1. Introduction
The cuticle is a vital adaptation of land plants that mediates their interactions with the external environment. As a continuous hydrophobic layer covering the aerial epidermis, it functions as a crucial barrier limiting uncontrolled water loss, shielding from UV radiation, and impeding pathogen and insect invasion [1]. Structurally, the cuticle is composed of a polyester cutin matrix embedded with and overlaid by cuticular waxes, which form the outermost layer. These waxes, through their specific chemical composition and microstructural organization, are responsible for many of the functional properties of the cuticle, including its water-repellent, reflective, and self-cleaning capacities. Despite their minimal visibility to the naked eye, cuticular waxes play disproportionately large roles in plant survival, particularly under stressful environmental conditions.
Cuticular waxes are chemically diverse mixtures of very-long-chain fatty acid (VLCFA) derivatives, including alkanes, alcohols, ketones, aldehydes, esters, and cyclic compounds such as triterpenoids. These molecules originate from complex biosynthetic pathways localized to epidermal cells, requiring the coordinated activity of fatty acid elongation enzymes, modification enzymes, and specialized transporters [2]. The specific composition and quantity of these waxes vary greatly depending on species, organ, developmental stage, and environmental stimuli. In many crops, especially within the Brassicaceae, these differences can have significant implications for performance and adaptation. A classic example is the glossy phenotype, which arises from defects in wax biosynthesis or deposition and is characterized by the absence or reduction of epicuticular crystals, resulting in a smooth, reflective leaf surface.
Kale (Brassica oleracea var. acephala), a non-heading member of the B. oleracea species complex, is globally valued for its nutritional density, containing abundant vitamins, minerals, fiber, and secondary metabolites such as glucosinolates and flavonoids. In recent years, it has also gained attention as a model system for studying leaf morphology, stress responses, and secondary metabolism in Brassicaceae crops, largely due to the availability of genomic resources and its extensive phenotypic diversity. Among these traits, naturally occurring glossy mutants of kale offer a unique opportunity to study the molecular and physiological impacts of wax deficiency. These mutants frequently exhibit increased water loss, reduced drought resistance, and greater susceptibility to pathogens and pests [3, 4]. Although initially treated as a simple visual trait, glossiness now serves as a window into a complex and highly regulated biosynthetic system.
The importance of understanding cuticular wax biology extends beyond basic plant science. Climate variability and increasing abiotic stress pose major threats to crop productivity worldwide. Enhancing traits like cuticular wax production holds potential for improving drought resilience, disease resistance, and post-harvest longevity. However, efforts to manipulate this trait in crops such as kale must consider not only the positive impacts on stress tolerance, but also potential trade-offs in growth, appearance, and consumer preference. Glossy phenotypes may reduce market value due to texture or shelf life concerns. Therefore, a deeper mechanistic understanding of the genetic, molecular, and environmental controls of wax production in kale is essential for guiding breeding and biotechnological strategies.
Despite significant progress in model species like Arabidopsis thaliana, the specific molecular basis of wax deficiency in Brassica oleracea remains incompletely understood. Homologs of key wax biosynthetic genes such as CER1, CER3, KCS, and FAR have been identified in kale and its relatives, yet causal mutations and regulatory pathways are still being elucidated. Additionally, the extent to which transcription factors, such as WIN1/SHN1 and MYB96, modulate wax biosynthesis in response to environmental cues in kale is an active area of research [5]. Advances in genomics, gene editing, and high-throughput phenotyping now offer powerful tools to dissect this trait more precisely and translate findings into practical breeding outcomes.
This review aims to synthesize current knowledge on the molecular mechanisms and physiological consequences of cuticular wax deficiency in glossy kale. We first detail the chemical and structural properties of cuticular waxes and summarize the biosynthetic and transport pathways involved in their formation. We then examine the specific genes and mutations known to contribute to the glossy phenotype, focusing on biosynthesis, export, and regulation. Next, we discuss the physiological and ecological implications of wax deficiency, particularly in relation to water relations, disease susceptibility, and post-harvest performance. Environmental and developmental regulation of wax production is addressed, followed by an evaluation of current and emerging strategies for improving this trait through breeding and biotechnology. By integrating molecular, physiological, and agronomic perspectives, this review provides a comprehensive framework for advancing research and improvement of wax-related traits in kale and other Brassica crops.
2. Composition, structure, and biosynthesis of cuticular wax
Cuticular waxes form the outermost barrier of the aerial surfaces of land plants. This layer is essential for maintaining water balance, resisting environmental assaults, and interacting with the biotic environment. In kale (Brassica oleracea var. acephala), the quantity, composition, and structure of cuticular wax have profound effects on physiological traits, including drought resistance and pest deterrence. A thorough understanding of the chemical heterogeneity, supramolecular structure, and biosynthetic machinery of wax is crucial for deciphering the mechanisms underlying wax deficiency in glossy mutants and for informing crop improvement strategies.
2.1. Chemical composition of epicuticular waxes
Cuticular waxes are complex mixtures of predominantly aliphatic compounds derived from very-long-chain fatty acids (VLCFAs), typically ranging from C20 to C34 or longer. These components include alkanes, primary and secondary alcohols, aldehydes, ketones, and wax esters, alongside cyclic compounds such as triterpenoids and flavonoids.
Alkanes are often the dominant class, especially in Brassicaceae species, and are primarily composed of odd-numbered straight-chain hydrocarbons (e.g., C29, C31, C33). These molecules contribute significantly to hydrophobicity and water barrier function. Primary alcohols, derived from the reduction of acyl-CoAs, are typically even-numbered (e.g., C28, C30), while secondary alcohols and ketones arise from hydroxylation or oxidation of alkanes, often by enzymes like MAH1. Wax esters result from esterification of primary alcohols with fatty acids and are critical for modulating wax melting point and mechanical properties.
In addition to these aliphatic compounds, cyclic constituents such as pentacyclic triterpenoids (e.g., lupeol, β-amyrin) and flavonoids are frequently embedded in or deposited on the surface, influencing UV protection, insect deterrence, and physical properties of the wax layer.
Kale cultivars exhibit variation in wax composition, with typical profiles dominated by C29 alkanes, ketones, and C28–C30 alcohols. Environmental conditions and genetic background both influence these profiles, contributing to inter-cultivar variability.
2.2. Supramolecular structure and epicuticular wax morphology
Beyond composition, the functional properties of cuticular wax are strongly influenced by their physical arrangement into epicuticular crystals. These structures form through self-assembly processes governed by the dominant chemical constituents and environmental cues.
Epicuticular crystals vary widely in shape and organization, appearing as platelets, tubules, rods, or amorphous films. For instance, waxes rich in alkanes and ketones often form tubular or rodlet structures, typical of Brassicaceae, whereas alcohol-rich waxes form platelets. This micro-topography alters leaf surface characteristics, affecting wettability, light reflection, and biotic interactions [6, 7].
Glossy phenotypes in kale often result from altered composition that prevents normal crystal formation, producing a smooth, reflective surface. Scanning electron microscopy (SEM) studies have shown that glossy mutants lack the characteristic wax tubules seen in wild-type plants, confirming the disruption of normal deposition patterns.
