1. Introduction
Under pressure from an increasingly severe energy crisis and environmental degradation, global efforts are rapidly moving toward developing innovative energy technologies [1]. As internal combustion engines consume an inordinate share of annual total demand energy consumption, their increased use has raised serious concerns regarding global warming and air contamination; today batteries offer an alternative energy solution. Today there are many types of batteries including zinc-air battery Li-ion battery and nickel-cadmium battery-with Li-ion being the best of these three options available to us. Li-ion batteries offer unmatched levels of both energy and power density, being portable rechargeable sources that have an expected lifecycle of more than 500 cycles. Now popularly utilized in smartphones, tablets and laptops alike-it's also widely utilized within electric car [2]. Li-ion batteries will significantly lower greenhouse gas emissions if electric automobiles displace most gasoline-powered transportation, according to an analysis released today by clean technical.
Li-ion batteries' high energy performance makes them suitable for various electrical grid applications, including improving energy gathered from wind, solar, geothermal, or other eco-friendly sources, thus expanding their usage further and helping promote an energy sustainable economy. Li-ion batteries operate under various and changing environments, leading them to experience efficiency degradation such as capacity reduction, lower endurance mileage and power fade-back over time [3]. Over time however, continuous operation leads to various efficiency impairments including capacity reduction, decreased endurance mileage and power fade back [1]. Batteries provide an alternative energy source. However, their limited ability to meet rising electricity needs prevents their full potential from being fully utilized by power suppliers. Recent analyses reveal that electric vehicles contribute significantly to rising greenhouse emissions due to their need for power sources-particularly those limited accessing renewable resources, leading to an overall increase in emissions [4]. Electric vehicles refer to three categories of automobiles; battery cars, fuel cell cars and serial hybrid (range extender) cars are included under this umbrella term. Within this research study battery cars will be considered electric vehicles-playing an instrumental role in supporting global sustainable development efforts. As noted above, electric vehicles have the power to drastically cut greenhouse gas emissions when replacing most conventional automobiles with them. From an environmental viewpoint, batteries do not produce greenhouse gas emissions during use or disposal and batteries can even be recycled after they no longer power any electric cars. Truth be told, though electric cars may produce zero emissions overall, their primary energy source consists of burning fossil fuels for electricity production, potentially increasing carbon emissions further. Battery recycling systems remain immature in many developing nations. Therefore, government action needs to be taken immediately in this regard in order to establish efficient recycling structures there as well.
Li-ion batteries hold great promise as an important tool in energy sustainability, according to previous studies [4]. Numerous books and research studies discuss Li-ion's development, such as health assessments of Li-ion batteries or its new materials used within them [1, 2]. However, such technologies do not come without issues either: for every 1 tons of lithium extracted requires 5-15 tons of carbon whereas their costs far surpass other forms. Additionally, their anode material LiCoO2 makes the anodes expensive products like these ones are more costly products as opposed other batteries. Beyond exploring current advances in Li-ion batteries, this research seeks to analyze their relationship to sustainable advancement as well as explore their application potential in electric vehicle applications.
2. Applying different methods to improve lithium-ion batteries performance
Sergio and Florin conducted extensive research to investigate both the present state of EV batteries as well as their prospects, breaking it down into three parts: state of technology for battery electric vehicles, life-cycle evaluation methods on electrical automobiles and batteries, and green chemistry approaches for novel battery technologies [4]. The fundamental working principle behind battery electric automobiles will be described. Numerous manufacturers utilize "energy recovery" technology, enabling their electrical motor to act both as propulsion source and generator when breaking or moving. Furthermore, comparisons exist between internal combustion engines and battery electric automobiles (BEV). Furthermore, scientists demonstrate there are two useful types of BEV. As shown in Figure 1, electric motors have replaced internal combustion engines as the source of power to move wheels through gearboxes, while each drive wheel also contains its own hub motor for transmission of drivetrain torque to drive wheels [4].
