1. Introduction
New energy is one of the decisive technical fields in the development of the world economy in the 21st century. Among them, solar photovoltaic power generation technology has received particular attention. In the past development, the photovoltaic industry has accumulated rich experience and has become a mature industry. Since the maximum output power of the photovoltaic array is affected by illumination time, intensity and temperature, its output power is intermittent and uncontrollable. In order to prevent fluctuations in the power grid, the introduction of energy storage devices can play a role in balancing, regulating and storing energy. When the light is sufficient, the energy storage device stores excess electric energy; when the light is insufficient or there is no light, it converts the stored energy into electric energy and continues to supply power to the load, thereby smoothing the power fluctuation and ensuring the continuity and stability of power supply [1].
As photovoltaic power generation gradually moves towards industrialization and scale, the requirements for energy storage are also getting higher and higher [2]. The market for photovoltaic power generation as well as the sustainable growth of the battery business both demand the development of long-life, low-cost, green, and environmentally-friendly unique batteries for power energy storage. New energy storage technology research will become a popular subject in the sector. The development of energy storage technologies will encourage the efficient use of clean energy sources like solar and wind power. The growth of the battery sector will also be aided by the advancement of technology and the development of photovoltaic and wind energy. After electric car batteries, the use of energy storage batteries in photovoltaic systems will emerge as a new area of battery research and development that is expected to increase exponentially.
In terms of research significance, this article could contribute to the understanding of ESS and RFBs, potentially leading to advancements in energy storage technology. It could also guide future research and development efforts in this field by highlighting the strengths and weaknesses of different battery solutions, particularly VRFBs. This could ultimately lead to more efficient, sustainable, and versatile energy storage systems.
2. The features and performance requirements of Energy Storage System
2.1. The features of Energy Storage System
Energy storage devices are one of the most important factors affecting the operating cost and efficiency of photovoltaic power generation systems. Choosing a suitable energy storage device is of great significance to the operation of photovoltaic power generation systems [3]. Its functions can be summarized in the following aspects [4]:
(1) Store excess electric energy when the sunshine is sufficient and use it at night or on rainy days, so that the photovoltaic power generation system can synchronize power generation with electricity consumption.
(2) The size of the electricity load changes with time, which makes photovoltaic power generation and load electricity consumption unable to naturally match. The energy storage capacity and charge and discharge performance of the energy storage device can well regulate the power and energy emitted by the system.
(3) The electrical load of large equipment such as water pumps and refrigerators not only has large capacity, but also generates surge current and impact current during startup and operation. The low internal resistance and good dynamic characteristics of the energy storage device can provide instantaneous large current.
(4) Photovoltaic power generation itself cannot be dispatched. The installation of energy storage devices can realize grid-connected operation with the large power grid for dispatching. Affected by natural factors such as climate, the photovoltaic power generation unit cannot perform power generation planning, but after configuring energy storage. According to the initial power generation plan, the photovoltaic power generation system is capable of producing power.
According to the characteristics of the photovoltaic power generation system and the role of the energy storage device, energy storage technology should meet the following performance requirements [5]:
(1) The device has a long cycle life, which is safe and reliable. The average lifespan of the primary components (photovoltaic cells and controllers) in a photovoltaic power generation system is over 15 years. As a result, the supporting energy storage device needs to have a lifespan of at least 5 years, which has a significant impact on the system's operating costs.
(2) The charging and discharging capabilities of the devices are good. In the photovoltaic system, the energy storage device's discharge depth is unstable. When it is continuously cloudy, it is easy to cause over-discharge, which affects the recovery of the capacity. This requires the energy storage device to have excellent recovery performance; small self-discharge and strong overcharge capability.
(3) The device has high energy efficiency and performance-price ratio, and large capacity.
(4) The device has wide applicability and low maintenance cost. Because photovoltaic power generation systems are mainly located in more remote areas.
