowercase portions underlined indicate the homologous arms complementary to the pGBKT7 vector sequence). Using rice cDNA as a template, the OsTLP16.5 gene was amplified and integrated into the bait vector pGBKT7 via homologous recombination, followed by transformation into E. coli competent cells. Positive single colonies were selected, verified by colony PCR, and sent for sequencing to confirm the accuracy of the insertion sequence.
2.2.2. Self-activation detection of bait vector
The recombinant bait vector 16.5-BD was used as the experimental group, and the empty pGBKT7 vector was used as the negative control. Both vectors were transformed into AH109 yeast cells by the LiAc method to test for self-activation of the bait vector. The transformation mixtures were plated onto SD/-Trp plates and incubated at 30°C for 3-5 days. PCR verification of the correct clones was performed using pGBKT7 vector primers, and the positive clones were plated on selective media: SD/-Trp, SD/-Trp/-His, SD/-Trp/-His/-Ade, and SD/-Trp/-His/-Ade+x-α-gal. The plates were incubated at 30°C for 3-5 days, and the growth status of the colonies was observed.
2.2.3. Screening of rice cDNA library
Competent cells of AH109 yeast containing the pGBKT7-OsTLP16.5 bait plasmid were prepared and used as the recipient strain. The cDNA library plasmid pGADT7-rice cDNA was then transformed into the yeast cells, and the transformation was plated on SD/-Trp/-Leu/-His screening plates.
2.2.4. Yeast positive clone sequencing and reverse verification
Positive clone genes were amplified from yeast cells and subjected to Sanger sequencing. The sequencing results were compared with the sequences in the GenBank database using BLAST. Positive clone plasmids were streaked onto SD/-Trp/-Leu-deficient plates, and the resulting positive transformants were diluted in sterile water and spotted onto SD/-Trp/-Leu, SD/-Trp/-Leu/-His, SD/-Trp/-Leu/-His/-Ade, and SD/-Trp/-Leu/-His/-Ade+x-α-gal deficient plates. The plates were incubated at 30°C for 3-5 days for reverse verification.
2.2.5. Combined next-generation sequencing (NGS) analysis
To identify more potential interacting proteins, all positive clones from the yeast screening plates were scraped into 2xYPDA liquid medium. After centrifugation to collect the yeast cells, colony PCR was performed, and the PCR products were subjected to NGS sequencing.
3. Results
3.1. Construction of the bait vector
The linearized vector was obtained by inverse PCR using BD-F/R primers. Using BD-16.5F/R primers, a fragment containing homologous arms was amplified from rice cDNA. After recovering the fragment, it was ligated into the pGBKT7 bait vector via homologous recombination. After transformation, seven positive clones were selected for colony PCR verification. The three clones with the most distinct bands were then subjected to Sanger sequencing. Sequence alignment using Snapgene software showed that the sequence of the recombinant plasmid from colony 7 completely matched the original OsTLP16.5 gene sequence, confirming the successful construction of the BD-16.5 bait plasmid. As shown in Figure 1 to 5.
