1 Introduction
Mycelium is a collection of mycelium which has a variety of functions such as consumption of mycelium can prevent Parkinson's disease Alzheimer's disease, diabetes, hypertension, stroke as well as anti-tumour, anti-bacterial, immune-boosting and cholesterol-lowering properties [1], and shiitake mushrooms, as a deep-cultured mycelium, have polysaccharides that can act as immune-boosting agents for macrophages by activating the MAPK pathway[2]. Inflammation is a natural protective response of the innate immune system in response to damage or harmful external stimuli such as pathogens, allergens, infections, irritants, and ultraviolet radiation [3]. A large number of studies at home and abroad have shown that mycelial polysaccharides have a role in anti-inflammation, such as monkey head mushroom polysaccharide extract[4], sidearm mycelial phosphorylated polysaccharides [5], and Poria cocos polysaccharides[6]. Now mycelial anti-inflammation has become a hot research object in the field in recent years, and this paper elaborates on the anti-inflammatory mechanism and application of mycelial polysaccharides.
2 Properties and extraction methods of mycelial polysaccharides
2.1. Structural and chemical properties of mycelial polysaccharides
The structural characteristics of mycelial polysaccharides are usually defined by their average molecular weight, monosaccharide composition and linkages[7]. Different kinds of mycelial polysaccharide extracts have different properties, by studying the aqueous extracts of shiitake mushrooms in which three polysaccharide fractions with different molecular weight sizes were isolated, it was found that the molecular weight was an important factor affecting the biological activity of mushroom polysaccharide fractions, and the polysaccharide with the smallest molecular weight was the most effective in regulating immunity[8]. Meanwhile, the biological activities of mushroom polysaccharides were mainly related to their immunomodulatory and anticancer properties. In addition, mycelial polysaccharides have antiviral effects, lower blood lipids or have antioxidant and antiproliferative activities[9]. Through the research of a large number of researchers at home and abroad, different types of mycelial polysaccharides have different effects. For example, polysaccharides extracted from edible mushroom polysaccharides have biological activities such as enhancing immunity, anti-tumour, anti-radiation, delaying aging and antioxidant activities;[10] Mycobacterium philippinarum has a wide range of health-promoting effects, including immunomodulation, anticancer, anti-inflammatory, hepatoprotective, hypoglycemic, hypolipidemic, antioxidant and other biological activities [11].
Table1: Molecular structure of mycelial polysaccharides
No. |
Name |
Mw (Da) |
Monosaccharide composition |
Structure features |
Bioactivities |
1 |
PLE |
1.20*103 |
Xyl, Man, Fuc, Glu, and Gal with a molar ratio of 2.3:1:6.4:22.1:19.83. |
ND |
Anti-tumor Immunomodulatory |
2 |
PLP |
2.07*104 |
Rha, Man, Ara, Gal, Xyl, and Glu with a molar ratio of 0.82:8.32:1.13:8.06:2.80:78.88. |
Glycosidic linkages were mostly 1 → 3, 1 → 6 or 1 → 3,6, main chain of → 3)-β-D Glcp-(1 → with → 6)-β-D-Glcp-(1 → side chain |
Immunomodulatory |
Table1: Continued
No. |
Name |
Mw (Da) |
Monosaccharide composition |
Structure features |
Bioactivities |
3 |
PIP |
1.85*104 |
Rha, Man, Ara, Gal, Xyl, and Glu with a molar ratio of 1.31:14.51:2.63:20.65:3.32:57.58. |
Glycosidic linkages were mostly 1 → 3, 1 → 6 or 1 → 3,6, main chain of → 3)-β-D Glcp-(1 → with → 6)-β-D-Glcp-(1 → side chain |
Immunomodulatory |
4 |
PNW1 |
3.30*104 |
Glu, Gal, Man, Ara, and Fuc with a molar ratio of 3.26:8.77:6.44:1:1.35. |
ND |
Anti-tumor、Immunomodulatory |
5 |
PNM1 |
2.90*104 |
Glu, Gal, Man, Ara, and Fuc with a molar ratio of 20.06:8.72:6.94:1:0.76. |
ND |
Anti-tumor、Immunomodulatory |
6 |
PNMP1 |
2.84*104 |
Glu, Gal, Man, and Xyl with a molar ratio of 18.65:41.37:35.41:4.57. |
ND |
Antioxidant Immunomodulatory |
7 |
PNMP2 |
3.15*104 |
Ara, Fuc, Glu, Gal, Man, and Xyl with a molar ratio of 5.78:7.24:14.42:41.57:28.62:2.37. |
ND |