2.3. Biosynthesis of cuticular wax: enzymatic pathways and regulatory nodes
Cuticular wax biosynthesis occurs predominantly in epidermal cells, with enzymes localized to the endoplasmic reticulum (ER). The pathway involves three major stages: (1) VLCFA elongation, (2) modification into final wax products, and (3) export across the cell wall and deposition onto the surface.
2.3.1. VLCFA elongation
The initial and rate-limiting step in wax biosynthesis is the elongation of C16/C18 acyl-CoAs into VLCFAs. This process is mediated by the fatty acid elongase (FAE) complex, composed of four core enzymes: β-ketoacyl-CoA synthase (KCS), β-ketoacyl-CoA reductase (KCR), β-hydroxyacyl-CoA dehydratase (HCD), and enoyl-CoA reductase (ECR).
KCS enzymes determine chain-length specificity and are encoded by multigene families. Mutations in CER6 (also known as KCS6) and KCS1 in Arabidopsis lead to wax deficiency and glossy phenotypes, and homologous genes have been identified in kale [8]. Other components such as PAS2 (encoding HCD) and CER10 (ECR) are equally essential. Disruption of these enzymes reduces VLCFA availability, thus impairing downstream wax formation (Bach et al., 2008; Zheng et al., 2005).
2.3.2. Modification into wax components
Following elongation, VLCFAs are modified into various wax products via two main branches: the alkane-forming pathway and the alcohol-forming pathway.
In the alkane pathway, VLCFAs are first reduced to aldehydes, then decarbonylated into alkanes by the CER1-CER3 complex. Secondary alcohols and ketones are derived from mid-chain hydroxylation and oxidation of alkanes, often catalyzed by the cytochrome P450 enzyme MAH1. Disruption in any of these genes leads to severe wax deficiencies and is a common cause of glossiness in Brassica crops.
In the alcohol-forming branch, VLCFAs are reduced to primary alcohols by enzymes like CER4, followed by esterification with fatty acyl-CoAs to generate wax esters via wax synthases such as WSD1. Although alcohols are less hydrophobic than alkanes, they still contribute to cuticle function and structure.
2.3.3. Export and deposition
The final stage involves transporting hydrophobic wax molecules from the ER to the cuticle surface. This is mediated by ATP-binding cassette (ABC) transporters, notably ABCG12 (CER5) and ABCG11. These proteins function as exporters at the plasma membrane, and their mutations cause intracellular accumulation of wax precursors.
The passage through the hydrophilic cell wall remains poorly understood but may involve lipid transfer proteins (LTPs) and vesicle-mediated mechanisms. Upon reaching the cuticle, wax molecules self-organize into crystals based on their chemical structure and external conditions
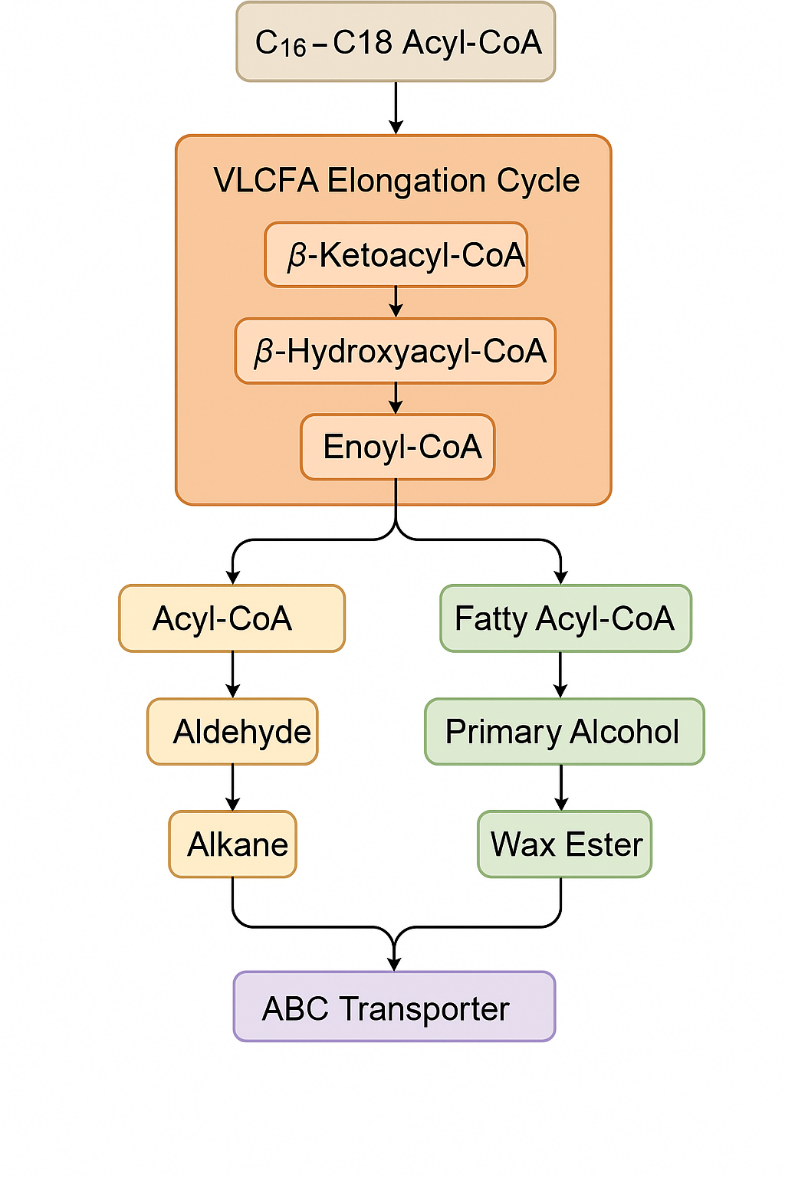
The pathway initiates with C16–C18 Acyl-CoA precursors, which are elongated into very-long-chain fatty acids (VLCFAs) through the iterative four-step VLCFA elongation cycle. Following elongation, VLCFAs are directed into two major modification branches. In the alkane-forming pathway, VLCFAs are converted first to aldehydes and subsequently to alkanes, which are major hydrophobic components of the cuticle. In the parallel alcohol-forming pathway, VLCFAs are reduced to form primary alcohols, which can be further esterified with other fatty acids to generate wax esters. Finally, the completed wax components from both branches are exported from the epidermal cell to the cuticle surface via ATP-binding cassette (ABC) transporters
3. Molecular mechanisms underlying wax deficiency in glossy kale
The glossy phenotype observed in certain kale cultivars is the visible manifestation of a disrupted cuticular wax layer. Characterized by a smooth, shiny leaf surface lacking epicuticular crystals, this trait is most often caused by mutations in genes involved in wax biosynthesis, transport, or regulation. Although much of our understanding stems from research in Arabidopsis thaliana, increasing evidence from Brassica species confirms the conservation of key pathways. Understanding the genetic architecture of wax deficiency is essential not only for elucidating fundamental plant biology but also for developing breeding strategies to improve stress resilience in kale.
3.1. Disruption of wax biosynthetic pathways
3.1.1. Mutations in VLCFA elongation genes
The elongation of C16 and C18 fatty acyl-CoAs to VLCFAs is foundational to wax biosynthesis. Mutations in genes encoding the four-step FAE complex—particularly KCS family genes—can severely curtail VLCFA production. In Arabidopsis, CER6 (KCS6) and KCS1 mutants exhibit drastically reduced wax loads and a glossy appearance (Millar et al., 1999; Todd et al., 1999). Homologous genes in kale, such as BoKCS6, show strong expression in leaf epidermal tissues and are candidate loci for glossiness in several cultivars [8].