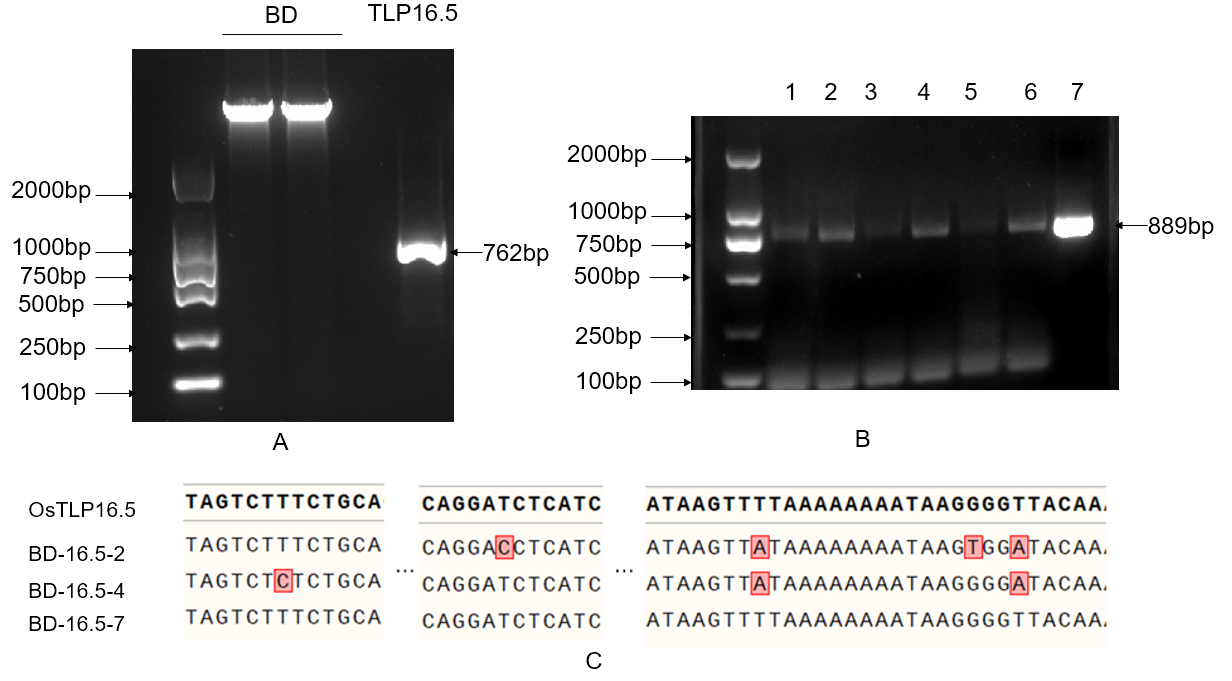
Figure 1. Sequencing analysis of the bait vector BD-16.5
A. Linearized pGBKT7 plasmid and amplification of OsTLP16.5;
B. Colony PCR verification;
C. Sequence alignment results.
3.2. Self-activation detection of the bait vector
Yeast cells containing the 16.5-BD vector and the empty pGBKT7 vector were plated on SD-Trp, SD/-Trp/-His, SD/-Trp/-His/-Ade, and SD/-Trp/-His/-Ade+x-α-gal plates for self-activation testing. The results showed that both the control strain and the negative control grew on SD-Trp, but did not grow on SD/-Trp/-His, SD/-Trp/-His/-Ade, or SD/-Trp/-His/-Ade+x-α-gal plates. The experimental group showed the same growth pattern as the control group, indicating that OsTLP16.5 protein can be normally expressed in yeast cells and does not exhibit self-activation.

Figure 2. Verification of self-activation of bait vector
3.3. Screening of rice cDNA library
The bait vector was co-transformed with the rice cDNA library vector into AH109 yeast competent cells. The transformation was then plated on SD/-Trp/-Leu/-His plates for screening, where 96 colonies grew on the plate.
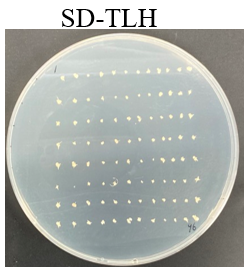
Figure 3. Screening of candidate interaction proteins for OsTLP16.5 in rice
All positive colonies were selected for PCR amplification, followed by DNA sequencing. The sequencing results were compared with sequences in the GenBank database via BLAST analysis. After removing empty vector and duplicate sequences, a total of 32 distinct rice proteins were identified.