3. Introduction of Redox-flow battery (RFB)
A flow battery’s active material, which is both an electrode active material and an electrolyte solution, is liquid, unlike typical batteries, which include the active material in a solid anode or cathode. It can be dissolved in two sizable liquid holding tanks. The solution flows through the flow battery by a pump, and on the electrodes on each side of the ion exchange membrane, reduction and oxidation reactions take place in turn. It is formed by two liquid pairs with different electrode potentials as positive and negative electrodes. Electric storage devices of different sizes. This kind of battery has no solid-state reaction and does not change the structure and form of the electrode material. Compared with other conventional batteries, it has obvious advantages.
According to the different particles participating in the reaction, RFB can also be divided into several types, and the typical ones are listed below and their characteristics are described as follows [6,7].
3.1. Vanadium Redox Flow Batteries
The system closest to commercialization at the moment with relatively developed technology is the all-vanadium system. The issue of cross-contamination between the positive and negative active materials is solved since both the positive and negative active materials are made of the same element [8]. However, due to the low solubility of V5+, the energy density of electricity is greatly limited, and the solubility of V5+ is negatively correlated with temperature. As the temperature increases, V2O5 will precipitate out, which reduces the operating temperature of the vanadium battery. Limit within 10~40℃. The current research work primarily focuses on the alteration of the electrolyte in order to raise the energy density and widen the operating temperature of all-vanadium Redox-flow batteries. Using a mixed solution of sulfuric acid and hydrochloric acid as a supporting solution, the operating temperature of the all-vanadium Redox-flow battery was extended to the range of -5~50℃at a vanadium concentration of 3.0mol/L, effectively expanding the operating temperature of the vanadium battery.
3.2. Iron-vanadium flow battery
The Fe-V system liquid flow battery is a newly proposed double-flow battery system. This kind of battery uses Fe3+/Fe2+ as the positive electrode pair and V3+/V2+ as the negative electrode pair [9]. The positive and negative electrodes are used to solve the liquid mixing method to avoid the capacity loss caused by the infiltration of the active material. Because the solubility of positive and negative electrode pairs is high, and the solubility increases with the increase of temperature, it effectively increases the energy density of the battery and broadens the working temperature of the battery, but the open-circuit voltage of this kind of battery is lower and the power density is low, and the charging state needs to be strictly controlled to prevent the V3+/V2+ in the positive electrolyte from being oxidized to a higher valence state and precipitate, which greatly limits the application of this kind of battery system.
3.3. Vanadium/air single flow battery
Vanadium/air single-flow battery is a new battery concept developed on the basis of all-vanadium flow battery and fuel cell technology [10]. The battery uses the negative electrode system of the all-vanadium flow battery as the negative half-cell, and adopts the air or oxygen diffusion electrode as positive half-cell.
Compared with the all-vanadium flow battery, since the vanadium/air single flow battery uses an air/oxygen diffusion electrode to replace the flow positive half-cell, the amount of vanadium electrolyte is reduced by half, and the energy density of the vanadium/air single flow battery system is increased. doubled, significantly reducing the cost of energy storage equipment.
Due to the different phase states of the active materials of the positive and negative electrodes, problems such as oxygen diffusion from the positive electrode side to the negative electrode side and solution penetration from the negative electrode side to the positive electrode side may occur. These problems not only cause the loss of battery capacity, but also greatly affect the battery life.
3.4. Full lead deposition single flow battery
The all-lead deposition single-flow battery is a typical representative of the all-deposition single-flow battery [11]. The battery uses acidic lead alkyl sulfonate aqueous solution as the electrolyte. During charging, the positive and negative electrodes deposit metal Pb and PbO2 on the inert matrix respectively. During discharge, the deposits are dissolved and converted into Pb2+ and returned to the solution. During the battery charging/discharging process, the positive and negative electrode materials need to be kept at a certain distance to avoid electrode contact and short circuit.