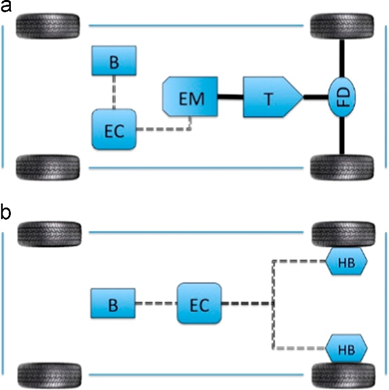
Figure 1. Two wheel drive battery electrical lorry building and construction types [4].
An appropriate drive and control strategy for electric cars are paramount components in optimizing energy management to extend running distance per battery charge. Therefore, this research study offers details about types of batteries as well as their advantages and disadvantages; specifically, research done into developing brand new materials for their cathodes and anodes; longevity impacts ultimately determines maintenance expenses of owning an electrical lorry.
Life cycle assessment (LCA) method is utilized to measure the environmental impacts associated with electrical lorries. Four significant steps comprise total LCA. LCA studies that investigate EV use are subject to significant constraints caused by battery design and type issues and conditions such as usage conditions or usage types, all which must be factored into any assessments of environmental, economic, or political sustainability of their use. Studies indicate that electric vehicles (EVs) may only become environmentally sustainable if their technology meets all criteria: battery advances, sustainable energy fashion trends and eco-driving. Some studies show that by considering European electrical power today, ecological impacts associated with each car type over their lifecycle can be determined at each phase of production, in-service usage, and disposal. Hybrid and battery electric cars produce greenhouse effects approximately 27-28% lower than that of petrol-driven automobiles, according to LCA studies. Furthermore, recycling procedures now use direct battery smelting. This makes an LCA analysis much more accurate. Organic compounds were investigated theoretically and experimentally as possible lithium rechargeable battery components. Lithium has long been utilized as the cathode material in rechargeable batteries, often as covalent crystals composed of organic substances based on carbonyl- and pyrene-containing groups such as Li2C2O6 or pyrene-4,5-9-10-tetraone (PLT). Four organic substances including PLT, tetracyanoquinodimethane (TCQDM), 2,5-dihydroxy benzoquinone and C35H20O10 are utilized as biological energy sources to develop new battery technologies. Enzymes and proteins remain underutilized within nano energetics as potential source materials to construct batteries from.
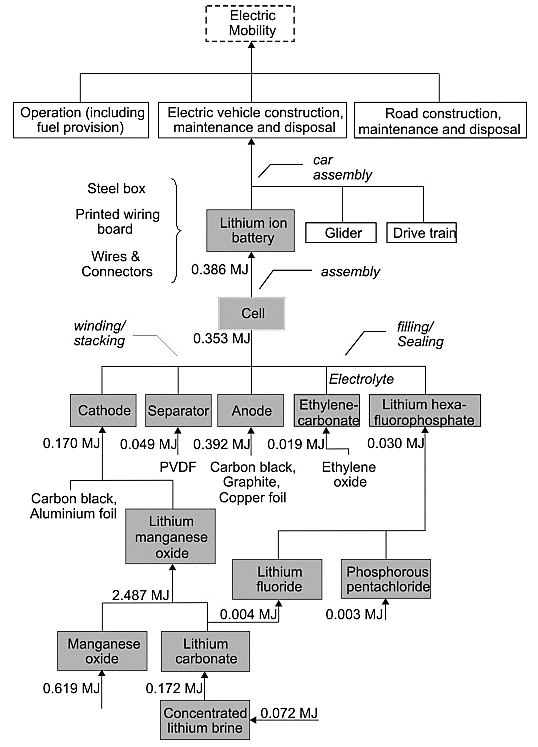
Figure 2. Depicts an electric transport design [5].
The LCA is widely employed today [5]. LCA is defined as providing an effective means for accounting and examining potential environmental impacts associated with specific items, processes, or activities [5]. LiMn2O4 battery was chosen for this study as its application provides ample environmental protection benefits [5]. Manganese will likely replace nickel and cobalt due to its lower costs and greater accessibility, and so manganese was chosen. An electric truck of similar size and power as that used by Volkswagen Golf cars. Research findings indicated the energy requirements of BEVs to be 17 kWh/100 km. By increasing or decreasing energy demands by 20%, their ecological burden was investigated. An internal combustion engine car was chosen for comparison due to their comparable technology with those used to manufacture BEVs. Figure 2 displays this process: lithium extraction and electrode manufacturing progress through battery pack manufacturing as well as movement with the electric vehicles.
