1. Introduction
With the appearance of the ozone hole in the Antarctic ozone layer, many scientists have found that the ozone layer is depleted. Also, the NASA Earth Observatory shows that the ozone layer gradually disappears with the blue area expanding. However, this year, the recovery of the ozone layer is announced. This essay aims to examine the impact of various factors on the ozone layer and identify the contribution of certain factors to its recovery. Specifically, the four factors that will be discussed are human activities, polar stratospheric cloud particles (PSCs), circulation or mixing changes, and volcanic eruptions. Additionally, the contribution of three factors to the ozone layer’s recovery will be identified, namely chemical reduction, kinetics, and temperature. It is worth noting that the specific effects of dynamics and temperature on the Antarctic ozone layer remain unknown. To explore the potential impact of greenhouse gases, a chemistry-climate model will be employed to simulate climate and chemical processes under different greenhouse gas emission scenarios. To address this issue, a new type of environmentally friendly insulation gas called fluoronitrile gas has been launched in recent years, which has the potential to replace sulfur hexafluoride. Several research papers provide observational data and model results on the disappearance of the Antarctic ozone hole. This information is significant in helping us understand the ozone layer’s recovery process and the impact of human activities on it. By raising awareness of these issues, we can emphasize the importance of environmental protection. The article also affirms the success and importance of the Montreal Protocol. Ultimately, this paper aims to encourage further exploration and research in the field of biogeochemistry on how to achieve ozone layer recovery more efficiently. By providing scientific guidance for its protection, we can mitigate the negative impact of human activities and safeguard the health of our planet.
2. Factors contributing to the destruction of the Antarctic ozone layer
Regarding the factors that may affect the ozone layer, they are human activities, polar stratospheric cloud particles (PSCs), and volcanic eruptions. Firstly, the diminution of reactive halogens is predominantly ascribed to the emission of anthropogenic halocarbons that contain chlorine and bromine. Consequently, individuals worldwide have initiated a concerted effort aimed at phasing out these harmful substances. For the PSCs, in the realm of chemical reactions, surfaces are often utilized as a catalyst for the process, and the formation of PSCs at low temperatures is the first step in the chlorine-catalyzed destruction of ozone [1]. However, it is essential to note that certain reactions can result in the production of chlorine free radicals, which have the potential to cause harm to the stratosphere and ultimately contribute to the depletion of the ozone layer. Moreover, another relevant factor worth considering is the impact that circulation or mixing changes can have on the transportation of ozone and other chemicals [2]. Eventually, one crucial aspect to consider is the frequency of volcanic eruptions. The inactive storage tanks mostly hold artificially produced chlorine, which is obtained by creating chlorofluorocarbon particles. When the chlorine atoms are reactivated, they can demolish up to 10,000 ozone molecules before they are eliminated from the catalytic process [3]. Such an event can trigger the release of specific PSCs and aerosol particles that possess the capacity to spark analogous chemical reactions, owing to the elevated levels of sulfur present in the stratosphere. Sulfate aerosols from volcanic activity have a dual impact on atmospheric chemistry and climate. They can serve as “chlorine activators,” meaning that they trigger chemical reactions that release chlorine atoms from previously inert reservoirs already in the stratosphere [4]. There is another danger associated with volcanic aerosols. They can lead to the destruction of ozone at higher temperatures in the atmosphere compared to Polar Stratospheric Clouds (PSC). As a result, the ozone destruction can move from the polar region to more densely populated areas [5].
Figure 1 shows that volcanic eruptions have impacted both the northern and southern hemispheres, especially at pressures between 70 and 300 hPa. Although temperatures above 100 hPa are usually too high for many PSCs to form, they can still occur due to the presence of enough water in cold polar environments that allows for effective heterogeneous chemistry. Based on the study’s simulations, Antarctica had the highest volcanic losses in 2011 and 2015, with losses reaching up to 30% and 55%, respectively.