Beyond KCS, mutations in other elongase components such as CER10 (ECR) and PAS2 (HCD) also lead to wax deficiency. These genes are essential for completing the elongation cycle and their loss-of-function results in accumulation of short-chain intermediates and reduced VLCFA output (Bach et al., 2008; Zheng et al., 2005). In Brassica napus, RNA interference or CRISPR-mediated disruptions in these orthologs reproduce glossy-like phenotypes (Pu et al., 2017; Wang et al., 2015).
3.1.2. Defects in wax component modification
Once VLCFAs are synthesized, they are processed into various wax components. Mutations in the alkane-forming pathway are especially associated with the glossy trait. The CER1-CER3 complex catalyzes aldehyde decarbonylation into alkanes, a critical step for forming hydrophobic, crystal-forming compounds. Loss of function in either gene leads to severe alkane deficiency and accumulation of upstream aldehydes (Aarts et al., 1995; Bernard et al., 2012). In Brassica oleracea, BoCER1 mutations have been directly linked to the glossy phenotype in cauliflower and cabbage, suggesting a similar mechanism in kale.
MAH1, encoding a cytochrome P450 hydroxylase, introduces mid-chain hydroxyl groups to alkanes, producing secondary alcohols and ketones. These modifications are critical for forming tubular epicuticular wax structures in Brassicaceae. Disruption of MAH1 reduces secondary alcohol content and alters crystal morphology, potentially contributing to modified or partial glossiness.
The alcohol-forming pathway, governed by CER4 (primary alcohol formation) and WSD1 (wax ester synthase), plays a supporting role. While defects here may not cause classic glossiness, they do contribute to reduced wax load and altered composition, with secondary physiological effects.
3.2. Impairment of wax transport and secretion
Even with normal biosynthesis, wax molecules must be transported across the plasma membrane and cell wall to reach the surface. This transport relies heavily on ATP-binding cassette (ABC) transporters of the G family.
ABCG12 (CER5) and ABCG11 form a functional complex responsible for exporting a broad range of wax components. In Arabidopsis, mutations in CER5 lead to glossy leaves and internal accumulation of wax precursors. ABCG11 appears to heterodimerize with CER5, and its disruption has similarly severe effects. Homologs in kale are yet to be functionally characterized, but genome comparisons suggest conservation and probable involvement in certain extreme glossy phenotypes [2].
Transport defects often result in stronger phenotypes than biosynthetic mutations, due to the systemic failure to deliver any wax to the surface. Thus, genes such as BoABCG12 and BoABCG11 remain key targets for future functional validation in kale.
3.3. Regulatory failures: transcriptional control of wax biosynthesis
Wax biosynthesis is under tight transcriptional control, responsive to both developmental cues and environmental stimuli. Transcription factors (TFs) form hierarchical networks that coordinate gene expression across the biosynthetic pathway.
One of the central regulators is WIN1/SHN1, an AP2/EREBP-type TF that directly upregulates KCS1, CER1, and CER4. Overexpression of WIN1 leads to increased wax deposition, whereas knockouts result in decreased wax and heightened cuticle permeability [9]. Brassica homologs of WIN1 have been identified and shown to enhance wax accumulation when overexpressed in transgenic systems.
MYB-type transcription factors are also critical. MYB96 is a drought-inducible regulator that activates multiple wax genes, often in concert with ABA signaling. MYB94, MYB30, MYB106, and MYB16 contribute to tissue-specific expression and developmental regulation of wax genes. Mutations in BoMYB96 or its network members could explain environmentally sensitive or developmentally variable glossiness.
An additional layer of control involves RNA-based regulation. For instance, CER7 influences wax biosynthesis by modulating small RNA levels that target regulatory TFs. Such mechanisms, though less explored in kale, offer promising directions for future discovery.
3.4. Gene discovery strategies in brassica
Identifying causal genes in kale requires integrative genetic and genomic approaches. Quantitative trait locus (QTL) mapping in segregating populations, fine-mapping, and synteny-based candidate gene analysis have been widely used [8]. Genome-wide association studies (GWAS) in diverse germplasm collections are also effective, especially when paired with high-resolution phenotyping and metabolomic profiling.
With the decreasing cost of sequencing, whole-genome resequencing of glossy mutants versus wild-type controls is becoming the method of choice. This approach allows rapid identification of nonsynonymous SNPs or indels in known wax-related genes and facilitates the development of tightly linked molecular markers for marker-assisted selection (MAS).
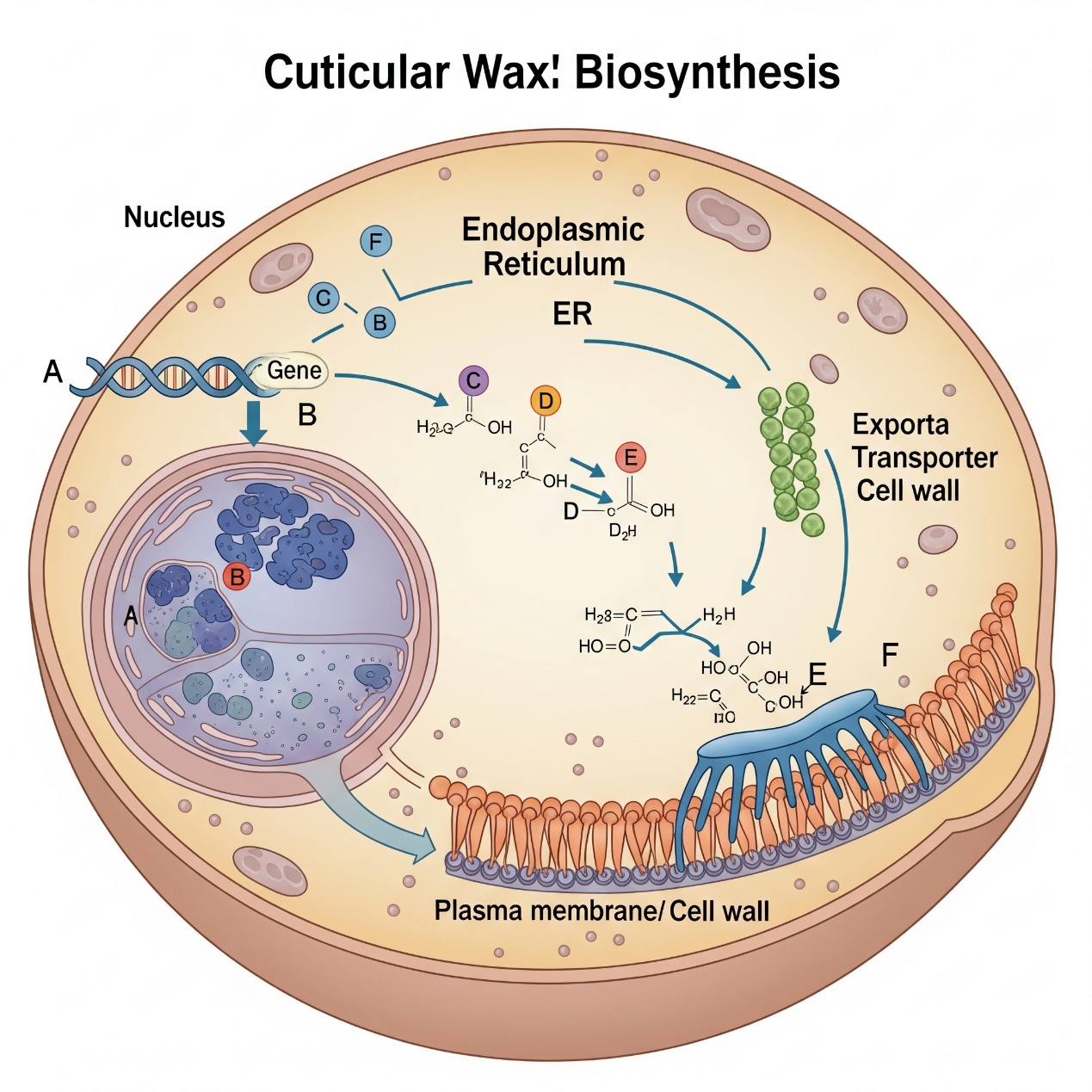
The diagram illustrates the three critical stages of cuticular wax production within a single plant epidermal cell—transcriptional regulation, biosynthesis, and transport—and highlights the key points where genetic defects can lead to the glossy phenotype. The process begins in the nucleus (1. Regulation), where environmental and developmental signals activate (A) Regulatory Transcription Factors (e.g., WIN1/SHN1, MYB96). These regulators induce the expression of (B) Wax Biosynthesis Genes (e.g., KCS, CER1). Subsequently, on the endoplasmic reticulum (2. Biosynthesis), the encoded enzymes catalyze the elongation of fatty acid precursors via (C) the VLCFA Elongase Complex and modify them into final wax components through (D) the alkane-forming pathway (e.g., CER1-CER3 complex) and (E) the alcohol-forming pathway (e.g., CER4). Finally, the synthesized wax molecules are exported from the cell (3. Transport) by (F) ABC Transporters (e.g., ABCG11/ABCG12) located at the plasma membrane. Mutations in any of these key genetic components (A–F) can disrupt the pathway, leading to a deficiency in epicuticular wax deposition and causing the characteristic glossy leaf surface.