Emissions and effects evaluation methods show that Li-ion batteries have only minimal impacts on E-mobility's ecological ramifications-regardless of which assessment methods were chosen to evaluate them in this research study (Figure 3). Four approaches used are displayed here as part of this investigation-these approaches can be seen below in Figure 3.
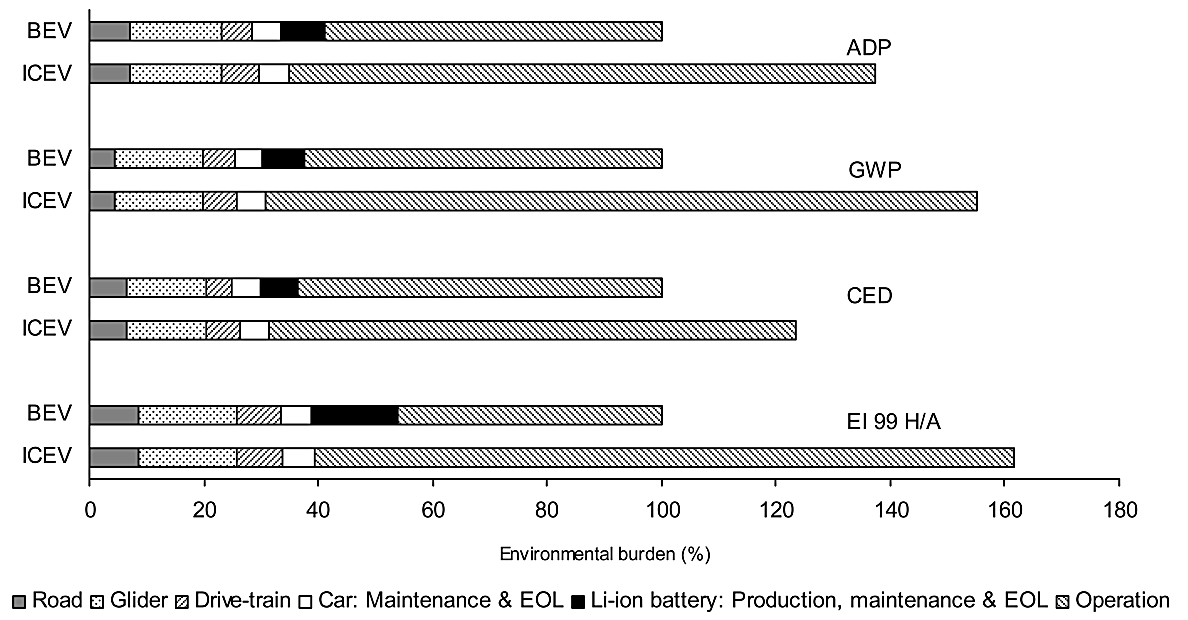
Figure 3. Depicts four assessment methodologies [5].
Under environmental measures related to road use (infrastructure, maintenance and disposal), an ICEV and BEV do not differ significantly in their environmental footprint and glider performance; there may however be small variations concerning drivetrain, maintenance and disposal issues between each type. Key differences exist when it comes to operating phase costs, which overshadow any influence from battery technology. LCA for E-mobility and mobility with an ICEV are greatly influenced by operating cost considerations; with costs for mobility with an ICEV being significantly more. Li-ion battery production typically involves producing its anode, cathode and battery pack. Other minor components may include single cells, separators, lithium salt solutions or solvents; battery packs consist of steel boxes with cables attached as well as printed wiring boards that comprise over 20%.
