1. Introduction
Lithium-ion batteries (LIBs) have undoubtedly transformed the modern world, powering an extensive range of portable electronic devices with the remarkable energy density and rechargeable capabilities. Since their commercialization, LIBs have become an integral part of our daily lives, providing the necessary energy for our communication, mobility, and convenience. The foundation of as lithium-ion battery technology can be traced back to the ground-breaking work of M. S. Whittingham in the 1970s. Whittingham's research on intercalation-based rechargeable batteries laid the groundwork for subsequent advancements in the field [1, 2]. Notably, the pivotal contributions of John B. Goodenough and Akira Yoshino in the 1980s and 1990s led to the commercialization of LIBs, propelling them to the forefront of energy storage technology in various applications. However, with the requirement for sustainable and efficient energy solutions, it is crucial to address the limitations of conventional LIBs. This introduction aims to provide a comprehensive overview of LIBs, their historical development, classification, and existing drawbacks. Additionally, it will explore the immense potential of nanomaterials in overcoming these limitations and significantly enhancing the performance of LIBs.
For classification of LIBs, they can be classified based on the materials used in their positive (cathode) and negative (anode) electrodes, which significantly influence their performance characteristics. Three main types of LIBs are distinguished based on their cathode materials. For lithium cobalt oxide (LiCoO2) cathodes, renowned for their high energy density, LiCoO2-based batteries are widely used in portable electronic devices such as smartphones and laptops, where energy density is critical for extended use. For lithium iron phosphate (LiFePO4) cathodes, LiFePO4-based batteries offer enhanced safety and thermal stability, making them the preferred choice for power tools, electric bikes, and medical devices, where safety is paramount. For lithium manganese oxide (LiMn2O4) cathodes, LiMn2O4-based batteries strike a balance between energy density and cost-effectiveness, making them suitable for power banks and consumer electronics.
Despite their widespread use and success, lithium-ion batteries are not without their challenges, and several limitations need to be addressed to fully unlock their potential. For capacity and energy density, the limited capacity and energy density of current LIBs pose challenges in meeting the escalating power for electric devises. Advancements in energy storage capacity are critical for supporting the transition to sustainable energy sources and decarbonizing various sectors. For safety concerns, while significant strides have been made to improve the safety of LIBs, incidents of thermal runaway and cell degradation remain a concern, particularly under extreme conditions or mechanical abuse. Ensuring the utmost safety is essential for broader acceptance and implementation across industries. For cycle life and degradation, LIBs undergo multiple charge and discharge cycles during their operational lifespan, leading to gradual capacity fade and performance degradation. This phenomenon limits the overall longevity of the battery and necessitates frequent replacements, raising concerns about environmental impact and cost-effectiveness.
In recent years, nanotechnology has emerged as a transformative approach to address the aforementioned limitations of LIBs. Nanomaterials, characterized by their nanoscale dimensions, offer unique properties that have the potential to revolutionize energy storage technology [3]. The integration of nanomaterials into various components of lithium-ion batteries has demonstrated several significant advantages. For increased surface area, nanomaterials possess an exceptionally high surface area per unit mass, providing more active sites for lithium-ion storage. This feature facilitates faster ion diffusion during charge and discharge processes, resulting in enhanced overall capacity and energy density of the battery. For improved electrode kinetics, the nanoscale dimensions of materials shorten the diffusion pathways for lithium ions, promoting faster charge and discharge rates. Consequently, this leads to improved electrode kinetics, higher power output, and reduced charging times for LIBs, addressing the need for quick-charging capabilities in various applications. For enhanced mechanical stability, certain nanomaterials exhibit enhanced mechanical stability, allowing them to withstand stress and volume changes during charge-discharge cycles. This feature minimizes degradation and extends the battery's cycle life, contributing to longer-lasting and more durable energy storage solutions, which can reduce waste and environmental impact. For enhanced safety, nanomaterials with improved thermal stability can improve the performance of LIBs, providing greater peace of mind to users and manufacturers alike.