4. Phenotypic consequences of wax deficiency in glossy kale
Glossy kale phenotypes, resulting from impaired cuticular wax biosynthesis or deposition, are associated with a cascade of physiological and ecological consequences. These go far beyond altered leaf appearance, affecting water regulation, pathogen resistance, pest interactions, abiotic stress responses, and post-harvest quality. Understanding these multifaceted effects is essential for evaluating the agronomic fitness of wax-deficient mutants and for making informed decisions in breeding programs.
4.1. Increased cuticular permeability and drought susceptibility
One of the most pronounced outcomes of wax deficiency is the loss of the cuticle’s barrier function against water loss. Epicuticular waxes, particularly alkanes and wax esters, significantly reduce non-stomatal transpiration by forming a hydrophobic surface layer. Glossy mutants, which lack sufficient wax load or proper crystal structure, exhibit elevated cuticular conductance even under non-stressful conditions [3, 4].
This increased water loss becomes critical during drought, when stomata close and non-stomatal pathways dominate transpiration. Glossy kale plants typically wilt earlier, show more severe desiccation symptoms, and suffer greater biomass reduction compared to wild-type counterparts under water-deficit conditions. Reduced wax accumulation also limits the plant's capacity to upregulate cuticle thickness or change composition adaptively in response to drought stress. Thus, the glossy trait substantially compromises drought tolerance, especially in climates with erratic rainfall or limited irrigation.
4.2. Heightened susceptibility to pathogens and insect pests
The wax layer acts as a first physical and chemical barrier to microbial infection. It restricts pathogen access by obstructing spore adhesion, germ tube penetration, and appressorial formation. Several studies in Arabidopsis and Brassica have linked wax deficiency to increased susceptibility to fungal and bacterial pathogens such as Botrytis cinerea, Alternaria brassicicola, and Pseudomonas syringae [10, 11].
In glossy kale, although direct studies remain limited, it is likely that similar mechanisms operate. A disrupted cuticle enables easier pathogen ingress and may also impair the perception and transmission of damage-associated molecular patterns (DAMPs), leading to delayed immune responses [12]. In addition, altered wax composition may reduce antimicrobial properties or fail to trigger resistance signaling.
Insect interactions are also affected. Epicuticular wax crystals influence insect movement, attachment, oviposition, and feeding behavior. Waxy surfaces often deter insects by creating a slippery texture and lacking chemical cues for host recognition. Glossy surfaces, in contrast, may enhance attachment or expose underlying epidermis to feeding [13]. Studies in Brassica species have shown increased aphid colonization, flea beetle damage, and diamondback moth oviposition in glossy lines [14, 15]. However, effects may vary depending on pest species, insect behavior, and environmental conditions.
4.3. Increased sensitivity to abiotic stresses
Beyond water loss, the cuticular wax layer contributes to tolerance against a range of abiotic stresses, including UV radiation, thermal extremes, and pollutant exposure. Wax crystals scatter and reflect solar radiation, reducing photodamage and UV-B penetration into leaf tissues [7]. Glossy mutants, with their diminished wax content and altered surface topography, are more susceptible to light-induced oxidative stress, particularly under high irradiance or low humidity conditions.
Wax also plays a role in the “Lotus effect,” enabling water droplets to roll off the leaf surface and carry away dust, spores, and pollutants [6]. Glossy leaves, lacking this self-cleaning mechanism, may accumulate particles that block stomata, interfere with photosynthesis, or harbor pathogens.
In extreme temperatures, wax serves as a thermal insulator, stabilizing leaf surface temperature. Disruption in wax properties may exacerbate heat stress or cold-induced membrane rigidification [16]. Although these aspects remain underexplored in kale, evidence from other Brassica species suggests that wax-deficient lines perform poorly in field conditions with fluctuating temperatures.
4.4. Reduced post-harvest quality and shelf life
For leafy vegetables like kale, post-harvest water loss is a key determinant of freshness, texture, and marketability. The cuticle and associated waxes limit transpirational loss after harvest, thereby maintaining turgor and visual appeal. Glossy leaves, due to their higher permeability, exhibit accelerated wilting, loss of firmness, and reduced shelf life during storage and transport [17].
Moreover, increased cuticle permeability may enhance susceptibility to post-harvest decay by saprophytic fungi and bacteria, further reducing product quality. These traits pose challenges for commercial production and consumer acceptance, particularly in markets that demand high standards of appearance and freshness.