Antioxidant Immunomodulatory |
8 |
PNMP3 |
2.61*104 |
Ara, Fuc, Glu, Gal, Man, and Xyl with a molar ratio of 3.45:8.44:21.55:36.42:26.58:3.56. |
ND |
Antioxidant Immunomodulatory |
2.2. Extraction and purification methods of mycelial polysaccharides
Currently, common mycelial polysaccharide extraction methods are water extraction, including hot water extraction, dilute alkaline water extraction and dilute acid water extraction[12]. Meanwhile, in order to improve the extraction efficiency of polysaccharides, microwave-assisted extraction (MAE), enzyme-assisted extraction (EAE), ultrasound-assisted extraction (UAE), and subcritical water extraction (SWE) are used[13].However, as an important step in obtaining polysaccharides, the extraction tends to affect polysaccharides yield, chemical structure, quality, and biological activity [14]. Processes (boiling, bleaching, fermentation) lead to a decrease in the polysaccharide content, the true intake of these macromolecules may vary between the raw and processed forms, and all processes lead to significant changes in the chemical composition of the polysaccharides (reduced content of proteins and phenolics), so that hot water extraction should not be used[15]. Studies have shown that microwave-assisted water two-phase extraction of polysaccharides from shiitake mushrooms is effective, the method is shorter in time, has higher yields and better selectivity, therefore, it provides a fast and efficient alternative to obtain a more diverse range of polysaccharides from shiitake mushrooms[16]. Different extraction methods were used with different extraction rates, Barbosa et al. used a binary method of hot water and supercritical carbon dioxide to extract polysaccharides from shiitake mushrooms with an extraction rate of 30.69%. Subcritical water extraction was used to extract polysaccharides from flat mushrooms. The highest extraction rate of 78.6% was achieved when the temperature reached 200ºC [17].
In conclusion, microwave-assisted aqueous phase extraction of polysaccharides and subcritical water extraction method of polysaccharides extraction has better extraction efficiency, and these two methods can be preferred in the extraction of polysaccharides, for the purification and extraction of polysaccharides the method not only to ensure the quantity at the same time but also to maintain the quality of polysaccharides.
3 Evaluation of anti-inflammatory activity and mechanism of mycelial polysaccharides
3.1. Study of anti-inflammatory mechanism of mycelial polysaccharides
The first immune response in the human body is inflammation (the onset of injury, injection and stress). Macrophages play an important role in pathogen resistance during inflammation [18],polysaccharides inhibit pro-inflammatory cytokines, iNOS, COX-2 and NF-κB related signalling pathways and interact with the individual's intestinal flora and the immune system to activate STAT3 and autophagy, thereby suppressing inflammation in the organism.Li and shah investigated polysaccharides extracted from apricot abalone [ PEPS and sulfo-PEPS (S-PEPS)] and found that S-PEPS had stronger anti-inflammatory activity against RAW 264.7 macrophages. The mycelial cell wall polysaccharide galactosamine galactoglucan (GAG) from Aspergillus fumigatus is a pathogen-associated molecular pattern (PAMP) that activates the host NLRP3 inflammasome, and GT4C is a potential synthase of GAG; mechanistically, the galactosamine subunit of the GAG molecule binds to glycosomal proteins, thereby blocking intracellular protein translation and inducing endoplasmic reticulum stress, leading to inflammasome GAG-induced inflammasome activation not only protects mice against Aspergillus fumigatus infection (as evidenced by strong virulence of strains lacking GAG and weak virulence of strains overexpressing GAG), but also attenuates DSS-induced colitis in mice by promoting IL-18 production [19].
In summary, mycelial polysaccharides can reduce the release of inflammatory factors by interfering with signalling in the organism as shown in Figure 1, and will also interfere with protein translation to inhibit inflammation.