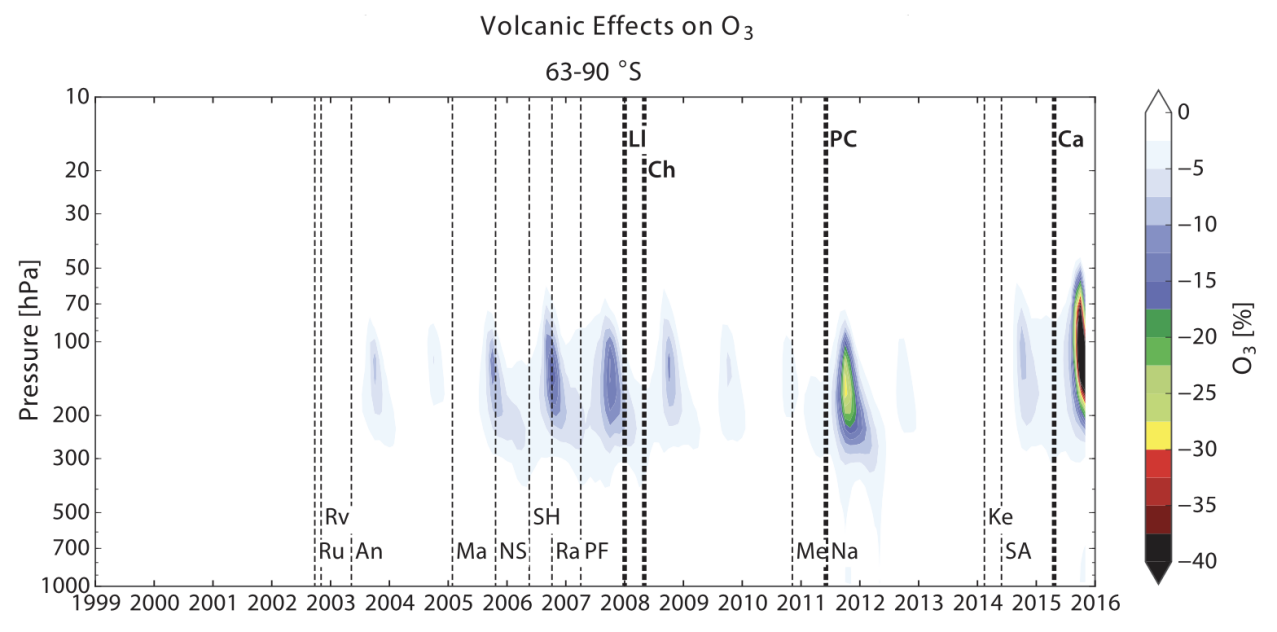
Figure 1. Model-calculated percentage changes in local concentrations of ozone due to a series of moderate volcanic eruptions.
These losses were primarily due to the eruptions of Puyehue-Cordón Caulle and Calbuco in Chile [1]. The study also indicates that tropical eruptions have contributed to ozone depletion in several earlier years. Therefore, it is clear from the research that the above factors have a significant impact on the polar ozone layer, and their effects should be carefully monitored and studied to mitigate any potential harm to the environment.
3. Factors contributing to the healing of the Antarctic ozone layer
Some articles present in-depth analyses of various observations and test cases, which include balloon and satellite ozone data, satellite data, chemistry-climate modeling, and volcanic aerosol measurements. The objective of this study is to distinguish the factors that are more likely to impact the polar ozone layer. Additionally, this essay presents important findings on the recovery of the Antarctic ozone layer, including trends, variations, and identifiable markers. These insights can help guide future research and actions in this area. As shown in Figure 2, the researchers use Total Ozone Mapping Spectrometer/Ozone Monitoring Instrument (TOMS/OMI) satellite observations together with numerical model simulations. When they calculated the monthly averaged October 2015 ozone hole that is 24.6 million km2 of volcanic aerosol include, namely the Chem-Dyn-Vol model, our calculated monthly averaged October 2015 ozone hole is 24.6 million km2, whereas the corresponding value excluding volcanic aerosols is much smaller, 21.1 million km2[6]
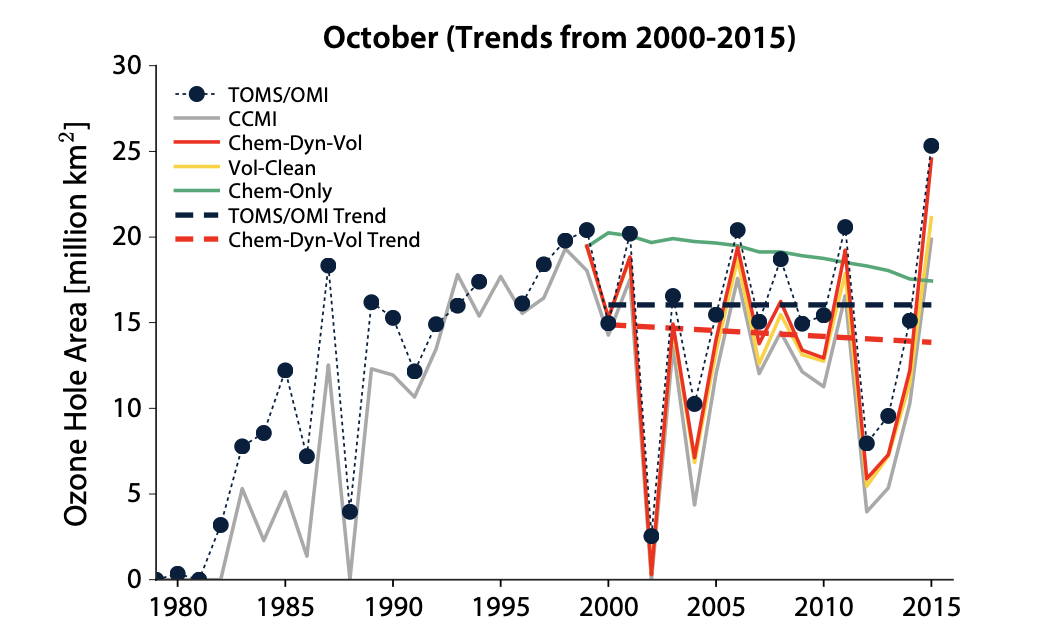
Figure 2. Annual size of the October monthly average ozone hole [6].