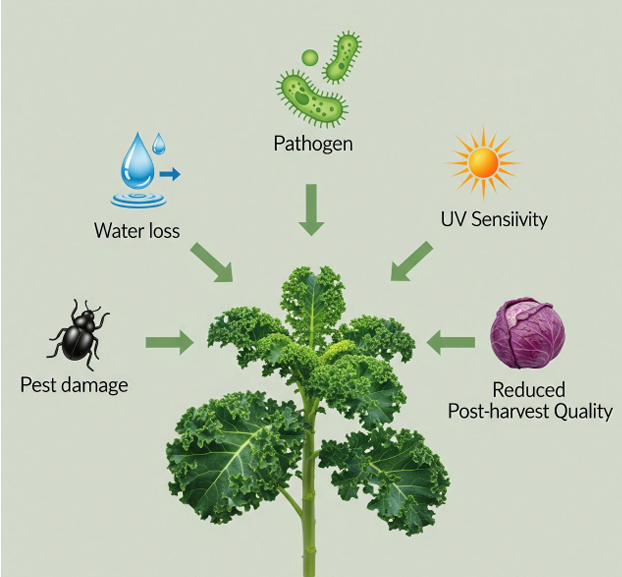
This diagram summarizes the cascade of detrimental effects resulting from a compromised cuticular wax layer. The deficiency directly impairs the cuticle's protective barrier function, leading to several negative outcomes for the plant. Most notably, it increases cuticular permeability, which causes accelerated water loss and significantly heightens susceptibility to drought stress. The weakened physical barrier also facilitates easier pathogen entry for fungi and bacteria and can increase pest damage by altering insect attachment, feeding, and oviposition behaviors. Furthermore, the lack of epicuticular wax crystals diminishes the reflection of solar radiation, leading to increased sensitivity to abiotic stresses such as UV damage. Finally, these vulnerabilities, particularly the high rate of transpiration, contribute to reduced post-harvest quality, manifesting as faster wilting, loss of firmness, and a shorter shelf life.
5. Environmental and developmental regulation of cuticular wax production
The biosynthesis and deposition of cuticular wax are not static processes but are tightly regulated by both environmental stimuli and internal developmental cues. This dynamic regulation enables plants to fine-tune wax load and composition in response to abiotic stresses and organ-specific demands. In kale, such regulation determines not only the degree of wax accumulation but also the severity and manifestation of glossy phenotypes under different conditions.
5.1. Environmental regulation: plasticity in response to abiotic cues
Among environmental factors, water availability is a major driver of wax production. Under drought stress, many plants—including kale and other Brassica species—enhance cuticular wax deposition, particularly alkanes and VLCFAs, which reduce non-stomatal water loss [2, 4]. This response is often mediated by abscisic acid (ABA), which induces the expression of transcription factors such as MYB96, MYB94, and WIN1/SHN1, ultimately upregulating wax biosynthetic genes like KCS1, CER1, and CER4 [5].
Conversely, high humidity environments suppress wax biosynthesis. Studies in tomato and Arabidopsis have shown that high atmospheric moisture leads to thinner cuticle layers and reduced expression of wax-related genes (Leide et al., 2011). For glossy kale mutants, this suppression may exacerbate the already impaired wax accumulation, making them even more vulnerable in humid yet pathogen-prone environments.
Light intensity and UV radiation also influence wax production. Exposure to strong illumination or UV-B can induce wax accumulation as a photoprotective strategy, improving leaf reflectance and reducing cellular oxidative stress [7]. The increased synthesis under light stress is thought to involve light signaling components such as HY5 and COP1, which intersect with ABA pathways. Glossy mutants, even if genetically impaired in biosynthesis, may show differential responses depending on their residual pathway activity and environmental conditions.
Temperature stress is another factor that modulates wax composition. Low temperatures often increase the proportion of saturated components and alcohols to stabilize membrane and cuticle fluidity, while high temperatures may trigger the deposition of more volatile compounds [16]. These modifications can alter wax crystallinity and physical properties, influencing both stress resistance and surface appearance.
Other environmental factors, including wind, mechanical abrasion, air pollution, and nutrient availability, have also been shown to influence wax accumulation, though their mechanisms remain less understood [18, 19]. In agricultural settings, these variables may explain intra-cultivar variation in glossiness and performance, even under genetically identical conditions.
5.2. Developmental regulation: temporal and spatial specificity
Wax deposition varies considerably during plant development. Young, expanding leaves actively synthesize cuticular waxes, while mature tissues often display plateaued or declining production [20]. In kale, mature outer leaves typically accumulate more wax than inner, younger leaves, reflecting differences in exposure and physiological demand.
Organ specificity is also evident. Stems, leaves, flowers, and siliques differ not only in wax load but also in composition and crystal morphology, tailored to their ecological function. For example, floral organs often contain more triterpenoids, while leaves emphasize alkanes and ketones for water retention and photoprotection. Such differences are regulated at the transcriptional level, with tissue-specific expression of wax pathway genes and transcription factors like MYB16, MYB30, and WIN1.
Leaf position, age, and epidermal cell differentiation status affect both wax composition and architecture. During leaf maturation, shifts in gene expression lead to compositional changes—e.g., increased alkane-to-alcohol ratio—that may affect physical structure and function. This developmental plasticity may partially explain why some glossy phenotypes are more apparent at specific growth stages or leaf positions.
Epidermal differentiation is intimately linked to cuticle formation. Genes that determine epidermal identity, such as GLABRA1 and TTG1, indirectly influence cuticle traits. Moreover, cell expansion during organogenesis may be required to establish the proper secretory machinery for wax export. Any disruptions in early epidermal development may therefore compromise later wax deposition, especially in mutants.
Taken together, the interaction between environmental and developmental regulation creates a highly plastic wax phenotype. In glossy kale mutants, the severity of wax deficiency—and its associated physiological impact—can vary considerably depending on age, light, water status, and temperature. Recognizing this complexity is critical for accurate phenotype assessment and for tailoring breeding strategies to specific environments.
6. Breeding and biotechnological approaches to modulate kale cuticular wax
Given the critical roles of cuticular wax in stress tolerance, pathogen defense, and post-harvest performance, improving wax characteristics through breeding or genetic engineering presents a promising strategy for enhancing kale resilience and quality. This section outlines current and emerging approaches—ranging from conventional phenotypic selection to precision gene editing—targeted at either suppressing undesirable glossy phenotypes or enhancing beneficial wax traits.
6.1. Conventional breeding and Marker-assisted Selection (MAS)
Traditional kale breeding programs have long employed phenotypic selection to eliminate severely glossy individuals, which often display compromised drought tolerance and increased pathogen susceptibility. While effective, this method is labor-intensive and environmentally confounded. Glossiness may not consistently manifest under high humidity or low light conditions, potentially obscuring genotype-phenotype associations.
The integration of molecular markers into breeding workflows allows for more efficient selection. Once causal mutations in genes such as BoCER1, BoKCS6, or BoABCG12 are identified, closely linked DNA markers can be developed to enable early selection of desirable alleles at the seedling stage, regardless of environmental variables [8]. Marker-assisted selection (MAS) is particularly valuable when targeting recessive glossy traits, which may segregate in large breeding populations.
Natural allelic variation in wax-related traits across Brassica oleracea germplasm also presents opportunities for quantitative improvement. Several QTLs associated with wax load, composition, and glossiness have been identified using segregating populations or genome-wide association studies (GWAS). By pyramiding favorable alleles, breeders may develop cultivars with increased wax deposition that maintain desirable appearance and consumer acceptance.
However, caution must be exercised to avoid overaccumulation of wax, which may impede gas exchange or negatively affect leaf texture. As with any multigenic trait, balancing yield, stress tolerance, and marketability remains a central breeding challenge.