Table 1. Putative proteins screened in the cDNA library of Oryza sativa interacting with OsTLP16.5
NO | Screening results |
0004 | Oryza sativa Japonica Group heavy metal-associated isoprenylated plant protein 7 (LOC4348282), transcript variant X1, mRNA |
0005 | Oryza sativa Japonica Group clone KCB766D02 s-adenosylmethionine synthase 1 mRNA, complete cds |
0006 | Oryza sativa Japonica Group DNA, chromosome 1, cultivar: Nipponbare, complete sequence |
0007 | Oryza sativa Japonica Group cDNA, clone: J065213C07, full insert sequence |
0008 | Oryza sativa Japonica Group citrate synthase 4, mitochondrial (LOC4328598), mRNA |
0009 | Oryza sativa Japonica Group dehydrin DHN1-like (LOC4330265), mRNA |
0010 | Oryza sativa Japonica Group clone KCS045C09 prolamin mRNA, complete cds |
0011 | Oryza sativa Japonica Group coilin (LOC4352003), mRNA |
0013 | Oryza sativa (japonica cultivar-group) DUF26-like protein mRNA, complete cds |
0014 | Oryza sativa Indica Group cultivar Zhenshan 97 chromosome 12 |
0015 | Oryza sativa Japonica Group phosphatidylinositol 4-kinase gamma 6 (LOC4337337), transcript variant X2, mRNA |
0016 | Oryza sativa Japonica Group cDNA clone:J023013H23, full insert sequence |
0017 | Oryza sativa Japonica Group plastocyanin, chloroplastic-like (LOC4339833), mRNA |
0018 | Pyricularia oryzae 70-15 uncharacterized protein (MGG_16629), mRNA |
0019 | Oryza sativa Japonica Group clone KCG181C08 protodermal factor-like protein mRNA, complete cds |
0020 | Oryza sativa Japonica Group sucrose synthase 1-like (LOC4333062), transcript variant X2, mRNA |
0022 | Oryza sativa Indica Group cultivar Shuhui498 chromosome 5 sequence |
0024 | Oryza sativa Japonica Group DNA-binding protein EMBP-1 (LOC4334523), transcript variant X5, mRNA |
0025 | Oryza sativa Japonica Group acyl-CoA-binding domain-containing protein 6-like (LOC4331191), transcript variant X1, mRNA |
0026 | Oryza sativa Japonica Group 17.9 kDa class I heat shock protein-like (LOC4332357), mRNA |
0027 | Oryza sativa Japonica Group glyceraldehyde-3-phosphate dehydrogenase 1, cytosolic-like (LOC4344564), mRNA |
0028 | Unknown |
0029 | Oryza sativa Japonica Group cDNA clone:J023058O18, full insert sequence |
0030 | Oryza rufipogon (W1943) cDNA clone: ORW1943C102K17, full insert sequence |
0032 | Oryza sativa Japonica Group NADH-cytochrome b5 reductase-like protein (LOC4327126), mRNA |
0036 | Pyricularia oryzae strain LpKY97 chromosome 3 |
0037 | Oryza sativa Japonica Group putative F-box/LRR-repeat protein 23 (LOC4344870), transcript variant X14, mRNA |
0040 | Oryza sativa Japonica Group probable V-type proton ATPase subunit H (LOC9272111), mRNA |
0056 | Oryza sativa Japonica Group DNA, chromosome 4, cultivar: Nipponbare, complete sequence |
0058 | Oryza sativa (japonica cultivar-group) clone KCS335A12 prolamin mRNA, complete cds |
0059 | Oryza sativa clone RRlibB00486 mRNA sequence |
0062 | Oryza sativa (indica cultivar-group) metallothionein 2b-like protein (MT2bL) mRNA, complete cds |
3.4. Confirmation of positive clones via reversal test
In the reversal test, the positive control grew on SD/-Trp/-Leu, SD/-Trp/-Leu/-His, SD/-Trp/-Leu/-His/-Ade, and SD/-Trp/-Leu/-His/-Ade+x-α-gal deficiency plates, and turned blue on SD/-Trp/-Leu/-His/-Ade+x-α-gal plates. The negative control only grew on the SD/-Trp/-Leu deficiency plate, and did not grow on SD/-Trp/-Leu/-His, SD/-Trp/-Leu/-His/-Ade, or SD/-Trp/-Leu/-His/-Ade+x-α-gal plates.