As a result, the effect of cold temperatures and dynamics on the ozone layer is much smaller than those of volcanic aerosols. At the same time, the paper examines chemical decline, dynamics, and temperature as three factors contributing to the Antarctic ozone layer. Through Figure 3, compared to October, the month of September exhibits a lower degree of variability in dynamics and experiences a robust chemical recovery. Consequently, it has emerged as the month with the most significant level of healing for the Antarctic ozone layer since the year 2000. This development provides more certainty that chemical factors have played an influential role in the observed trends. Based on the model analysis, it is estimated that roughly fifty percent of the ozone layer healing in September is attributable to chemical factors, while the remaining half is attributed to dynamics and temperature. Consequently, it concludes that the healing of the Antarctic ozone hole is emerging. Furthermore, it is obvious that the healing of the ozone layer frequently happened in September. However, it more recently happened in winter, where the rate in percent is highest at present [7]. In winter, the lifespan of the ozone layer is notably extended due to the fact that the photochemical lifetime of ozone is lengthened during this season, as compared to summer. This is particularly true at lower concentrations of NO, where the lifespan is further prolonged. According to winter data, the lifespan of ozone is typically 21 days (at 10 ppbv NO concentration) or 180 days (at 0.1 ppbv NO concentration), while in summer, these values are only 4.5 and 12 days, respectively [7]. This phenomenon occurs because NO persists for a longer duration during winter, allowing it to travel further both horizontally and vertically, thus extending the lifespan of ozone. Despite the slower photochemical production rate of ozone in winter, the reduced presence of NO can produce more ozone molecules (nonlinear effects), resulting in an increased overall production of ozone. Consequently, ozone gradually accumulates during winter and spreads throughout the Northern Hemisphere, which accounts for the observed peak ozone concentrations in the troposphere during the spring season in the Northern Hemisphere.