Ghassan Zubi and others conduct extensive research in lithium-ion battery technologies that utilize electrical energy storage (EES), an essential technology [6]. EES systems convert electrical power to another kind of energy for storage before returning it back as electricity when required. Electrochemical energy storage solutions such as lead-acid, lithium-ion and metal air batteries are among the most frequently deployed EES innovations. A research paper explores both their pros and cons [6]. The main types of batteries being utilized by EES technologies include lead-acid, lithium ion NiMH NiCd. EES systems have many applications in everyday life, from power supply systems and portable electrical/electronic gadgets, to portable electrical/electronic appliances and security. Each EES innovation offers distinctive efficiency features which make them specifically suitable for certain tasks or uses. Lithium stands out among metals as being particularly promising when it comes to battery chemistry, with high energy density, specific energy consumption, and particular power capacities. Lithium's electropositive nature and readily-availability makes it particularly promising as an electrode material. Additionally, it remains nontoxic during battery use. However, lithium being so reactive makes creating safe lithium-ion battery cells an enormous technological challenge. One solution has been the use of particle capable of emitting lithium ions rather than metallic lithium to circumvent this issue. Li-ion battery cells use a chemical process in which ions move back and forth between electrodes in an irreversible cycle, creating four main components: cathode, anode, electrolyte and separator. Lithium ions flow from the cathode to anode through electrolyte during charging and back again during discharge, the cathode material consisting of lithium metal oxide such as lithium cobalt oxide (LCO), manganese oxide and iron phosphate being utilized as cathodes respectively. Electrolytes for lithium batteries typically consist of lithium salts mixed with organic solvents such as LIPF6, LiClO4, or LIAsF6; organic solvents play an essential role in improving mobility of lithium ions that facilitate battery performance. Lithium battery cells come either cylindrical or stack designs (Figure 4). Battery management systems (BMSs) can ensure Li-ion cells remain effective and safe by tracking vital performance factors and managing performance parameters, according to specific user needs [6].
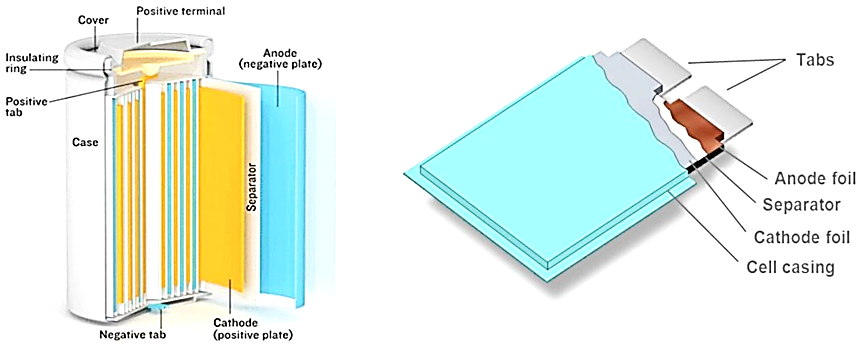
Figure 4. Li-ion battery cell setups [6].
Li-ion batteries have become ubiquitous across a range of applications and new ones are constantly emerging. Portable electronic gadgets were once their largest market. However, roadway transport now demands even more batteries from Li-ion cells than before, including mobile phones, tablets, laptops, cordless phones, digital video cameras, camcorders, MP3 gamers, video games and toys to name but a few examples of portable electronic gadgets; phones account for most battery market share while hybrid electric vehicles (HEVs) integrate traditional internal combustion engine with electric propulsion (battery + electric engine) technologies into one engine-driven chassis system (battery+electric engine).
Kiruthika Sundar Raj and others conducted research highlighting different techniques of making Li-ion battery from food waste, with an ultimate aim to recycle food waste before turning it into Li-ion batteries [7]. Biomass made from food waste has long been utilized as an abundant and cost-effective source of carbon for use in Li-ion battery production [8]. According to the Food Waste Index Report, approximately 931 million tons of food waste was created worldwide in 2019, divided roughly equally among residential garbage (61 percent), food service waste (26%), and retail waste (13%). Carbonization remains at the core of creating carbon materials; subsequent events include hydrogen transfer, condensation, dehydrogenation and isomerization events that all help form part of carbon substance synthesis.