Among the 32 yeast positive clones selected, 14 grew on the SD/-Trp/-Leu/-His deficiency plate, and 3 clones turned blue on SD/-Trp/-Leu/-His/-Ade+x-α-gal plates (Figure 4). This indicates that OsTLP16.5 interacts with the three candidate proteins in yeast cells.
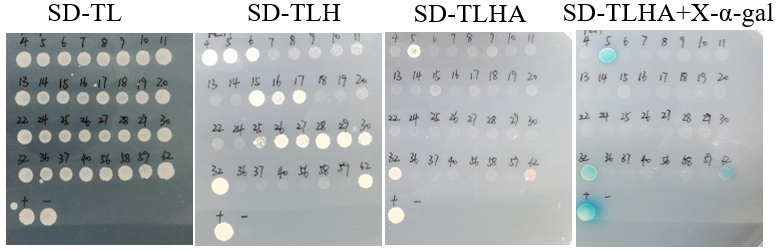
Figure 4. Confirmation of positive interactors
(+) is the positive control pGADT7-largeT+pGBKT7-p53
(-) is the negative control pGADT7-largeT+pGBKT7-laminC
3.5. Next-generation sequencing (NGS) analysis
To screen more potential interacting proteins, a total of 977 possible interacting proteins were identified through next-generation sequencing. GO enrichment analysis was performed using the Metascape online tool (http://metascape.org/), and the results are shown in Figure 5. In terms of cellular component, the interacting proteins were mainly located in the cytosolic ribosome, vacuole, thylakoid, chloroplast stroma, and proton transport ATPase complex, among others. A total of 51 chloroplast-related proteins were identified.
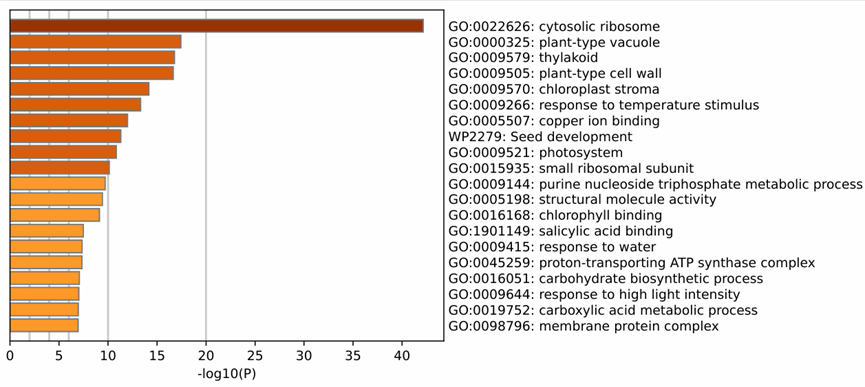
Figure 5. GO enrichment results of Y2H-seq screened proteins
4. Discussion
Arabidopsis MPH2 protein is a key factor for Arabidopsis growth under fluctuating light and high-light conditions [14] . Rice OsTLP16.5 is a homolog of MPH2, with a high degree of sequence similarity between the two proteins. Therefore, it is reasonable to speculate that OsTLP16.5 may affect rice growth and development by regulating PSII repair.