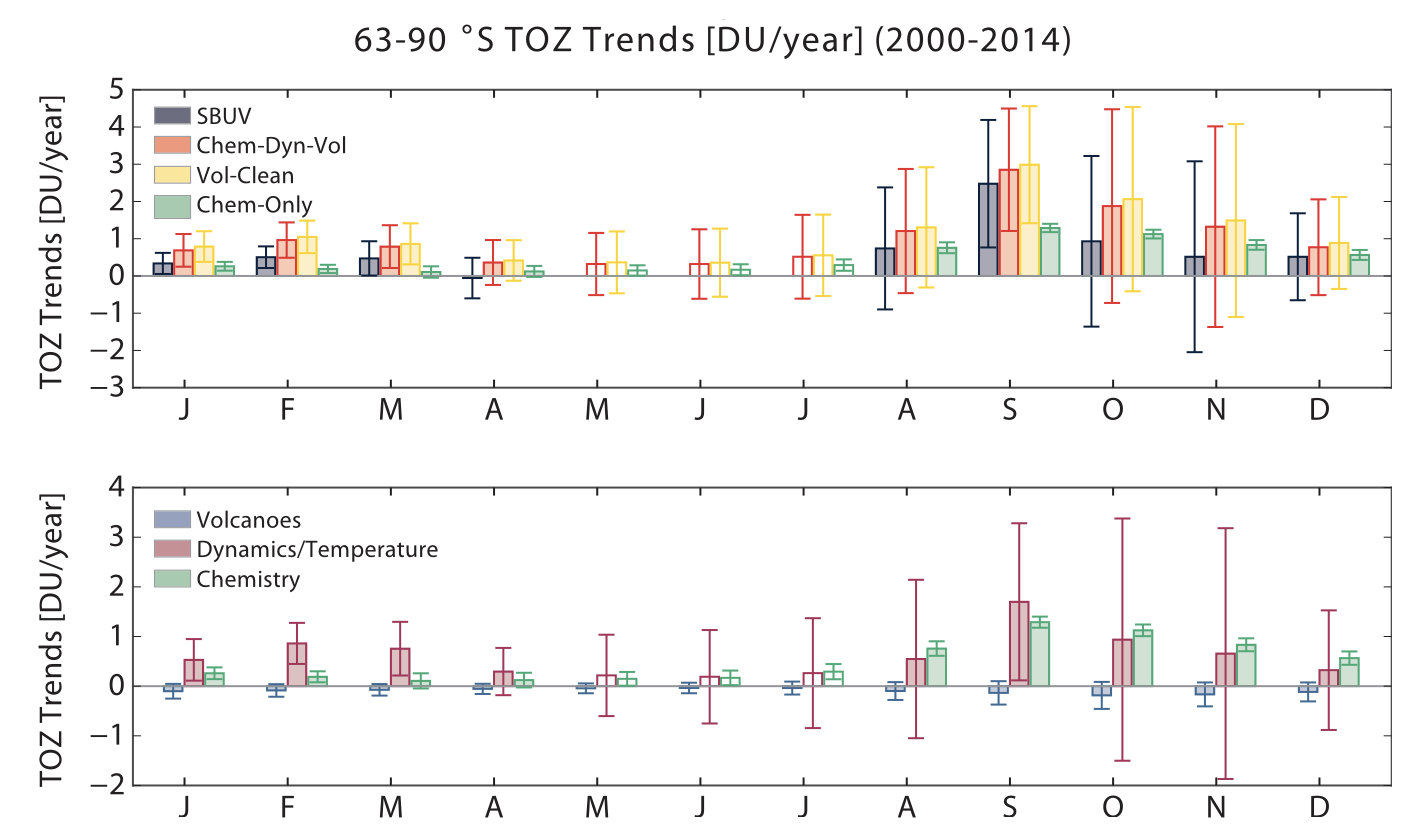
Figure 3. Trends in total ozone abundance (TOZ) by month from 2000 to 2014.
4. The suggestion for addressing the global warming
Based on this conclusion, this research indicates that it is possible to expand our understanding of significant environmental issues, such as global warming. Additionally, chemistry-climate models can be applied to simulate climate and chemical processes under various greenhouse gas emission scenarios, as demonstrated in this report. This approach allows people to evaluate the potential impact of greenhouse gases with greater accuracy. Sulfur Hexafluoride (SF6) is a synthetic fluorinated compound with an extremely stable molecular structure. In the world of electric power systems, SF6 is a highly important component. Its unique dielectric properties make it an ideal choice for voltage electrical insulation, current interruption, and arc quenching during the transmission and distribution of electricity. Even though the content of SF6 is not particularly high, it is still considered to be one of the most harmful substances when it comes to global warming. SF6 is a very strong greenhouse gas with a Global Warming Potential (GWP) of 23,500 times that of carbon dioxide (CO2) [8]. But if people continue to use SF6, then global warming will become more serious. Therefore, the next step is to find out a replacement for SF6. Fluoronitrile gas (C4F7N) is a new type of environmentally friendly insulation gas launched in recent years and one of the most promising candidates as an insulating and breaking medium in high and medium voltage electrical equipment. Its insulation is more than twice that of SF6 and can effectively reduce the use of SF6. Alise Chachereau and other researchers found that C4F7N has a large electron attachment cross section at thermal energies. The present research indicates that the determination of electron rate and transport coefficients is feasible at low pressures. This represents a significant milestone towards more accurate modeling endeavors in the future [9]. However, nowadays, studies on C4F7N normally focus on the electrical performance and there are few reports considering its safety problems and application risks. One research published by Chinese people uses mice experiments to test the acute toxicity of C4F7N through exposure test, blood cell analysis, and Pathological section analysis, and they find that the application risk of C4F7N is higher than that of SF6 because of its potential health effect. To be more specific, it will trigger respiratory frequency attenuation, progressive loss of moving ability, and abnormal blood cells, which is significant for people to pay attention to. As a result, people should learn that safety measures are required when coming back into contact with these gases.For instance, they introduce the concentration monitoring and warming system that is built into the test area or the equipment surrounding it. For individual protection equipment, people should use the full-face mask air-purifying respirator or the powered air-supplied respirator, ventilation goggles, and Nitrile-Butadiene gloves [8]. It’s important to note that C4F7N/N2 gas has a lower toxicity level than SF6 gas in the same conditions. Therefore, utilizing C4F7N/N2 gas as an insulator is a relatively safe option [10].