6.2. Transgenic approaches for enhanced wax biosynthesis
Transgenic overexpression of positive regulators or biosynthetic enzymes has been used in model systems and some crops to increase wax content. For instance, constitutive expression of WIN1/SHN1, MYB96, or CER1 in Arabidopsis and tomato significantly enhanced wax accumulation and conferred improved drought tolerance [5, 9]. These results demonstrate the potential of transcriptional reprogramming to activate entire wax biosynthetic pathways.
In Brassica napus, heterologous expression of BnWSD1 or BnCER1 in Arabidopsis confirmed functional conservation and led to increased wax ester or alkane content. These findings suggest that transgenic manipulation of wax pathways is feasible in Brassica oleracea as well. However, public concerns about GMOs, regulatory hurdles, and potential pleiotropic effects—such as reduced leaf surface gloss or metabolic burdens—must be considered before deployment in commercial kale cultivars.
Furthermore, transgenic strategies must be tailored to the target environment and consumer market. In some culinary applications, moderate glossiness may be acceptable or even desirable. Thus, fine-tuning expression levels using tissue-specific or stress-inducible promoters may provide a balanced solution.
6.3. Genome editing: precision engineering of wax traits
CRISPR-Cas technologies now enable highly precise modifications of endogenous genes, offering a powerful alternative to transgenic approaches. In the context of kale wax improvement, several strategies can be envisioned:
Gene knock-in or repair: Correcting known loss-of-function mutations in genes such as BoCER1 in glossy mutants can restore wax production while preserving elite cultivar backgrounds.
Promoter editing: Tuning expression levels of key wax genes (CER4, WSD1) via cis-regulatory modification can optimize composition without introducing new genes.
Allele replacement: Introducing favorable alleles identified from diverse B. oleracea lines can broaden wax diversity while avoiding linkage drag associated with conventional introgression.
Such approaches offer the advantage of minimal genomic disruption and may be exempt from strict GMO regulation in certain jurisdictions. Nevertheless, the success of genome editing in kale requires robust transformation protocols, which are still under optimization in many Brassica vegetables.
6.4. Considerations and future challenges
While enhancing wax content can improve stress tolerance and shelf life, it is essential to balance potential trade-offs. Excessive wax may reduce leaf gas exchange or light absorbance, impairing photosynthesis and growth. Moreover, consumer preferences for leaf texture, color, and sheen vary across markets, necessitating region-specific breeding targets.
Metabolic costs also warrant attention. Biosynthesis of cuticular wax is energetically expensive, requiring substantial carbon and ATP investment. Redirecting resources toward wax production under non-stressful conditions could reduce biomass accumulation or alter secondary metabolism. Future strategies should therefore integrate multi-trait genomic selection models, optimizing wax traits in conjunction with growth, yield, and nutritional quality.
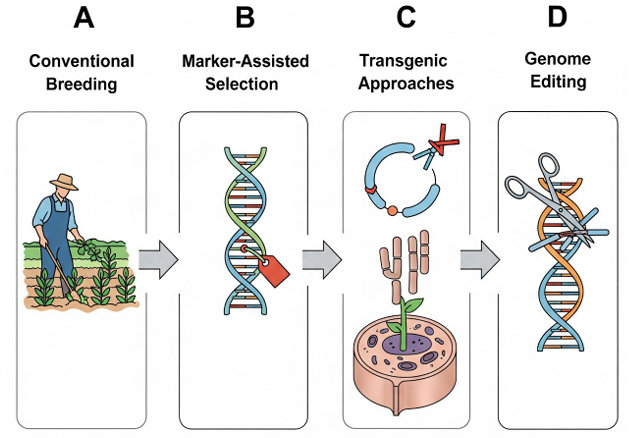
This diagram illustrates the progression of methodologies aimed at modulating wax characteristics, moving from traditional breeding to modern biotechnology. (A) Conventional Breeding relies on direct phenotypic selection of individuals with desirable wax traits (e.g., non-glossy, high-wax) from large, segregating populations. (B) Marker-Assisted Selection (MAS) enhances breeding efficiency by using DNA markers linked to specific genes to select for favorable alleles at an early stage, regardless of environmental influences. (C) Transgenic Approaches involve the introduction and/or overexpression of key positive regulators or biosynthetic enzymes (e.g., WIN1/SHN1) to systematically enhance wax production and confer improved stress tolerance. Representing the frontier of precision, (D) Genome Editing technologies like CRISPR-Cas enable the targeted repair of faulty genes, fine-tuning of gene expression via promoter editing, or the direct replacement of alleles to optimize wax traits with minimal disruption to the native genome.
7. Conclusion and future perspectives
Cuticular wax is a critical component of the kale leaf surface. It forms a complex, multifunctional barrier that protects the plant against desiccation, pathogen invasion, insect herbivory, and damaging ultraviolet radiation. In glossy kale varieties, this protective barrier is structurally and functionally compromised due to mutations affecting the intricate processes of wax biosynthesis, chemical modification, transport, or regulation. As a direct consequence, these mutants exhibit elevated cuticular water loss, a greater susceptibility to a range of environmental and biological stresses, and reduced post-harvest performance. Together, these traits negatively impact both the agronomic resilience and the commercial quality of the crop.
This review has synthesized the current understanding of the molecular architecture of cuticular wax biosynthesis in Brassica oleracea var. acephala. We have highlighted the central role of very-long-chain fatty acid (VLCFA) elongation enzymes (e.g., KCS, CER10), the alkane- and alcohol-forming pathways (e.g., CER1, CER3, MAH1, CER4), and the essential ABCG transporters that deliver wax components to the surface (e.g., CER5, ABCG11). Furthermore, we examined the sophisticated regulatory networks governed by transcription factors like WIN1/SHN1 and MYB96, which integrate developmental signals and environmental cues to fine-tune wax production. Environmental variables such as drought, light intensity, and humidity, together with developmental factors like leaf age and organ identity, jointly modulate wax accumulation, composition, and crystal structure. These findings collectively reveal a dynamic and highly coordinated system that ultimately determines the cuticle’s integrity and its capacity for plastic adaptation.
Recent advances in molecular genetics, high-throughput sequencing, and precision gene editing offer promising new opportunities for the targeted manipulation of wax traits in kale. Marker-assisted selection and quantitative trait locus (QTL) mapping can accelerate the elimination of undesirable glossy alleles from breeding programs, while transgenic and CRISPR-based strategies may enable the introduction or correction of specific gene variants to enhance wax production. However, breeding for optimal cuticular wax traits is not a simple task and requires navigating potential trade-offs. These can include altered photosynthetic efficiency, significant metabolic costs, or changes to leaf appearance that affect consumer preference. Ultimately, the successful improvement of wax-related traits will require integrative approaches that combine deep molecular understanding with rigorous, practical evaluations under field conditions.
To further advance research and application in this field, several key future directions should be prioritized:
Comprehensive Genetic Characterization. There is a pressing need to identify and functionally validate the causal mutations underlying the glossy phenotype across diverse kale germplasm collections. Integrating genomics, transcriptomics, and metabolic profiling will be essential for the fine-mapping of trait-associated loci, which will in turn provide precise targets for modern, marker-assisted breeding and gene editing efforts.