4. Introduction of Vanadium Redox Flow battery (VRFB)
The University of New South Wales created the Vanadium Redox Flow battery in 1985 [12]. Based on this, VRB Power Systems developed the vanadium redox flow battery system, a sort of energy storage that can combine chemical and electrical energy. Different valence states of vanadium ions can store chemical energy. Electrochemical reactions take place while the electrolyte solution flows perpendicular to the electrode surface. Through the two electrode plates, the current is gathered and carried. The working principle of VRB is shown in Figure 1. Vanadium ions have multiple valence states of V5+, V4+, V3+, V2+, and they can all exist stably. Among them, V5+/V4+ is the positive active point pair, and V3+/V2+ is the negative active point pair, which are stored in two storage tanks as positive and negative electrolytes respectively. During operation, the electrolytic hydraulic pressure is continuously put into the battery stack by two rotating liquid flow pumps, and the potential difference between the two pairs promotes the redox reaction, which is realized by the directional migration of H+ ion through the ion exchange diaphragm.
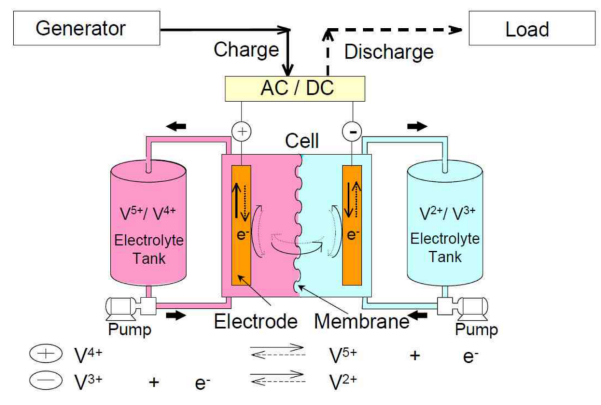
Figure 1. Standard vanadium redox flow battery schematic [8].
4.1. Main characteristics of VRFB
Vanadium battery uses the redox reaction of different valence vanadium ions to realize the conversion of electric energy and chemical energy, which has the following main characteristics [13].
(1) The battery’s power and capacity can be separately designed. It is practical to increase the capacity because the stack determines battery power and a single electrolyte determines capacity. (2) The rate of self-discharge is low. Vanadium batteries have a very low self-discharge rate between them and may be stored for a very long period since the positive and negative electrolytes are placed apart when they are not in use. (3) Strong capacity for overdischarge. The vanadium battery system's performance is unaffected by the deep discharge, and the vanadium battery can be fully recharged and placed back to use. (4) The electrolyte of the battery is circulating, and the battery does not have the problem of thermal runaway. At the same time, it also reduces the electrochemical polarization, so that the battery can charge and discharge at high current. (5) The effect of temperature on vanadium battery is much smaller than that on lead-acid battery, and with the recovery of temperature, the performance of vanadium battery is completely restored. (6) The cycle life is longer than 6 years. (7) The electrolyte can be recycled without restriction and will not cause environmental pollution. (8) Can be made into a megawatt energy storage system. (9) The cost is low and the maintenance is simple.
The above advantages show that vanadium battery is suitable to be used as the energy storage system of solar photovoltaic system and has a broad market prospect in solar photovoltaic system.
4.2. Application fields of VRFB
Vanadium flow batteries are widely used in many fields because of their unique advantages:
4.2.1. Wind power market. In order to protect the wind blades in an emergency, wind turbines currently need to be fitted with lead-acid batteries that have a power rating of about 1% of their total power [14]. Additionally, each wind turbine must include a dynamic battery with a power output that ranges from 10% to 50% of its own. A greater amount of dynamic energy storage batteries are required for off-grid wind turbine power generation. In order to create a dynamic energy storage system for wind farms, conventional lead-acid batteries can be totally replaced by vanadium batteries, which have numerous exceptional advantages.