In conclusion, the advent of lithium-ion batteries has revolutionized our world, powering an extensive range of devices and vehicles that define our modern lifestyle. However, their existing limitations necessitate continuous research and innovation to meet the growing demand for cleaner and more efficient energy storage solutions. The integration of nanomaterials offers great promise in overcoming these limitations and unlocking the full potential of lithium-ion battery technology. In this research, the vast potential of various nanomaterials and their specific roles in enhancing the performance of LIBs will be studied, providing valuable insights into effective sustainable technology advancement.
2. Application of nanomaterials in LIBs
LIBs hold a pivotal position in the realm of electric vehicles, and nanomaterials, owing to their distinctive physical and chemical attributes, prove indispensable in downsizing, lightening, and curbing the power consumption of lithium-ion batteries. Simultaneously, electrodes at the nanoscale exhibit minimal impedance growth, mitigating power dissipation throughout the cycling process. Additionally, the increased contact area between nanomaterial electrodes and electrolytes enhances charge and discharge rates to deliver maximum power. ArunaBharathi et al. have developed a novel nanoscale cathode material that enhances the performance of LIBs [4]. Using a co-precipitation chemical synthesis method, they prepared electroactive spinel LiNi0.5Mn1.5O4, which, according to the test, exhibited good physical property in crystallinity. Concurrently, M. ArunaBharathi and colleagues accomplished the accurate forecasting of the configuration and electrochemical attributes of spinel LiNi0.5Mn1.5O4 through the application of first-principles methodologies relying on density functional theory (DFT) computations. Lithium manganese spinel, distinguished by its lack of toxicity, ease of synthesis, cost-effectiveness, environmental friendliness, impressive cycling stability, and storage capacity, holds a prominent position as a cathode material for LIBs. Experimental results have demonstrated that this nanoscale metal oxide cathode material can enhance the specific power density of LIBs. The research approach is also considered a crucial tool for manufacturing nanoelectrode materials for LIBs used in electric vehicles.
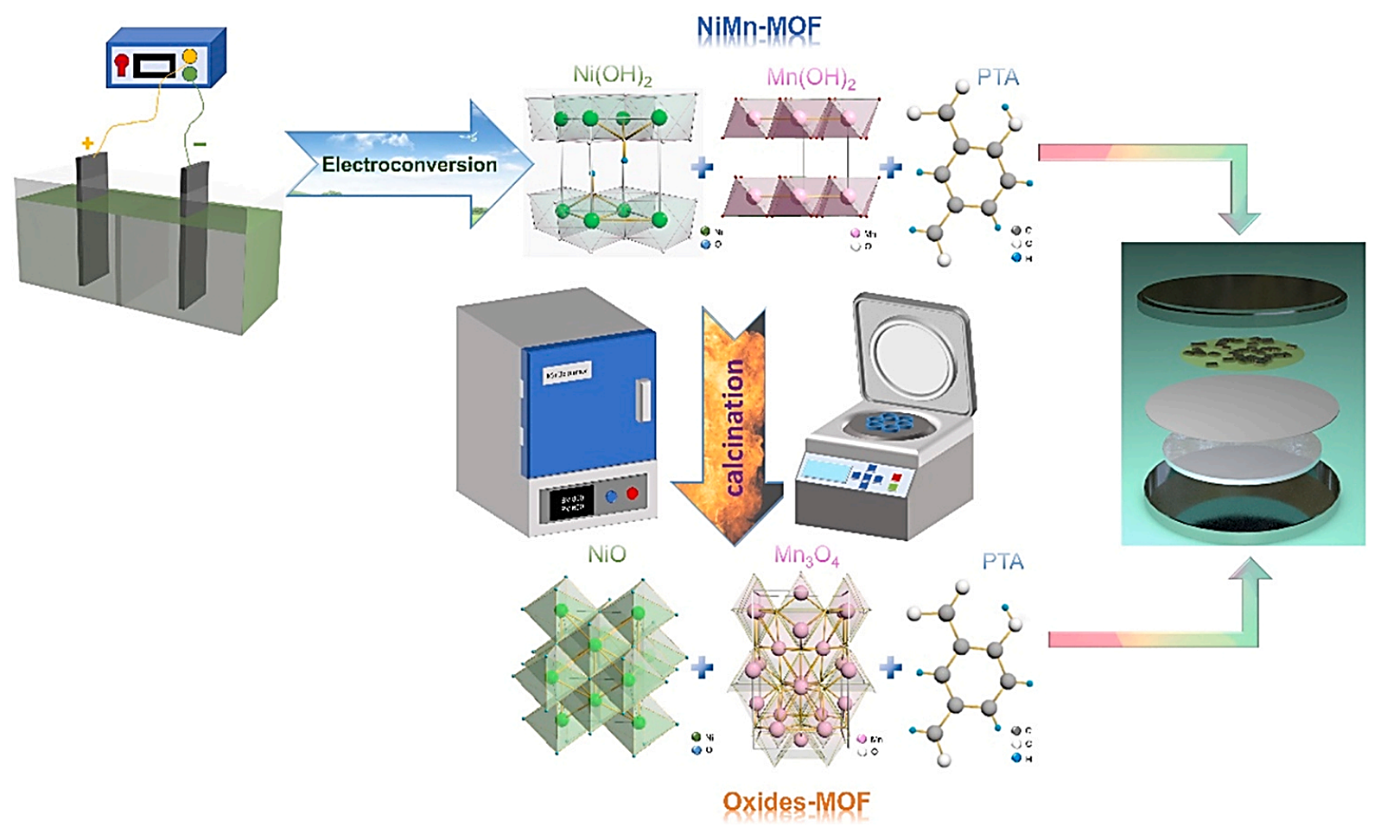
Figure 1. Material synthesis route of NiMn-MOF [5].