Darjazi et al. reported anodes made of coffee ground waste as an anode material combined with green biners such as polyacrylic acid (PAA), Na-carboxymethyl cellulose (NMC) or alginate (Alg) [8]. Electrochemical studies reveal that PAA-based anodes significantly enhance charge/discharge efficiency and retention capacity, while CMC electrodes as LIBs anodes offer optimal discharge capability and cycle stability. Producing effective Li-ion and Na-ion batteries using anode materials derived from food waste appears to be a viable solution. Biomass items derived from biological wastes, including agricultural debris, leftover food scraps and chicken waste such as eggshells are an environmental problem in large quantities. Economic sustainability and treatment require intensive efforts when recycling eggshell trash; each year around 1 billion eggshell waste products from food businesses are left behind on agricultural lawns for disposal, leading to environmental pollution and increasing greenhouse emissions. Eggshell can be an unorthodox battery material.
Zhang et al. developed an easy hydrothermal technique for manufacturing batt-like MnO2 nanoplatelet balls within eggshell holes (Figure 5) [9]. Eggshell was ground into powder and sieved through various mesh sizes in order to prepare MnO2 samples for correlation using an identical process. MnO2 produced using an eggshell-free approach boasts an exceptional specific charge/discharge capacity of 1008.2/2156.6 m2/g at present density of 100 mA/g at initial density. Following an initial small drop after 30 cycles, its charge/discharge capability slowly increased and eventually peaked out at 1024.80/1021.21 mAh/g by 100 cycles-far exceeding anything ever observed when producing MnO2 [9].
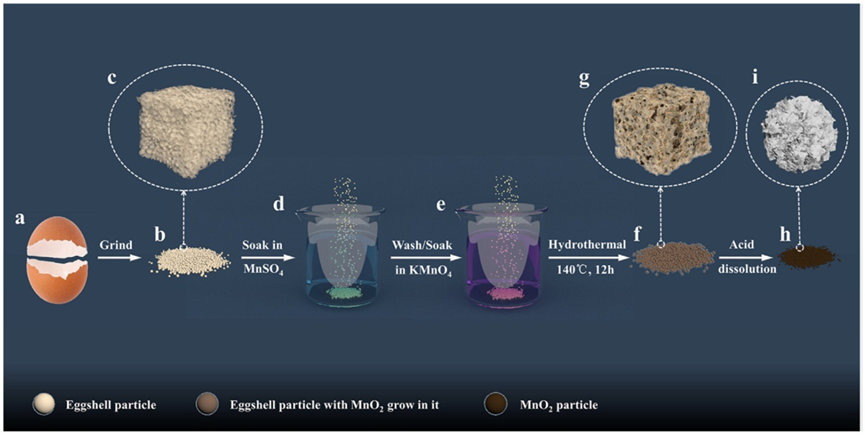
Figure 5. Preparation of MnO2 particles using eggshell as a rigid template for their production [7].
Due to activated carbon's microcrystalline structure, it is an amorphous, high-porous, and adaptable chemical with numerous applications [10]. It features greater interlayer area and aromatic sheets stacked disarray when compared with graphite, to produce activated carbon from biomass requires five stages: pre-treatment, carbonization, actuation, heat treatment and finally cleaning. Every year there is an accumulation of food waste from industrial sources as well as household garbage which cannot be eaten directly but which could instead be recycled and turned into activated carbon for use in manufacturing Li-S cells, thus helping both real world food waste reduction while simultaneously benefiting emerging Li-ion battery enterprises alike!
According to published accounts, carbon material can be manufactured through easy and non-activating pyrolysis using "after-boiling waste" from beer brewing. A sulfur-carbon composite was then utilized as the cathode in Li-S batteries due to its high Sulphur loading of over 70% at biomass-derived carbon via melt diffusion method; and performed extremely well, producing 847 mAh/g at 1 C, 586 mAh/g at 2 C and even 498 mAh/g at 5C capacity dropped only slightly after 400 cycles and now only drops by 0.01% each cycle [7].