4.2.2. Photovoltaic generation. Existing lead-acid batteries have very low power, capacity, and longevity. Vanadium batteries are anticipated to be the preferred option for solar energy storage batteries due to their numerous notable advantages [15].
4.2.3. Power grid peak regulation. A pumped storage power station has traditionally served as the primary method of peak management of the electrical grid since it requires the construction of upper and lower reservoirs, which is constrained by geographic constraints. In a plain location, it is difficult to occupy a sizable space and the expense of maintenance is considerable [16]. The vanadium battery energy storage power plant has an unrestricted location, little land use, minimal maintenance costs, and no restrictions on geography. Vanadium battery energy storage power stations are anticipated to gradually replace pumped storage power stations as vanadium battery technology advances and play a significant role in power system peak regulation.
4.2.4. Electric vehicle power supply. Due to the vanadium battery’s particular structure and high charging capabilities, which include high specific power and high specific energy, it is appropriate for use as an electric vehicle power source. Additionally, it can address the issue of air pollution brought on by vehicle exhaust emissions. Vanadium batteries have the advantage of “instant charging” (direct electrolyte replacement or replenishment) when used as an automobile’s power source.
4.2.5. Uninterruptible power supply and emergency power supply. It can be used as a UPS for emergency lighting in buildings like offices, theatres, hospitals, etc. as well as a backup power source for electronics like computers and some military hardware.
4.2.6. Power supply system. In places like islands and isolated areas, it is expensive to construct traditional power plants or develop and erect transmission lines [17]. Vanadium batteries used in conjunction with solar, wind, and other power producing equipment can provide a consistent power supply in these regions. Vanadium batteries can also be utilized as power supply systems for radio broadcasting, railway signaling, and postal and telecommunications.
5. Conclusion
The article provides a comprehensive analysis of Energy Storage Systems (ESS) and Redox Flow Batteries (RFB), with a special focus on Vanadium Redox Flow Batteries (VRFB). It elucidates the functions and characteristics of ESS, emphasizing their critical role in energy management. The chemical principles of various RFB battery solutions are explored, offering insights into their efficiency, capacity, and potential applications. The unique characteristics and application fields of VRFBs are summarized, highlighting their long lifespan, flexible design, and ability to deliver large amounts of power. These features make VRFBs suitable for a range of applications, including grid storage and renewable energy integration.
Looking ahead, the future of ESS and RFBs is promising. Continued research in this field could lead to more efficient, sustainable, and versatile energy storage systems, paving the way for technological progress and a more sustainable energy future. At present, there are very few energy storage technologies that have excellent performance in terms of energy density, power density, response speed, cycle life, and environmental friendliness. As a result, it is important to pick an energy storage technology that works for various power applications while also taking into account the needs of both parties. Vanadium redox flow batteries are ideal for use as energy storage devices for independent photovoltaic power generation systems based on the needs of the photovoltaic power generation system for energy storage devices and the benefits of vanadium redox flow batteries. At the same time, they are also competitive in cost compared with lead-acid batteries. Independent photovoltaic power generation systems have broad market prospects.
References
[1]. Hiksas M. M., Aninditio M. L.: Redox Flow Batteries for small scale energy storage. 2016 IEEE Conference on Technologies for Sustainability (SusTech), 2016, 134–139.
[2]. C. Choi, S. Kim, R. Kim, Y. Choi, S. Kim, H. Jung, J.H. Yang, H.T. Kim: A review of vanadium electrolytes for vanadium redox flow batteries. Renew. Sustain. Energy Rev., 69 (2017), pp. 263-274.
[3]. P. Vasko, A. Verboviy, M. IbragImova and S. Pazych, Pumped hydroelectric energy storage - technological basis of integration of powerful wind and photoelectric power stations into the pow-er system of Ukraine, Hidroenerhetyka Ukrainy, no. 1–2, pp. 20-25, 2017.