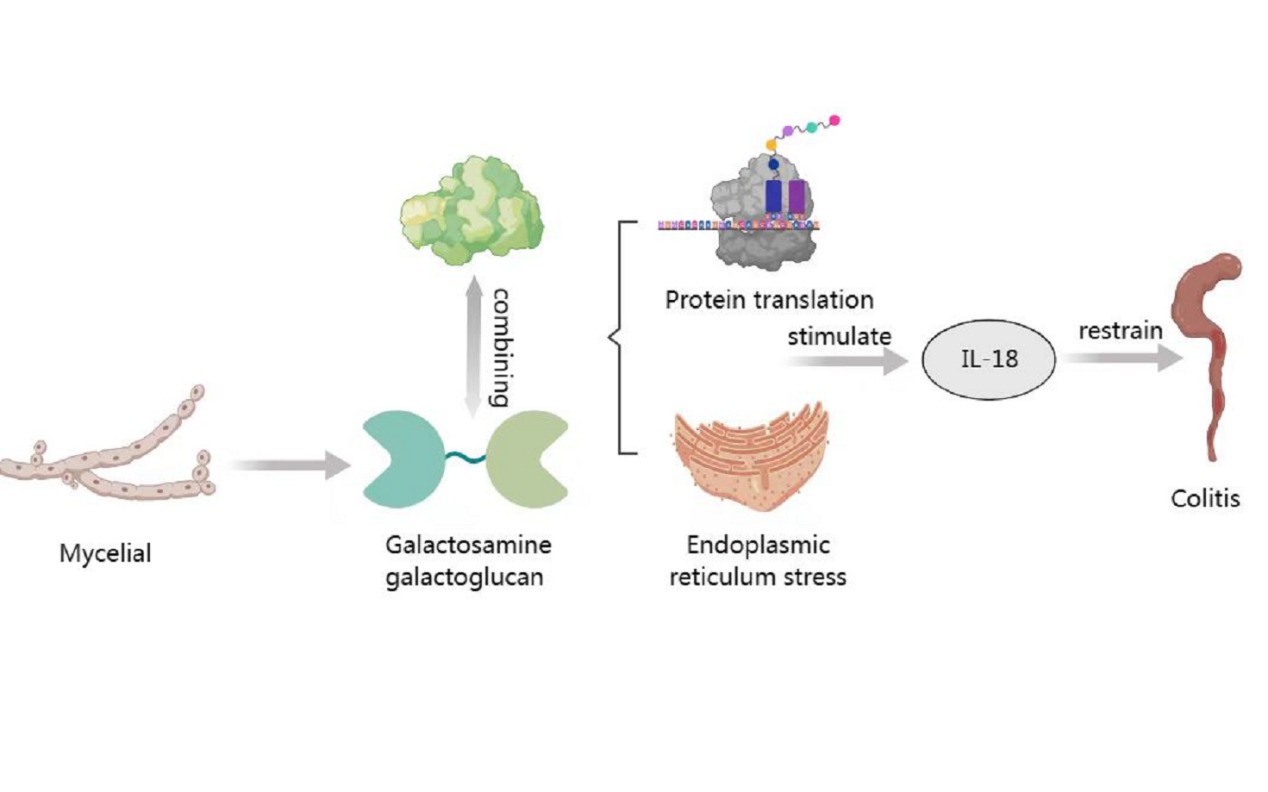 Figure 1: Mechanisms of mycelial inhibition of inflammation
Figure 1: Mechanisms of mycelial inhibition of inflammation
4 Research on the application of mycelial polysaccharides in inflammation models
4.1. Anti-inflammatory effects of mycelial polysaccharides in animal models
Studies have shown that the anti-inflammatory effects of mycelium in animal models present different effects under different conditions, e.g. Ying Yang,Jing Ji et al. conducted a study on natural polysaccharides against alcoholic liver injury and found that Polysaccharides of L. barbarum (LBP) mediated ethanol-induced hepatic inflammation through inhibition of the thioredoxin-interacting protein (TXNIP) and nod-like receptor 3 (NLRP3) inflammatory vesicles, which mediates the attenuation of ethanol-induced liver injury, and is thus resistant to alcoholic liver injury.[20] Meng Meng et al. studied Ganoderma lucidum polysaccharides (GLP) against Rheumatoid arthritis (RA) and showed that GLP inhibits the proliferation and migration of RA synovial fibroblasts (RASF) and regulates the proliferation and differentiation of dendritic cells, which are the antigen presenting cells. and finally promotes the formation of osteoblasts, thereby protecting bone and articular bone.[21] Huaping Li, Yanbo Feng, and Wenxue Sun concluded that phosphorylated Lateralia spp. have better anti-inflammatory effects than ordinary Lateralia spp. injected into mice in controlled experiments. Xuejing Jia, Lishuai Ma et al. studied Poria coconut polysaccharides (pcp) and found that PC-II (a water-soluble (1,3)-a-d-galactose) in pcp could inhibit the production of IFN-induced inflammation marker IP-10 in a dose-dependent manner, thereby anti-inflammatory.