Yeast two-hybrid (Y2H) technology is an important tool for studying protein-protein interactions. In this study, Y2H and Sanger sequencing were used to screen a rice cDNA library with OsTLP16.5 as the bait protein. Three stable interacting proteins were successfully identified, including S-adenosylmethionine synthetase 1 (SAMS1), NADH-cytochrome b5 reductase-like protein, and Type 2 metal-thiol protein (MT2bL). SAMS1, a widely expressed core metabolic enzyme, catalyzes the synthesis of S-adenosylmethionine (SAM), which serves as both a precursor for polyamine biosynthesis and a universal donor for methylation modifications [17]. Overexpression of SAMS1 has been shown to significantly enhance plants’ water retention ability and photosynthetic efficiency under drought/salt stress [18, 19]. NADH-cytochrome b5 reductase-like proteins participate in maintaining the NADH/NAD+ balance and regulating cellular redox homeostasis [20]. MT2b is a cysteine-rich metal-binding protein that can chelate essential metals such as zinc/copper, as well as toxic heavy metals like cadmium/mercury, to exert dual antioxidant functions through free radical scavenging [21]. High light stress can cause over-excitation of the PSII reaction center, leading to oxidative damage of the D1 protein and accumulation of reactive oxygen species. It is hypothesized that OsTLP16.5 may indirectly regulate PSII repair by interacting with the above-mentioned target proteins. Traditional Sanger sequencing is limited by the interference effects of highly abundant genes, which reduces the rate of positive clone identification. To overcome this, whole-sample next-generation sequencing (NGS) was innovatively performed on the library colonies, systematically improving the detection rate of low-abundance interaction genes. NGS analysis identified 977 possible interacting proteins, and the enrichment results suggest that OsTLP16.5 may be involved in the plant photosystem’s response to high light intensity, which aligns with the hypothesis that OsTLP16.5 influences rice growth and development through PSII repair.
5. Conclusion
In this study, the yeast two-hybrid bait vector BD-16.5 was constructed. After screening the rice cDNA library, 32 potential interacting proteins were identified via Sanger sequencing, and three positive interacting proteins were confirmed through reversal testing. Additionally, next-generation sequencing (NGS) identified 977 potential interacting proteins, with 51 located in the chloroplast. These results lay the foundation for future research into the specific molecular mechanisms underlying OsTLP16.5’s interactions.
References
[1]. Li, Q., & Jiang, X. (2022). Genetic studies on rice yield-related traits. Journal of Anhui Agricultural Sciences, 50(13), 10-13, 20.
[2]. Guo, X., Wang, C., Zhu, Q., et al. (2024). Albino lethal 13, a chloroplast-imported protein required for chloroplast development in rice. Plant Direct, 8(6), e610.
[3]. Kang, Z. (2019). The role of NADPH-dependent thioredoxin reductase C in plant plastids. Chinese Journal of Biochemistry and Molecular Biology, 35(02), 121-130.
[4]. Yokono, M., Takabayashi, A., & Kishimoto. (2019). The PSI-PSII megacomplex in green plants. Plant Cell Physiology, 60(5), 1098-1108.
[5]. Lan, Y., Chen, Q., & Kong, M. (2021). PetM is essential for the stabilization and function of the cytochrome b6f complex in Arabidopsis. Plant Cell Physiology, 62(10), 1603-1614.
[6]. Gao, Y. S., Wang, Y. L., & Wang, X. (2020). Hexameric structure of the ATPase motor subunit of magnesium chelatase in chlorophyll biosynthesis. Protein Science, 29(4), 1040-1046.
[7]. Sheng, X., Liu, Z.,& Kim, E. (2021). Plant and algal PSII-LHCII supercomplexes: Structure, evolution, and energy transfer. Plant Cell Physiology, 62(7), 1108-1120.
[8]. Gollan, P. J., Trotta, A., & Bajwa, A. A. (2021). Characterization of the free and membrane-associated fractions of the thylakoid lumen proteome in Arabidopsis thaliana. International Journal of Molecular Sciences, 22(15), 8126.
[9]. Anderson, J. M., Chow, W. S., & De Las Rivas, J. (2008). Dynamic flexibility in the structure and function of photosystem II in higher plant thylakoid membranes: The grana enigma. Photosynthesis Research, 98(1-3), 575-587.