3. Conclusion
From research, lithium-ion battery systems have some inherent limitations; but solutions exist. Green chemistry solutions use aromatic rings quickly providing charge transfer capability using carbon, nitrogen, and oxygen components as primary constituents. Unfortunately, few testable carbon-based technologies have yet been developed into battery systems; more are necessary in order to keep portable energy supplies available and electric vehicle power sustainable.
Li-ion batteries used in BEVs for transportation services exert only minimal environmental impacts when considering environmental impact analyses; in-depth sensitivity analyses revealed only minor variations among various lithium-based cathode materials during environmental impact evaluation; for simplicity's sake it would seem best to neglect specific active materials when making assessments with general measures; this helps keep battery chemistry assessments straightforward and reduce complexity. Comparative reviews with conventional vehicles revealed them eco-friendlier overall.
Food waste contains hard carbon material which can be utilized as battery cathodes. Though non-valuable materials like this would normally make this approach unfeasible financially, its collection and transportation challenges make this approach attractive. Li-ion batteries cannot be considered ecologically friendly in an absolute sense; therefore, businesses and related sectors that deal with Li-ion batteries should understand how best to maximize its environmental advantages while remaining mindful of all environmental considerations throughout their value chains; from mining raw material through fabrication of cells and batteries packs before collecting and recycling spent cells/batteries for recycling. Li-ion batteries could play an essential part in contributing to global energy sustainability. Key actions for accomplishing this include decreasing electricity sector carbon footprint, developing an effective collection and recycling system for Li-ion cells and decreasing cobalt dependence.
References
[1]. Li Y, Luo L, Zhang C and Liu H 2023 World Elctr. Veh. J. 14(7) 188
[2]. Wakihara M 2001 Materials Science and Engineering: Reports 33(4) 109-134
[3]. Nitta N, Wu F, Lee J T, and Yushin G 2015 Materials today 18(5) 252-264
[4]. Manzetti S and Mariasiu F 2015 Renewable and Sustainable Energy Reviews 51 1004-1012
[5]. Notter D A, Gauch M, Widmer R et al. 2010 Environmental Science & Technology 44 (17) 6550-6556
[6]. Zubi G, Dufo-López R, Carvalho M, and Pasaoglu G 2018 Renewable and Sustainable Energy Reviews 89 292-308
[7]. Raj K S, Baskaran N, Nair P P et al. 2023 Journal of Hazardous Material Advances 10 100317
[8]. Darjazi H, Staffolani A, Sbrascini L et al. 2020 Energies 13(23) 6216
[9]. Zhang W, Zhang B, Jin H et al. 2018 Ceramics International 44(16) 20441-20448
[10]. Bacilla A C C, Futamura R, Sugiyama Y et al. 2022 Carbon 193 88-97
Cite this article
Cao,J. (2024). Applying different methods to improve the performance of lithium-ion batteries. Applied and Computational Engineering,58,119-125.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Li Y, Luo L, Zhang C and Liu H 2023 World Elctr. Veh. J. 14(7) 188
[2]. Wakihara M 2001 Materials Science and Engineering: Reports 33(4) 109-134
[3]. Nitta N, Wu F, Lee J T, and Yushin G 2015 Materials today 18(5) 252-264
[4]. Manzetti S and Mariasiu F 2015 Renewable and Sustainable Energy Reviews 51 1004-1012
[5]. Notter D A, Gauch M, Widmer R et al. 2010 Environmental Science & Technology 44 (17) 6550-6556
[6]. Zubi G, Dufo-López R, Carvalho M, and Pasaoglu G 2018 Renewable and Sustainable Energy Reviews 89 292-308
[7]. Raj K S, Baskaran N, Nair P P et al. 2023 Journal of Hazardous Material Advances 10 100317
[8]. Darjazi H, Staffolani A, Sbrascini L et al. 2020 Energies 13(23) 6216
[9]. Zhang W, Zhang B, Jin H et al. 2018 Ceramics International 44(16) 20441-20448
[10]. Bacilla A C C, Futamura R, Sugiyama Y et al. 2022 Carbon 193 88-97