Functional Dissection of Wax Composition. While total wax load is a commonly measured trait, the specific functional roles of distinct chemical constituents—such as alkanes, alcohols, ketones, and esters—remain underexplored in kale. Elucidating how these individual molecules differentially contribute to stress resistance, surface interactions, and post-harvest quality will be critical for true trait optimization, moving beyond simply increasing total wax content.
Environmental Regulation and Plasticity Studies. The effects of combined, realistic environmental stresses (e.g., simultaneous drought and heat, or high light and UV exposure) on wax production and function are not yet fully understood in B. oleracea. Dedicated studies under both controlled and field-relevant settings are necessary to improve our understanding of context-dependent wax expression and to build crops that are genuinely resilient in fluctuating agricultural environments.
Exploring Cuticle-Microbiome Interactions. An exciting frontier of research is the interplay between the cuticle and the leaf’s microbial inhabitants. Altered wax composition may significantly influence the assembly and function of the phyllosphere microbiome, with profound consequences for plant health and disease resistance. Investigating these interactions may reveal novel layers of plant defense or identify indirect breeding targets related to promoting beneficial microbial communities.
Trait Integration and Multi-objective Breeding. Future breeding programs must aim to integrate wax-related traits with other essential agronomic objectives, including yield, nutrient content, and resistance to other pests. This holistic approach is necessary to avoid detrimental trade-offs. Predictive genomic selection models, aided by high-throughput phenomics and metabolomics, will be instrumental in achieving the complex goal of optimizing multiple traits simultaneously.
In conclusion, the glossy trait in kale, though visually subtle, provides a powerful lens through which to understand the fundamental principles of plant surface biology and their broad implications for crop performance. By leveraging both traditional knowledge and emerging technologies, researchers and breeders are now better equipped than ever to develop kale cultivars that are not only more resilient and resource-efficient but are also tailored to meet the evolving demands of agriculture and consumers in a rapidly changing global climate.
Author contributions
Shuyuan Kong is the sole author and responsible for all aspects of this work, including Conceptualization, Investigation, Data Curation, Formal Analysis, Funding Acquisition, and Writing – Original Draft, Review & Editing.
Acknowledgments
The authors would like to express their sincere gratitude to the members of China Agricultural University for their insightful discussions and technical support throughout this research. We also thank the reviewers for their constructive comments, which significantly improved the quality of this manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
[1]. Yeats, T. H., & Rose, J. K. C. (2013). The formation and function of plant cuticles.Plant Physiology,163(1), 5–20. https: //doi.org/10.1104/pp.113.222737
[2]. Lee, S. B., & Suh, M. C. (2015). Advances in the understanding of cuticular waxes in Arabidopsis thaliana and crop species.Plant Cell Reports,34(4), 557–572. https: //doi.org/10.1007/s00299-015-1772-2Frontiers
[3]. Leide, J., Hildebrandt, U., Reussing, K., Riederer, M., & Vogg, G. (2007). The developmental pattern of tomato fruit wax accumulation and its impact on cuticular transpiration barrier properties: Effects of a deficiency in a β-ketoacyl-coenzyme A synthase (LeCER6).Plant Physiology,144(3), 1667–1679. https: //doi.org/10.1104/pp.107.099481.
[4]. Kosma, D. K., Bourdenx, B., Bernard, A., Parsons, E. P., Lü, S., Joubès, J., & Jenks, M. A. (2009). The impact of water deficiency on leaf cuticle lipids of Arabidopsis.Plant Physiology,151(4), 1918–1929. https: //doi.org/10.1104/pp.109.141911
[5]. Seo, P. J., Lee, S. B., Suh, M. C., Park, M. J., Go, Y. S., & Park, C. M. (2011). The MYB96 transcription factor regulates cuticular wax biosynthesis under drought conditions in Arabidopsis.The Plant Cell, 23(3), 1138–1152. https: //doi.org/10.1105/tpc.111.083485
[6]. Barthlott, W., & Neinhuis, C. (1997). Purity of the sacred lotus, or escape from contamination in biological surfaces.Planta, 202(1), 1–8. https: //doi.org/10.1007/s004250050096Frontiers.
[7]. Holmes, M. G., & Keiller, D. R. (2002). Effects of pubescence and waxes on the reflectance of leaves in the ultraviolet and photosynthetic wavebands: A comparison of a range of species.Plant, Cell & Environment,25(1), 85–93. https: //doi.org/10.1046/j.0016 8025.2001.00800.x
[8]. Jessen, D., Roth, C., Wiermer, M., & Fulda, M. (2011). Arabidopsis long-chain acyl-CoA synthetase 1 is involved in peroxisomal fatty acid degradation and cuticle biosynthesis.The Plant Cell, 23(3), 1132–1147. https: //doi.org/10.1105/tpc.110.082602
[9]. Aharoni, A., Dixit, S., Jetter, R., Thoenes, E., van Arkel, G., & Pereira, A. (2004). The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis.The Plant Cell,16(9), 2463–2480. https: //doi.org/10.1105/tpc.104.022897
[10]. Bessire, M., Borel, S., Fabre, G., Carrac, L., Efremova, N., Yephremov, A., & Nawrath, C. (2007).A member of the PLEIOTROPIC DRUG RESISTANCE family of ATP binding cassette transporters is required for the export of cuticular wax components in Arabidopsis.The Plant Cell,19(3), 1148–1161. https: //doi.org/10.1105/tpc.106.042390
[11]. Isaacson, T., Kosma, D. K., Matas, A. J., Buda, G. J., He, Y., Yu, B., ... & Jenks, M. A. (2009). Cutin deficiency in the tomato fruit cuticle consistently affects resistance to mic2009robial infection and biomechanical properties, but not transpirational water loss.The Plant Journal, 60(3), 363–377. https: //doi.org/10.1111/j.1365-313X.2009.03969.x
[12]. Serrano, M., Coluccia, F., Torres, M., L’Haridon, F., & Métraux, J. P. (2014). The cuticle and plant defense to pathogens.FrontiersinPlant Science, 5, 274. https: //doi.org/10.3389/fpls.2014.00274
[13]. Eigenbrode, S. D., & Espelie, K. E. (1995). Effects of plant epicuticular lipids on insect herbivores.Annual Review of Entomology, 40(1), 171–194. https: //doi.org/10.1146/annurev.en.40.010195.001131
[14]. Stoner, K. A. (1990). Glossy leaf wax and host-plant resistance to insects in Brassica oleracea.Environmental Entomology,19(3), 730–739. https: //doi.org/10.1093/ee/19.3.730.
[15]. Eigenbrode, S. D., & Jetter, R. (2009). Attachment to plant surface waxes by an insect predator.Integrative and Comparative Biology,49(6), 820–831. https: //doi.org/10.1093/icb/icp041: contentReference [oaicite: 67]{index=67}
[16]. Jetter, R., & Riederer, M. (2016). Localization of the transpiration barrier in the epi- and intracuticular waxes of eight plant species: Water transport resistances are associated with fatty acyl rather than alicyclic components.Plant Physiology, 170(2), 921–934. https: //doi.org/10.1104/pp.15.01699
[17]. Paull, R. E. (1999). Effect of temperature and relative humidity on fresh commodity quality.Postharvest Biology and Technology,15(3), 263–277. https: //doi.org/10.1016/S0925 5214(98)00088-7
[18]. Baker, E. A. (1982). Chemistry and morphology of plant epicuticular waxes. InAdvances in Plant Cuticle Research(pp. 139–165). Butterworths.