[4]. Shigematsu T., Kumamoto T., Deguchi H. Applications of a vanadium redox-flowbattery to maintain power quality [C]. Trasimission and Distribution Conference and Exhibition2002: 1065-1070
[5]. Garg, H.P. (1987). Storage of Solar Energy. In: Advances in Solar Energy Technology. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-0659-9_4
[6]. Weber, A.Z., Mench, M.M., Meyers, J.P. et al. Redox flow batteries: a review. J Appl Electrochem 41, 1137–1164 (2011). https://doi.org/10.1007/s10800-011-0348-2
[7]. T. Khan, A.K. Garg, A. Gupta, A.K. Madan, P.K. Jain. Comprehensive review on latest advances on rechargeable batteries. J. Energy Storage, 57 (2023), pp. 38, 10.1016/j.est.2022.106204
[8]. J.H. Vinco, A. Domingos, D.C.R. Espinosa, J.A.S. Tenorio, M.D.G. Baltazar. Unfolding the vanadium redox flow batteries: an indeep perspective on its components and current operation challenges. J. Energy Storage, 43 (2021), pp. 31.
[9]. Zhao, L., Ma, Q., Xu, Q. et al. Performance improvement of non-aqueous iron-vanadium flow battery using chromium oxide–modified graphite felt electrode. Ionics 27, 4315–4325 (2021).
[10]. Diwakar, K., Rajkumar, P., Subadevi, R. et al. A study on high rate and high stable sodium vanadium phosphate electrode for sodium battery alongside air exposure treatment. J Mater Sci: Mater Electron 32, 14186–14193 (2021).
[11]. Sun, Y., Guo, S., Wang, Y. et al. A new lead single flow battery in a composite perchloric acid system with high specific surface capacity for large-scale energy storage. J Solid State Electrochem 21, 3533–3543 (2017).
[12]. H. Deguchi, N. Tokuda, T. Kanno, K. Motoi, T. Kumamoto, T. Itoh, et al., Development of a 450kW Vanadium Redox Flow Battery System, 33 rd lntersociety Engineering on Energy Conversion, pp. 1074-1079, Aug. 1998.
[13]. Jung, BY., Ryu, CH. & Hwang, GJ. Characteristics of the all-vanadium redox flow battery using ammonium metavanadate electrolyte. Korean J. Chem. Eng. 39, 2361–2367 (2022).
[14]. D. D. Banham-Hall, G. A. Taylor, C. A. Smith and M. R. Irving, Flow Batteries for Enhancing Wind Power Integration, in IEEE Transactions on Power Systems, vol. 27, no. 3, pp. 1690-1697, Aug. 2012.
[15]. Ashurov, K.B., Abdurakhmanov, B.M., Iskandarov, S.C. et al. Solving the Problem of Energy Storage for Solar Photovoltaic Plants (Review). Appl. Sol. Energy 55, 119–125 (2019).
[16]. Chuang, SY., Leu, CH., Hsueh, KL. et al. Stability of Vanadium Electrolytes in the Vanadium Redox Flow Battery. MRS Online Proceedings Library, 1492, 138–144 (2012).
[17]. A. Cunha, F.P. Brito, J. Martins, N. Rodrigues, V. Monteiro, J.L. Afonso, P. Ferreira. Assessment of the use of vanadium redox flow batteries for energy storage and fast charging of electric vehicles in gas stations Energy, 115 (2016), pp. 1478-1494, 10.1016/j.energy.2016.02.118
Cite this article
Yu,Y. (2024). Vanadium redox flow battery: Characteristics and application. Applied and Computational Engineering,58,267-273.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Hiksas M. M., Aninditio M. L.: Redox Flow Batteries for small scale energy storage. 2016 IEEE Conference on Technologies for Sustainability (SusTech), 2016, 134–139.
[2]. C. Choi, S. Kim, R. Kim, Y. Choi, S. Kim, H. Jung, J.H. Yang, H.T. Kim: A review of vanadium electrolytes for vanadium redox flow batteries. Renew. Sustain. Energy Rev., 69 (2017), pp. 263-274.