[10]. Järvi, S., Suorsa, M., & Aro, E.-M. (2015). Photosystem II repair in plant chloroplasts—Regulation, assisting proteins and shared components with photosystem II biogenesis. Biochimica et Biophysica Acta, 1847, 900–909. https://doi.org/10.1016/j.bbabio.2015.01.006
[11]. Liu, J., & Last, R. L. (2017). A chloroplast thylakoid lumen protein is required for proper photosynthetic acclimation of plants under fluctuating light environments. Proceedings of the National Academy of Sciences of the United States of America, 114(38), E8110-E8117.
[12]. Nickelsen, J., & Rengstl, B. (2013). Photosystem II assembly: From cyanobacteria to plants. Annual Review of Plant Biology, 64, 609-635.
[13]. Wang, Y. L., & Kang, Z. (2023). Metabolomic analysis of the thylakoid lumen protein gene OsTLP16.5 mutant in rice. Genomics and Applied Biology, 42(4), 359-372.
[14]. Liu, J., & Last, R. L. (2017). A chloroplast thylakoid lumen protein is required for proper photosynthetic acclimation of plants under fluctuating light environments. Proceedings of the National Academy of Sciences, 114(38). https://www.pnas.org/doi/10.1073/pnas.1712206114
[15]. Fields, S., & Song, O. K. (1989). A novel genetic system to detect protein-protein interactions. Nature, 340(6230), 245-246.
[16]. Erffelinck, M. L., Ribeiro, B., Perassolo, M., et al. (2018). A user-friendly platform for yeast two-hybrid library screening using next-generation sequencing. PLoS ONE, 13(12), e0201270.
[17]. Zhou, Q., Zheng, X., He, H., et al. (2017). New functional perspectives of plant S-adenosylmethionine synthetase. Chemical Life, 37(04), 521-527. https://doi.org/10.13488/j.smhx.20170409
[18]. Zhang, X., Bao, Z., Gong, B., et al. (2020). S-adenosylmethionine synthetase 1 confers drought and salt tolerance in transgenic tomato. Environmental and Experimental Botany, 179, 104226.
[19]. M. W., Wang, Y., Wu, J. Q., et al. (2019). Isolation and characterization of S-adenosylmethionine synthase gene from cucumber and its response to abiotic stress. Plant Physiology and Biochemistry, 141, 431-445.
[20]. Hall, R., Yuan, S., Wood, K., et al. (2022). Cytochrome b5 reductases: Redox regulators of cell homeostasis. Journal of Biological Chemistry, 298(12), 102654.
[21]. Pirzadeh, S., & Shahpiri, A. (2016). Functional characterization of a type 2 metallothionein isoform (OsMTI-2b) from rice. International Journal of Biological Macromolecules, 88, 491-496.
Cite this article
Deng,L.;Zheng,J.;Gong,L.;Li,Y.;Kang,Z. (2025). Utilizing Y2H-seq to screen interacting proteins of rice OsTLP16.5. Journal of Food Science, Nutrition and Health,4(1),24-30.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Journal:Journal of Food Science, Nutrition and Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Li, Q., & Jiang, X. (2022). Genetic studies on rice yield-related traits. Journal of Anhui Agricultural Sciences, 50(13), 10-13, 20.
[2]. Guo, X., Wang, C., Zhu, Q., et al. (2024). Albino lethal 13, a chloroplast-imported protein required for chloroplast development in rice. Plant Direct, 8(6), e610.
[3]. Kang, Z. (2019). The role of NADPH-dependent thioredoxin reductase C in plant plastids. Chinese Journal of Biochemistry and Molecular Biology, 35(02), 121-130.
[4]. Yokono, M., Takabayashi, A., & Kishimoto. (2019). The PSI-PSII megacomplex in green plants. Plant Cell Physiology, 60(5), 1098-1108.