Binary transition metals are also well-suited as cathode materials for LIBs because their low-cost and theoretically good performance in Li storage capacity. Nonetheless, the utilization of these materials as energy storage components is hampered by challenges stemming from insufficient conductivity and the detrimental effects of volume fluctuations during energy cycles, which lead to poor cycling stability. As shown in Figure 1, Zhang et al. have pioneered a one-step electrochemical conversion method to successfully synthesize Ni/Mn binary compounds and achieve to produce metal-organic framework (MOF) nanosheets with good physical and chemical properties [5]. This method harnesses the property of cathodic generation of hydroxide ions, dissolving the organic framework (terephthalic acid, PTA) in an alkaline solution, enabling uniform coating of hydroxides onto PTA and successfully producing NiMn-MOF nanomaterials. NiMn-MOF demonstrates outstanding capabilities when employed as an anode material for LIBs, boasting a remarkable charge-discharge specific capacity of 1024/1554mAh·g-1. This is coupled with a minimal charge transfer impedance, although it retains the relatively suboptimal rate performance characteristic of transition metals. Impressively, even after undergoing 1000 cycles, the Coulombic efficiency remains at a steadfast 100%. Even has stable performance after hundreds of cycles. Th structure derived from the MOF not only serves as pathways for enhanced conductivity, enhancing electron and lithium-ion transport, but also helps alleviate volume expansion issues. This method of successful in-situ electrochemical conversion synthesis of NiMn-MOF, pioneered for the first time, holds promise for large-scale applications in the field of LIBs, since it is low cost and strong electrochemical performance.
Zhang et al. utilized biomass to synthesize porous rutile titanium dioxide nanomaterials, which has crucial effects on enhancing the performance of lithium-ion batteries [6]. They successfully synthesized porous titanium dioxide nanomaterials assembled from rutile titanium dioxide nanoparticles using sodium alginate. Alginate assists in forming small titanium dioxide nanoparticles, and the SO42- in the precursor partially inhibits the crystal phase transition from rutile to anatase during the calcination with high-temperature. The thermal decomposition products of alginate ions also prevent forming larger titanium dioxide nanoparticle aggregates. The concentration of the reactants and the proportion of sodium alginate to Ti(SO4)2 have a substantial impact on physical and chemical performance of the material. These nanoporous titanium dioxide nanomaterials find application as anode materials in LIBs, attaining a reversible capacity of 223 mAh/g after 20 cycles at a 1 C rate. Even in harder cycling condition, a substantial reversible capacity will be maintained. Due to their efficient nanoscale structure and good crystallinity, these nanoporous titanium dioxide nanomaterials exhibit outstanding electrochemical performance.