[3]. P. Vasko, A. Verboviy, M. IbragImova and S. Pazych, Pumped hydroelectric energy storage - technological basis of integration of powerful wind and photoelectric power stations into the pow-er system of Ukraine, Hidroenerhetyka Ukrainy, no. 1–2, pp. 20-25, 2017.
[4]. Shigematsu T., Kumamoto T., Deguchi H. Applications of a vanadium redox-flowbattery to maintain power quality [C]. Trasimission and Distribution Conference and Exhibition2002: 1065-1070
[5]. Garg, H.P. (1987). Storage of Solar Energy. In: Advances in Solar Energy Technology. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-0659-9_4
[6]. Weber, A.Z., Mench, M.M., Meyers, J.P. et al. Redox flow batteries: a review. J Appl Electrochem 41, 1137–1164 (2011). https://doi.org/10.1007/s10800-011-0348-2
[7]. T. Khan, A.K. Garg, A. Gupta, A.K. Madan, P.K. Jain. Comprehensive review on latest advances on rechargeable batteries. J. Energy Storage, 57 (2023), pp. 38, 10.1016/j.est.2022.106204
[8]. J.H. Vinco, A. Domingos, D.C.R. Espinosa, J.A.S. Tenorio, M.D.G. Baltazar. Unfolding the vanadium redox flow batteries: an indeep perspective on its components and current operation challenges. J. Energy Storage, 43 (2021), pp. 31.
[9]. Zhao, L., Ma, Q., Xu, Q. et al. Performance improvement of non-aqueous iron-vanadium flow battery using chromium oxide–modified graphite felt electrode. Ionics 27, 4315–4325 (2021).
[10]. Diwakar, K., Rajkumar, P., Subadevi, R. et al. A study on high rate and high stable sodium vanadium phosphate electrode for sodium battery alongside air exposure treatment. J Mater Sci: Mater Electron 32, 14186–14193 (2021).
[11]. Sun, Y., Guo, S., Wang, Y. et al. A new lead single flow battery in a composite perchloric acid system with high specific surface capacity for large-scale energy storage. J Solid State Electrochem 21, 3533–3543 (2017).
[12]. H. Deguchi, N. Tokuda, T. Kanno, K. Motoi, T. Kumamoto, T. Itoh, et al., Development of a 450kW Vanadium Redox Flow Battery System, 33 rd lntersociety Engineering on Energy Conversion, pp. 1074-1079, Aug. 1998.
[13]. Jung, BY., Ryu, CH. & Hwang, GJ. Characteristics of the all-vanadium redox flow battery using ammonium metavanadate electrolyte. Korean J. Chem. Eng. 39, 2361–2367 (2022).
[14]. D. D. Banham-Hall, G. A. Taylor, C. A. Smith and M. R. Irving, Flow Batteries for Enhancing Wind Power Integration, in IEEE Transactions on Power Systems, vol. 27, no. 3, pp. 1690-1697, Aug. 2012.
[15]. Ashurov, K.B., Abdurakhmanov, B.M., Iskandarov, S.C. et al. Solving the Problem of Energy Storage for Solar Photovoltaic Plants (Review). Appl. Sol. Energy 55, 119–125 (2019).
[16]. Chuang, SY., Leu, CH., Hsueh, KL. et al. Stability of Vanadium Electrolytes in the Vanadium Redox Flow Battery. MRS Online Proceedings Library, 1492, 138–144 (2012).
[17]. A. Cunha, F.P. Brito, J. Martins, N. Rodrigues, V. Monteiro, J.L. Afonso, P. Ferreira. Assessment of the use of vanadium redox flow batteries for energy storage and fast charging of electric vehicles in gas stations Energy, 115 (2016), pp. 1478-1494, 10.1016/j.energy.2016.02.118