[5]. Lan, Y., Chen, Q., & Kong, M. (2021). PetM is essential for the stabilization and function of the cytochrome b6f complex in Arabidopsis. Plant Cell Physiology, 62(10), 1603-1614.
[6]. Gao, Y. S., Wang, Y. L., & Wang, X. (2020). Hexameric structure of the ATPase motor subunit of magnesium chelatase in chlorophyll biosynthesis. Protein Science, 29(4), 1040-1046.
[7]. Sheng, X., Liu, Z.,& Kim, E. (2021). Plant and algal PSII-LHCII supercomplexes: Structure, evolution, and energy transfer. Plant Cell Physiology, 62(7), 1108-1120.
[8]. Gollan, P. J., Trotta, A., & Bajwa, A. A. (2021). Characterization of the free and membrane-associated fractions of the thylakoid lumen proteome in Arabidopsis thaliana. International Journal of Molecular Sciences, 22(15), 8126.
[9]. Anderson, J. M., Chow, W. S., & De Las Rivas, J. (2008). Dynamic flexibility in the structure and function of photosystem II in higher plant thylakoid membranes: The grana enigma. Photosynthesis Research, 98(1-3), 575-587.
[10]. Järvi, S., Suorsa, M., & Aro, E.-M. (2015). Photosystem II repair in plant chloroplasts—Regulation, assisting proteins and shared components with photosystem II biogenesis. Biochimica et Biophysica Acta, 1847, 900–909. https://doi.org/10.1016/j.bbabio.2015.01.006
[11]. Liu, J., & Last, R. L. (2017). A chloroplast thylakoid lumen protein is required for proper photosynthetic acclimation of plants under fluctuating light environments. Proceedings of the National Academy of Sciences of the United States of America, 114(38), E8110-E8117.
[12]. Nickelsen, J., & Rengstl, B. (2013). Photosystem II assembly: From cyanobacteria to plants. Annual Review of Plant Biology, 64, 609-635.
[13]. Wang, Y. L., & Kang, Z. (2023). Metabolomic analysis of the thylakoid lumen protein gene OsTLP16.5 mutant in rice. Genomics and Applied Biology, 42(4), 359-372.
[14]. Liu, J., & Last, R. L. (2017). A chloroplast thylakoid lumen protein is required for proper photosynthetic acclimation of plants under fluctuating light environments. Proceedings of the National Academy of Sciences, 114(38). https://www.pnas.org/doi/10.1073/pnas.1712206114
[15]. Fields, S., & Song, O. K. (1989). A novel genetic system to detect protein-protein interactions. Nature, 340(6230), 245-246.
[16]. Erffelinck, M. L., Ribeiro, B., Perassolo, M., et al. (2018). A user-friendly platform for yeast two-hybrid library screening using next-generation sequencing. PLoS ONE, 13(12), e0201270.
[17]. Zhou, Q., Zheng, X., He, H., et al. (2017). New functional perspectives of plant S-adenosylmethionine synthetase. Chemical Life, 37(04), 521-527. https://doi.org/10.13488/j.smhx.20170409
[18]. Zhang, X., Bao, Z., Gong, B., et al. (2020). S-adenosylmethionine synthetase 1 confers drought and salt tolerance in transgenic tomato. Environmental and Experimental Botany, 179, 104226.
[19]. M. W., Wang, Y., Wu, J. Q., et al. (2019). Isolation and characterization of S-adenosylmethionine synthase gene from cucumber and its response to abiotic stress. Plant Physiology and Biochemistry, 141, 431-445.
[20]. Hall, R., Yuan, S., Wood, K., et al. (2022). Cytochrome b5 reductases: Redox regulators of cell homeostasis. Journal of Biological Chemistry, 298(12), 102654.
[21]. Pirzadeh, S., & Shahpiri, A. (2016). Functional characterization of a type 2 metallothionein isoform (OsMTI-2b) from rice. International Journal of Biological Macromolecules, 88, 491-496.