The porous in activated carbon (AC) configuration significantly increases the surface area of the carbon, resulting in a substantial adsorption capacity. The utilization of AC as an anode material in LIBs is crucial because of its ability to efficiently store Li+ and provide good mechanical stability. In addition, AC demonstrates exceptional electrical conductivity and possesses the ability to accommodate volumetric alterations during cycling, consequently enhancing the cycle performance of the battery. Nanostructured AC can improve the performance of LIBs. Hence, it is imperative to explore environmentally friendly and efficient methods for obtaining such nanostructured activated carbon. An innovative approach introduced by Ashraf et al. involved utilizing solid biopulp from watermelon seeds as an alternative precursor for nanostructured activated carbon production [7]. Initially, the researchers subjected the seeds to carburization and subsequently activated them using potassium hydroxide through a ball milling process. The activated carbon derived from watermelon seeds demonstrated an impressive capacity of approximately 360 milliampere-hours per gram (mAh/g) after more than 100 cycles, and the Coulombic efficiency of it was 98%. The selection of watermelon seeds as a biowaste source was based on their abundance and ready availability as a by-product of the food industry, making them a cheap and environmentally friendly carbon source for LIBs anodes. Moreover, the unique structure of watermelon seeds facilitated their conversion into carbon compounds. Inside the seed husk there is a porous material suitable for carbonization, offering a better physical property. The composition of watermelon seed husks, including 39.17% cellulose, 30% lignin, approximately 20% hemicellulose, and some mineral content, makes them an ideal carbon source. Through meticulous management of the conditions of the reactions, the synthesis process of the material can be controlled. The research demonstrates the feasibility of using watermelon seeds as precursors for the synthesis of nanomaterials intended for LIBs anodes. This discovery holds substantial significance for the deeper development of nanostructured AC materials in LIBs.
The need for high-performance batteries has become an urgent issue in the realm of energy storage, largely driven by the depletion of oil resources and mounting environmental concerns. Transition-metal oxides featuring a spinel structure have attracted considerable interest as potential candidate anode materials for LIB. These materials are distinguished by their unique crystal structure and the ability to exhibit various oxidation states. Notable examples of such transition-metal oxides include MnCo2O, NiCo2O4, and Co3O. Nonetheless, Certain defects have hindered the practical application of spinel-structured transition metal oxides including their electronic insulator characteristics and issues with ion transport stability, leading to less-than-optimal battery cycle stability. Ternary metal oxides hold immense promise for LIBs due to their special chemical properties, which can significantly enhance the energy efficiency of Li-ion batteries. Building on this potential, Ren et al. devised a method to synthesize Mn0.5Zn0.5Co2O4/C nanospheres featuring a unique yolk-double shell structure [8]. This accomplishment was realized by introducing manganese (Mn) into zinc cobalt oxide (ZnCo2O4) via a self-templating solvothermal process. Within the Mn-doped ZnCo2O4 structure, manganese (Mn) atoms fill the octahedral gaps within the spinel structure, thus amplifying the pseudocapacitive attributes of the anode material. The resultant material exhibits a distinctive special structure, characterized by an outer shell-layer with good physical properties. This unique configuration effectively mitigates volume expansion and shortens the diffusion path for Li+, thereby substantially enhancing the electrochemical performance. Notably, even after undergoing 100 cycles at a current density of 200 mA/g, the material retained a specific capacity of 1001 mAh/g. Consequently, the Mn0.5Zn0.5Co2O4/C nanospheres exhibit outstanding electrochemical performance, which is characterized by a high reversible capacity and superior cycling stability.