5 Clinical application and prospect of mycelial polysaccharides
At this stage, mycelial polysaccharide anti-inflammatory has entered into clinical use with a large scope for development, e.g., the pharmacological effects of APSs have been demonstrated at the molecular, cellular, and animal levels, however, a few cases have been reported of their use in clinical adjuvant therapy or in the treatment of major diseases. [22] Although much progress has been made in the study of methods for the extraction, isolation and purification of EFPs, it is necessary to continue the search for simpler, more efficient and cheaper methods for the large-scale production of high-quality EFPs in the pharmaceutical and functional food industries. [23] Since EFP has high biological activity, it is important to establish an efficient and economical extraction method. At the same time, attention should be paid to improving the purity of polysaccharides, obtaining homogeneous polysaccharides, and studying their physicochemical properties and bioactivities in order to maximise the value of EFP Since EFP has high biological activity, it is important to establish an efficient and economical extraction method. At the same time, attention should be paid to improving the purity of polysaccharides, obtaining homogeneous polysaccharides, and studying their physicochemical properties and biological activities to maximise the value of EFP.
6 Conclusion
At present, with the in-depth study of mycelial polysaccharides, the anti-inflammatory effect of mycelial polysaccharides has become clearer, and different extraction methods affect the properties of mycelial polysaccharides to varying degrees, which in turn affects their anti-inflammatory effect. Different mycelial polysaccharides alleviate or resist different inflammation in different ways, and there are many differences in their mechanism of action, but studies have shown that mycelial polysaccharides have a certain effect on anti-inflammation. Therefore, it is now possible to explore in more depth how the extraction of polysaccharides can reduce polysaccharide loss, as well as reduce the effect of extraction on polysaccharide properties. Some of the mycelia have only been tested for anti-inflammatory effects for the time being, but the specific polysaccharides have not been detected, so this area can also be studied in depth at this time.
References
[1]. Berger R G, Bordewick S, Krahe N K, et al. Mycelium vs. fruiting bodies of edible fungi—A comparison of metabolites[J]. Microorganisms, 2022, 10(7): 1379.
[2]. Wang T, He H, Liu X, et al. Mycelial polysaccharides of Lentinus edodes (shiitake mushroom) in submerged culture exert immunoenhancing effect on macrophage cells via MAPK pathway[J]. International journal of biological macromolecules, 2019, 130: 745-754.
[3]. Sheng K, Wang C, Chen B, et al. Recent advances in polysaccharides from Lentinus edodes (Berk.): Isolation, structures and bioactivities[J]. Food chemistry, 2021, 358: 129883.
[4]. Zhu Jiamin, Wu Yi, Zhao Linjing, et al. Progress on polysaccharide structure and regulation of intestinal microflora [J]. Food and Fermentation Industry, 2023,49 (14): 311-320.
[5]. Li H, Feng Y, Sun W, et al. Antioxidation, anti-inflammation and anti-fibrosis effect of phosphorylated polysaccharides from Pleurotus djamor mycelia on adenine-induced chronic renal failure mice[J]. International Journal of Biological Macromolecules, 2021, 170: 652-663.
[6]. Jia X, Ma L, Li P, et al. Prospects of Poria cocos polysaccharides: Isolation process, structural features and bioactivities[J]. Trends in Food Science & Technology, 2016, 54: 52-62.
[7]. Lu Xingrui, Wu Rui, Ai Honghu, etc. Effect of hydrangea polysaccharides on oxidative stress and autophagy damage in lead-induced mouse kidneys [J / OL]. Journal of Animal Nutrition: 1-14 [2023-12-14]. http://kns.cnki.net/kcms/detail/11.5461.S. 20231205.1734.006.html.
[8]. Chen S, Liu C, Huang X, et al. Comparison of immunomodulatory effects of three polysaccharide fractions from Lentinula edodes water extracts[J]. Journal of Functional Foods, 2020, 66: 103791
[9]. Radzki W, Ziaja-Sołtys M, Nowak J, et al. Effect of processing on the content and biological activity of polysaccharides from Pleurotus ostreatus mushroom[J]. LWT-Food Science and Technology, 2016, 66: 27-33.
[10]. Guo Q, Liang S, **ao Z, et al. Research progress on extraction technology and biological activity of polysaccharides from Edible Fungi: A review[J]. Food Reviews International, 2023, 39(8): 4909-4940.