5. Conclusion
In conclusion, there is an elaborating description of four factors that could impact the Antarctic ozone layer, which are human activities, the polar stratospheric cloud, circulation or mixing changes, and volcanic eruptions. Then, these papers found that the primary cause of the depletion of the ozone layer is attributed to the emission of chlorofluorocarbon that is caused by human activities. In this essay, through various kinds of research and reports, some of them use some observations and mode-test cases, like balloon and satellite ozone data, to determine which factors are more likely to affect the polar ozone layer and to derive trends, variability, and signs of healing in the Antarctic ozone layer. They proved that the effect of cold temperatures and dynamics on the ozone layer is much smaller than those of volcanic aerosols, and the reduction of chemistry plays a crucial role in the healing of the Antarctic ozone layer. Furthermore, some papers also did some deep research about the seasonal trend in the healing of the ozone layer, showing that the concentration of ozone increases in spring and summer; however, more recently, in winter, the rate of healing is the highest. This is because NO has a longer lifespan during the winter, causing it to spread farther horizontally and vertically. Ultimately, people can use this knowledge to some extent to solve the global warming issue by using the chemistry-climate model to assess the different levels of threat from greenhouse gases, showing that the most harmful gas is SF6. Following that, this paper found that some research showed that there is an alternative gas, which is C4F7N, with more environmentally friendly properties, a larger electronic attachment, and more effective insulation. Nevertheless, there have been reports indicating that the use of C4F7N gas may lead to respiratory issues, mobility loss, and abnormal blood cells in mice experiments. While it may serve as a potential alternative to SF6, caution must be exercised with regards to its potential toxicity and risks, and it is imperative that appropriate personal protective equipment be worn at all times. On the other hand, there is still a knowledge gap in understanding how dynamics and temperature factors influence the healing of the Antarctic ozone layer. The data suggests a possible link between the springtime depletion of the ozone layer and the variations in dynamical and temperature contributions to its recovery. This implies the existence of potential feedback mechanisms that warrant further exploration.
References
[1]. Brune, W. H., Anderson, J. G., Toohey, D. W., Fahey, D. W., Kawa, S. R., Jones, R. L., McKenna, D. S., & Poole, L. R. (1991). The Potential for Ozone Depletion in the Arctic Polar Stratosphere. Science, 252(5010), 1260–1266. https://doi.org/10.1126/science.252.5010.1260
[2]. Assessment for Decision-Makers: Scientific Assessment of Ozone Depletion: 2014, World Meteorological Organization, Global Ozone Research and Monitoring Project—Report No. 56, Geneva, Switzerland, 2014.