Silicon has attracted considerable interest as a highly prospective contender for the forthcoming generation of LIBs. It offers numerous advantages over traditional graphite anodes, such as a high capacity, abundant availability, and sustainability. However, silicon faces significant challenges due to its substantial volume expansion (approximately 300%) during lithiation, leading to the pulverization of silicon particles and the instability associated with the solid electrolyte interphase (SEI) layers developed on their surfaces. Consequently, the stability during cycling of silicon anodes is compromised. Additionally, silicon's low intrinsic conductivity results in suboptimal rate capabilities. To tackle these issues, a significant body of work has been dedicated to the development of Si/metal composites aimed at bolstering conductivity. However, these composites have limited effectiveness in alleviating the substantial volume expansion problem. Conversely, carbon nanolayers have found utility in electrode materials to ameliorate their performance, bolstering mechanical resilience, and forge enduring interfaces with the electrolyte. The nanostructured silicon which has a conformal carbon coating, often referred to as Si@C, emerges as a new anode material for the futural LIBs. Nevertheless, the conventional synthesis of silicon nanostructures and carbon nanocoatings typically involves separate processing steps, resulting in a costly, complex, and time-consuming overall synthesis process. In response to this challenge, Huang et al. proposed a novel approach [9]. They initially convert silica nanomaterials into Mg2Si, and subsequently, when the converted Mg2Si reacts with CaCO3, a conformal carbon nanolayer (measuring 1–5 nm) forms on the newly created Si nanostructures spontaneously, resulting in Si@C composite materia. Significantly, Si@C materials derived from diatomite exhibit impressive electrochemical capabilities, showcasing a reversible capacity of 1359.7 mAh/g at 4 A/g, with a sustained capacity of 764.6 mAh/g even after 500 cycles. This innovative approach offers a scalable and cost-efficient means of crafting high-performance Si@C anode materials for LIBs, promising advancements in lithium-ion battery technology. Given the abundant availability of diatomaceous earth and the natural generation of carbon coating, the prepare method described in this research holds the potential for widespread production of nanoscale Si@C materials, with profound implications for the evolution of lithium-ion batteries.
LIBs are the dominant choice for energy storage facilities because of their high performance in energy density and minimal self-discharge. However, many electrode materials used in LIBs suffer from inherent limitations that hinder their advancement. Two critical challenges are the substantial volume changes they undergo during operation and their low electrical conductivity. To address these issues, researchers have commonly employed two clever strategies: creating various silicon (Si) nanostructures with internal voids and incorporating conductive materials. Nevertheless, these methods often involve expensive raw materials and complex preparation techniques, making them less suitable for large-scale commercial applications. In a notable development, Benzait et al. successfully synthesized a coral-like nanoporous Si/reduced graphene oxide (rGO) composite using cost-effective raw materials, graphite, and aluminum silicon (Al-Si) powders [10]. They employed straightforward methods that didn't require high temperatures or sophisticated equipment. Significantly, this method streamlined the preparation process by simultaneously carrying out the Al-etching and graphene oxide (GO) reduction reactions, eliminating the need for separate steps. The performance of LIBs half-cells constructed using this composite was further enhanced through the incorporation of additional carbon nanomaterials, thereby increased cycle and rate capabilities with synergistic effects. Remarkable electrochemical outcomes were attained, including a reversible capacity of 1080 mAh/g when operating at 0.2 A/g after 250 cycles. Moreover, when tested in full batteries with lithium cobalt oxide (LCO) cathodes, promising results were obtained, with a reversible capacity of more than 50 mAh/g at 36 mA/g after more than 20 cycles. This study showcased a straightforward synthesis method for Si/rGO composite materials using low-cost Al-Si and graphite precursors. It resulted in Si-based LIBs with outstanding electrochemical performance. Future research should focus on improving the batteries' first coulombic efficiency and capacity retention by achieving more uniform coral-like Si nanostructures, investigating the size and shape effects of both Si particles and carbon nanomaterials (0D, 1D, and 2D, like carbon nanotubes, carbon black, and reduced graphene oxide), gaining a deeper understanding of the synergistic effects of each component, and optimizing their proportions for integration into commercial LIBs.