[11]. Luan F, Peng X, Zhao G, et al. Structural diversity and bioactivity of polysaccharides from medicinal mushroom Phellinus spp.: A review[J]. Food Chemistry, 2022: 133731.
[12]. Wei Q, Zhou F, Cai B, et al. Extraction and Antioxidant Activities of Polysaccharides Produced by Submerged Mycelia Culture of Oudemansiella raphanipes[J]. Current Topics in Nutraceutical Research, 2022, 20(3).
[13]. Wang P, Chen D, Jiang D, et al. Alkali extraction and in vitro antioxidant activity of Monascus mycelium polysaccharides[J]. Journal of food science and technology, 2014, 51: 1251-1259.
[14]. Wang Z, Wang C, Quan Y. Extraction of polysaccharides from Phellinus nigricans mycelia and their antioxidant activities in vitro[J]. Carbohydrate polymers, 2014, 99: 110-115.
[15]. ao J, Sun J, Yao L, et al. Physicochemical characteristics of ultrasonic extracted polysaccharides from cordyceps cephalosporium mycelia[J]. International Journal of Biological Macromolecules, 2012, 51(1-2): 64-69.
[16]. Radzki W, Ziaja-Sołtys M, Nowak J, et al. Effect of processing on the content and biological activity of polysaccharides from Pleurotus ostreatus mushroom[J]. LWT-Food Science and Technology, 2016, 66: 27-33.
[17]. Lin Y, Zeng H, Wang K, et al. Microwave-assisted aqueous two-phase extraction of diverse polysaccharides from Lentinus edodes: Process optimization, structure characterization and antioxidant activity[J]. International Journal of Biological Macromolecules, 2019, 136: 305-315.
[18]. Sharma A, Sharma A, Tripathi A. Biological activities of Pleurotus spp. polysaccharides: A review[J]. Journal of Food Biochemistry, 2021, 45(6): e13748.
[19]. Briard B, Fontaine T, Samir P, et al. Galactosaminogalactan activates the inflammasome to provide host protection[J]. Nature, 2020, 588(7839): 688-692.
[20]. Yang Y, Ji J, Di L, et al. Resource, chemical structure and activity of natural polysaccharides against alcoholic liver damages[J]. Carbohydrate polymers, 2020, 241: 116355.
[21]. Meng M, Yao J, Zhang Y, et al. Potential Anti-Rheumatoid Arthritis Activities and Mechanisms of Ganoderma lucidum Polysaccharides[J]. Molecules, 2023, 28(6): 2483.
[22]. Dai J, Liu B, Ji D, et al. Extraction, isolation, identification, and bioactivity of polysaccharides from Antrodia cinnamomea[J]. Quality Assurance and Safety of Crops & Foods, 2023, 15(4): 60-76.
[23]. Wei X, Yao J, Wang F, et al. Extraction, isolation, structural characterization, and antioxidant activity of polysaccharides from elderberry fruit[J]. Frontiers in Nutrition, 2022, 9: 947706.
Cite this article
Li,C.;Ouyang,S.;Luo,C.;Liang,B.;Wu,Z. (2023). Anti-inflammatory mechanism and application of mycelial polysaccharides. Journal of Food Science, Nutrition and Health,1,27-33.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Journal:Journal of Food Science, Nutrition and Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Berger R G, Bordewick S, Krahe N K, et al. Mycelium vs. fruiting bodies of edible fungi—A comparison of metabolites[J]. Microorganisms, 2022, 10(7): 1379.
[2]. Wang T, He H, Liu X, et al. Mycelial polysaccharides of Lentinus edodes (shiitake mushroom) in submerged culture exert immunoenhancing effect on macrophage cells via MAPK pathway[J]. International journal of biological macromolecules, 2019, 130: 745-754.
[3]. Sheng K, Wang C, Chen B, et al. Recent advances in polysaccharides from Lentinus edodes (Berk.): Isolation, structures and bioactivities[J]. Food chemistry, 2021, 358: 129883.
[4]. Zhu Jiamin, Wu Yi, Zhao Linjing, et al. Progress on polysaccharide structure and regulation of intestinal microflora [J]. Food and Fermentation Industry, 2023,49 (14): 311-320.