[19]. Bourgault, R., Matschi, S., Vasquez, M., Qiao, P., Sonntag, A., Charlebois, C., ... & Molina, I. (2020). Constructing functional cuticles: Analysis of relationships between cuticle lipid composition, ultrastructure and water permeability in developing adult maize leaves.Frontiers in Plant Science, 11, 594. https: //doi.org/10.3389/fpls.2020.00594: contentReference [oaicite: 73]{index=73}
[20]. Suh, M. C., Samuels, A. L., Jetter, R., Kunst, L., Pollard, M., Ohlrogge, J., & Beisson, F. (2005). Cuticular lipid composition, surface structure, and gene expression in Arabidopsis stem epidermis.Plant Physiology, 139(4), 1649–1665. https: //doi.org/10.1104/pp.105.070805: contentReference [oaicite: 76]{index=76}
Cite this article
Kong,S. (2025). Cuticular wax deficiency in glossy kale: molecular mechanisms and physiological consequences. Journal of Food Science, Nutrition and Health,4(1),70-81.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Journal:Journal of Food Science, Nutrition and Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Yeats, T. H., & Rose, J. K. C. (2013). The formation and function of plant cuticles.Plant Physiology,163(1), 5–20. https: //doi.org/10.1104/pp.113.222737
[2]. Lee, S. B., & Suh, M. C. (2015). Advances in the understanding of cuticular waxes in Arabidopsis thaliana and crop species.Plant Cell Reports,34(4), 557–572. https: //doi.org/10.1007/s00299-015-1772-2Frontiers
[3]. Leide, J., Hildebrandt, U., Reussing, K., Riederer, M., & Vogg, G. (2007). The developmental pattern of tomato fruit wax accumulation and its impact on cuticular transpiration barrier properties: Effects of a deficiency in a β-ketoacyl-coenzyme A synthase (LeCER6).Plant Physiology,144(3), 1667–1679. https: //doi.org/10.1104/pp.107.099481.
[4]. Kosma, D. K., Bourdenx, B., Bernard, A., Parsons, E. P., Lü, S., Joubès, J., & Jenks, M. A. (2009). The impact of water deficiency on leaf cuticle lipids of Arabidopsis.Plant Physiology,151(4), 1918–1929. https: //doi.org/10.1104/pp.109.141911
[5]. Seo, P. J., Lee, S. B., Suh, M. C., Park, M. J., Go, Y. S., & Park, C. M. (2011). The MYB96 transcription factor regulates cuticular wax biosynthesis under drought conditions in Arabidopsis.The Plant Cell, 23(3), 1138–1152. https: //doi.org/10.1105/tpc.111.083485
[6]. Barthlott, W., & Neinhuis, C. (1997). Purity of the sacred lotus, or escape from contamination in biological surfaces.Planta, 202(1), 1–8. https: //doi.org/10.1007/s004250050096Frontiers.
[7]. Holmes, M. G., & Keiller, D. R. (2002). Effects of pubescence and waxes on the reflectance of leaves in the ultraviolet and photosynthetic wavebands: A comparison of a range of species.Plant, Cell & Environment,25(1), 85–93. https: //doi.org/10.1046/j.0016 8025.2001.00800.x
[8]. Jessen, D., Roth, C., Wiermer, M., & Fulda, M. (2011). Arabidopsis long-chain acyl-CoA synthetase 1 is involved in peroxisomal fatty acid degradation and cuticle biosynthesis.The Plant Cell, 23(3), 1132–1147. https: //doi.org/10.1105/tpc.110.082602
[9]. Aharoni, A., Dixit, S., Jetter, R., Thoenes, E., van Arkel, G., & Pereira, A. (2004). The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis.The Plant Cell,16(9), 2463–2480. https: //doi.org/10.1105/tpc.104.022897
[10]. Bessire, M., Borel, S., Fabre, G., Carrac, L., Efremova, N., Yephremov, A., & Nawrath, C. (2007).A member of the PLEIOTROPIC DRUG RESISTANCE family of ATP binding cassette transporters is required for the export of cuticular wax components in Arabidopsis.The Plant Cell,19(3), 1148–1161. https: //doi.org/10.1105/tpc.106.042390
[11]. Isaacson, T., Kosma, D. K., Matas, A. J., Buda, G. J., He, Y., Yu, B., ... & Jenks, M. A. (2009). Cutin deficiency in the tomato fruit cuticle consistently affects resistance to mic2009robial infection and biomechanical properties, but not transpirational water loss.The Plant Journal, 60(3), 363–377. https: //doi.org/10.1111/j.1365-313X.2009.03969.x
[12]. Serrano, M., Coluccia, F., Torres, M., L’Haridon, F., & Métraux, J. P. (2014). The cuticle and plant defense to pathogens.FrontiersinPlant Science, 5, 274. https: //doi.org/10.3389/fpls.2014.00274
[13]. Eigenbrode, S. D., & Espelie, K. E. (1995). Effects of plant epicuticular lipids on insect herbivores.Annual Review of Entomology, 40(1), 171–194. https: //doi.org/10.1146/annurev.en.40.010195.001131
[14]. Stoner, K. A. (1990). Glossy leaf wax and host-plant resistance to insects in Brassica oleracea.Environmental Entomology,19(3), 730–739. https: //doi.org/10.1093/ee/19.3.730.
[15]. Eigenbrode, S. D., & Jetter, R. (2009). Attachment to plant surface waxes by an insect predator.Integrative and Comparative Biology,49(6), 820–831. https: //doi.org/10.1093/icb/icp041: contentReference [oaicite: 67]{index=67}
[16]. Jetter, R., & Riederer, M. (2016). Localization of the transpiration barrier in the epi- and intracuticular waxes of eight plant species: Water transport resistances are associated with fatty acyl rather than alicyclic components.Plant Physiology, 170(2), 921–934. https: //doi.org/10.1104/pp.15.01699
[17]. Paull, R. E. (1999). Effect of temperature and relative humidity on fresh commodity quality.Postharvest Biology and Technology,15(3), 263–277. https: //doi.org/10.1016/S0925 5214(98)00088-7
[18]. Baker, E. A. (1982). Chemistry and morphology of plant epicuticular waxes. InAdvances in Plant Cuticle Research(pp. 139–165). Butterworths.
[19]. Bourgault, R., Matschi, S., Vasquez, M., Qiao, P., Sonntag, A., Charlebois, C., ... & Molina, I. (2020). Constructing functional cuticles: Analysis of relationships between cuticle lipid composition, ultrastructure and water permeability in developing adult maize leaves.Frontiers in Plant Science, 11, 594. https: //doi.org/10.3389/fpls.2020.00594: contentReference [oaicite: 73]{index=73}
[20]. Suh, M. C., Samuels, A. L., Jetter, R., Kunst, L., Pollard, M., Ohlrogge, J., & Beisson, F. (2005). Cuticular lipid composition, surface structure, and gene expression in Arabidopsis stem epidermis.Plant Physiology, 139(4), 1649–1665. https: //doi.org/10.1104/pp.105.070805: contentReference [oaicite: 76]{index=76}