3. Conclusion
In the field of LIBs, the application of nanomaterials shows great potential. This article summarizes various nanomaterials, including two-dimensional materials, metal oxides, and carbon-based materials, whose introduction significantly improves battery performance, including capacity, cycle life, and charge and discharge rates. The tunability of these materials makes them uniquely advantageous in battery design. However, challenges that need to be addressed include issues of large-scale production, cost-effectiveness, and safety. This study highlights the potential applications of nanomaterials in renewable energy storage and electric vehicles, providing important support for the development of clean energy technologies. Although in-depth research and engineering innovation are still needed, we believe that through continuous efforts, nanomaterials will continue to promote the improvement of LIBs performance and make greater contributions to the realization of a sustainable energy future. This research has important implications for reducing carbon emissions, improving the environment, and improving energy storage efficiency.
References
[1]. Scrosati, B., & Garche, J. 2010 Journal of Power Sources 195(9) 2419–2430.
[2]. Li, C., Zhang, H., Ding, P., Yang, S., & Bai, Y. 2023 Renewable & Sustainable Energy Reviews 184 113576–113576.
[3]. Lee, K.-T., & Cho, J. 2011 Nano Today 6(1) 28–41.
[4]. ArunaBharathi M, Sushama M, Rao K V, et al. 2023 Materials Today: Proceedings.
[5]. Lin, S., & Zhang, T. 2023 Journal of Alloys and Compounds 942 168926–168926.
[6]. Li, X., Zhang, F., Zhai, B., Wang, X., Zhao, J., & Wang, Z. 2020 Journal of Physics and Chemistry of Solids 145 109552–109552.
[7]. Ashraf, H., & Karahan, B. D. 2023 Materials Research Bulletin 112492.
[8]. Ren, Y.-X., Li, X., Wang, Y., Gu, S., Yang, C., Gao, T., Cao, P., & Zhou, G. 2022 Applied Materials Today 27 101452–101452.
[9]. Huang, X., Ding, Y., Li, K., Guo, X., Zhu, Y., Zhang, Y., & Bao, Z. 2021 Journal of Power Sources 496 229833–229833.
[10]. Benzait, Z., & Yuca, N. 2020 Electrochimica Acta 339 135917.
Cite this article
Wang,W. (2024). Application of nanomaterials in Li-ion batteries. Applied and Computational Engineering,58,31-36.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Scrosati, B., & Garche, J. 2010 Journal of Power Sources 195(9) 2419–2430.
[2]. Li, C., Zhang, H., Ding, P., Yang, S., & Bai, Y. 2023 Renewable & Sustainable Energy Reviews 184 113576–113576.
[3]. Lee, K.-T., & Cho, J. 2011 Nano Today 6(1) 28–41.
[4]. ArunaBharathi M, Sushama M, Rao K V, et al. 2023 Materials Today: Proceedings.
[5]. Lin, S., & Zhang, T. 2023 Journal of Alloys and Compounds 942 168926–168926.
[6]. Li, X., Zhang, F., Zhai, B., Wang, X., Zhao, J., & Wang, Z. 2020 Journal of Physics and Chemistry of Solids 145 109552–109552.
[7]. Ashraf, H., & Karahan, B. D. 2023 Materials Research Bulletin 112492.
[8]. Ren, Y.-X., Li, X., Wang, Y., Gu, S., Yang, C., Gao, T., Cao, P., & Zhou, G. 2022 Applied Materials Today 27 101452–101452.
[9]. Huang, X., Ding, Y., Li, K., Guo, X., Zhu, Y., Zhang, Y., & Bao, Z. 2021 Journal of Power Sources 496 229833–229833.
[10]. Benzait, Z., & Yuca, N. 2020 Electrochimica Acta 339 135917.