[3]. McCormick, M., Thomason, L. & Trepte, C. Atmospheric effects of the Mt Pinatubo eruption. Nature 373, 399–404 (1995). https://doi.org/10.1038/373399a0
[4]. Coffey, M. T. (1996). Observations of the impact of volcanic activity on stratospheric chemistry. Journal of Geophysical Research: Atmospheres, 101(D3), 6767–6780. https://doi.org/10.1029/95JD03763
[5]. Tie, X., & Brasseur, G. (1995). The response of stratospheric ozone to volcanic eruptions: Sensitivity to atmospheric chlorine loading. Geophysical Research Letters, 22(22), 3035–3038. https://doi.org/10.1029/95GL03057
[6]. Solomon, S., Ivy, D. J., Kinnison, D., Mills, M. J., Neely, R. R., & Schmidt, A. (2016). Emergence of healing in the Antarctic ozone layer. Science, 353(6296), 269–274. https://doi.org/10.1126/science.aae0061
[7]. Janach, W. E. (1989). Surface ozone: Trend details, seasonal variations, and interpretation. Journal of Geophysical Research, 94(D15), 18289. https://doi.org/10.1029/JD094iD15p18289
[8]. Li, Y., Zhang, X., Zhang, J., Xiao, S., Xie, B., Chen, D., Gao, Y., & Tang, J. (2019). Assessment on the toxicity and application risk of C4F7N: A new SF6 alternative gas. Journal of Hazardous Materials, 368, 653–660. https://doi.org/10.1016/j.jhazmat.2019.01.100
[9]. Chachereau, A., Hösl, A., & Franck, C. M. (2018). Electrical insulation properties of the perfluoronitrile C 4 F 7 N. Journal of Physics D: Applied Physics, 51(49), 495201. https://doi.org/10.1088/1361-6463/aae458
[10]. Li, Y., Zhang, X., Xiao, S., Chen, Q., Tang, J., Chen, D., & Wang, D. (2018). Decomposition Properties of C 4 F 7 N/N 2 Gas Mixture: An Environmentally Friendly Gas to Replace SF 6. Industrial & Engineering Chemistry Research, 57(14), 5173–5182. https://doi.org/10.1021/acs.iecr.8b00010
Cite this article
Zhang,E. (2024). The recovery of the Antarctic ozone layer and suggestions for addressing the global warming. Applied and Computational Engineering,58,112-118.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Brune, W. H., Anderson, J. G., Toohey, D. W., Fahey, D. W., Kawa, S. R., Jones, R. L., McKenna, D. S., & Poole, L. R. (1991). The Potential for Ozone Depletion in the Arctic Polar Stratosphere. Science, 252(5010), 1260–1266. https://doi.org/10.1126/science.252.5010.1260
[2]. Assessment for Decision-Makers: Scientific Assessment of Ozone Depletion: 2014, World Meteorological Organization, Global Ozone Research and Monitoring Project—Report No. 56, Geneva, Switzerland, 2014.
[3]. McCormick, M., Thomason, L. & Trepte, C. Atmospheric effects of the Mt Pinatubo eruption. Nature 373, 399–404 (1995). https://doi.org/10.1038/373399a0
[4]. Coffey, M. T. (1996). Observations of the impact of volcanic activity on stratospheric chemistry. Journal of Geophysical Research: Atmospheres, 101(D3), 6767–6780. https://doi.org/10.1029/95JD03763
[5]. Tie, X., & Brasseur, G. (1995). The response of stratospheric ozone to volcanic eruptions: Sensitivity to atmospheric chlorine loading. Geophysical Research Letters, 22(22), 3035–3038. https://doi.org/10.1029/95GL03057
[6]. Solomon, S., Ivy, D. J., Kinnison, D., Mills, M. J., Neely, R. R., & Schmidt, A. (2016). Emergence of healing in the Antarctic ozone layer. Science, 353(6296), 269–274. https://doi.org/10.1126/science.aae0061
[7]. Janach, W. E. (1989). Surface ozone: Trend details, seasonal variations, and interpretation. Journal of Geophysical Research, 94(D15), 18289. https://doi.org/10.1029/JD094iD15p18289
[8]. Li, Y., Zhang, X., Zhang, J., Xiao, S., Xie, B., Chen, D., Gao, Y., & Tang, J. (2019). Assessment on the toxicity and application risk of C4F7N: A new SF6 alternative gas. Journal of Hazardous Materials, 368, 653–660. https://doi.org/10.1016/j.jhazmat.2019.01.100
[9]. Chachereau, A., Hösl, A., & Franck, C. M. (2018). Electrical insulation properties of the perfluoronitrile C 4 F 7 N. Journal of Physics D: Applied Physics, 51(49), 495201. https://doi.org/10.1088/1361-6463/aae458
[10]. Li, Y., Zhang, X., Xiao, S., Chen, Q., Tang, J., Chen, D., & Wang, D. (2018). Decomposition Properties of C 4 F 7 N/N 2 Gas Mixture: An Environmentally Friendly Gas to Replace SF 6. Industrial & Engineering Chemistry Research, 57(14), 5173–5182. https://doi.org/10.1021/acs.iecr.8b00010