[5]. Li H, Feng Y, Sun W, et al. Antioxidation, anti-inflammation and anti-fibrosis effect of phosphorylated polysaccharides from Pleurotus djamor mycelia on adenine-induced chronic renal failure mice[J]. International Journal of Biological Macromolecules, 2021, 170: 652-663.
[6]. Jia X, Ma L, Li P, et al. Prospects of Poria cocos polysaccharides: Isolation process, structural features and bioactivities[J]. Trends in Food Science & Technology, 2016, 54: 52-62.
[7]. Lu Xingrui, Wu Rui, Ai Honghu, etc. Effect of hydrangea polysaccharides on oxidative stress and autophagy damage in lead-induced mouse kidneys [J / OL]. Journal of Animal Nutrition: 1-14 [2023-12-14]. http://kns.cnki.net/kcms/detail/11.5461.S. 20231205.1734.006.html.
[8]. Chen S, Liu C, Huang X, et al. Comparison of immunomodulatory effects of three polysaccharide fractions from Lentinula edodes water extracts[J]. Journal of Functional Foods, 2020, 66: 103791
[9]. Radzki W, Ziaja-Sołtys M, Nowak J, et al. Effect of processing on the content and biological activity of polysaccharides from Pleurotus ostreatus mushroom[J]. LWT-Food Science and Technology, 2016, 66: 27-33.
[10]. Guo Q, Liang S, **ao Z, et al. Research progress on extraction technology and biological activity of polysaccharides from Edible Fungi: A review[J]. Food Reviews International, 2023, 39(8): 4909-4940.
[11]. Luan F, Peng X, Zhao G, et al. Structural diversity and bioactivity of polysaccharides from medicinal mushroom Phellinus spp.: A review[J]. Food Chemistry, 2022: 133731.
[12]. Wei Q, Zhou F, Cai B, et al. Extraction and Antioxidant Activities of Polysaccharides Produced by Submerged Mycelia Culture of Oudemansiella raphanipes[J]. Current Topics in Nutraceutical Research, 2022, 20(3).
[13]. Wang P, Chen D, Jiang D, et al. Alkali extraction and in vitro antioxidant activity of Monascus mycelium polysaccharides[J]. Journal of food science and technology, 2014, 51: 1251-1259.
[14]. Wang Z, Wang C, Quan Y. Extraction of polysaccharides from Phellinus nigricans mycelia and their antioxidant activities in vitro[J]. Carbohydrate polymers, 2014, 99: 110-115.
[15]. ao J, Sun J, Yao L, et al. Physicochemical characteristics of ultrasonic extracted polysaccharides from cordyceps cephalosporium mycelia[J]. International Journal of Biological Macromolecules, 2012, 51(1-2): 64-69.
[16]. Radzki W, Ziaja-Sołtys M, Nowak J, et al. Effect of processing on the content and biological activity of polysaccharides from Pleurotus ostreatus mushroom[J]. LWT-Food Science and Technology, 2016, 66: 27-33.
[17]. Lin Y, Zeng H, Wang K, et al. Microwave-assisted aqueous two-phase extraction of diverse polysaccharides from Lentinus edodes: Process optimization, structure characterization and antioxidant activity[J]. International Journal of Biological Macromolecules, 2019, 136: 305-315.
[18]. Sharma A, Sharma A, Tripathi A. Biological activities of Pleurotus spp. polysaccharides: A review[J]. Journal of Food Biochemistry, 2021, 45(6): e13748.
[19]. Briard B, Fontaine T, Samir P, et al. Galactosaminogalactan activates the inflammasome to provide host protection[J]. Nature, 2020, 588(7839): 688-692.
[20]. Yang Y, Ji J, Di L, et al. Resource, chemical structure and activity of natural polysaccharides against alcoholic liver damages[J]. Carbohydrate polymers, 2020, 241: 116355.
[21]. Meng M, Yao J, Zhang Y, et al. Potential Anti-Rheumatoid Arthritis Activities and Mechanisms of Ganoderma lucidum Polysaccharides[J]. Molecules, 2023, 28(6): 2483.
[22]. Dai J, Liu B, Ji D, et al. Extraction, isolation, identification, and bioactivity of polysaccharides from Antrodia cinnamomea[J]. Quality Assurance and Safety of Crops & Foods, 2023, 15(4): 60-76.
[23]. Wei X, Yao J, Wang F, et al. Extraction, isolation, structural characterization, and antioxidant activity of polysaccharides from elderberry fruit[J]. Frontiers in Nutrition, 2022, 9: 947706.









