1. Introduction
The conversion of stored chemical energy into electricity reveals the general working mechanism of any electric battery. The advent of electric batteries can be dated back in 1800, which was invented by Alessandro Volta, an Italian physicist [1]. This over epoch invention has long paved the way for the modern era now with electric cars. The carbon neutral vision is shared among many countries in the near future, and in China, electric car sales increased by 80% in 2022, with demand in battery grew over 70% [2]. Therefore, the all-electric future is in envision. Most electric vehicles (EVs) nowadays use lithium-ion battery inside, and those batteries will slowly encounter battery degradation overtime, resulting in reduced energy capacity, range, power and overall efficiency. The batteries equipped nowadays featuring bulky battery pack and low capacity, which not only cuts the range but also is expensive to manufacture. Thus, improving mechanical strength through battery even under harsh environment and reducing car body weight has become a crucial problem. The question remains how to maximize the EV’s range and performance while maintaining a relatively low cost, which is the main goal among EV players to gain a bigger share in the entire market.
Li-ion batteries tend to have highest energy densities compared to other commercialized batteries that have energy density of 75 Wh/kg [1]. The application of nanotechnology has opened many new opportunities to discover novel materials as they can extend the longevity of batteries. They can be used as coating to act as a barrier to separate the electrodes from the electrolytes in the battery [1]. With the increased surface area, more current can flow between the electrodes; With doped elements, the current cathode materials can possess extra stability for Ni-rich cathodes like NCA and NMC [1].
For anodes that has been relying on graphite power as the intercalation material, the battery capacity and lithium removal or insertion rate can be enhanced by alternating power that’s micrometer-sized with carbon nanotubes (CNT) [2]. CNT can be applied to obtain higher specific power, enlarged cycle life and larger capacity. Carbon nanotubes are also constructive of their large surface area to a perfect battery build-up. Furthermore, achieving further mechanical strength and battery weight, lightweight nanocomposites like Si/C anodes can be applied on structural application of EVs as well [3]. The usage of nanostructured materials helps improving electrodes’ current density not only lies in reducing the Li+ diffusion path but also increasing mobility and electrical conductivity for quicker electrochemical reactions to take place [3].
Electrolyte can be divided into solid and liquid organic solvents, and all solid state batteries (ASSBs) have been attracted for research attentions lately to provide a more stable approach into the manufacturing of the batteries. Yet the current manufacturing process still faces challenges to be able to design superior ASSBs due to instability caused by side interfacial reactions, dendrites formation at the lithium metal anode interface, and deficient cathode interface physical contact.
Therefore, this research aims to study different nanomaterials imposed on different components of the battery, such that the batteries performance can be maximized to provide a lighter weighted and safer battery based on its working mechanism.
2. Application of nanomaterials in batteries
2.1. Cathode
The gravimetric energy density of a modern lithium-ion batteries (LIBs) is at 250 Wh/kg, which is equivalent to a typical EV with a range of 440 km with a 900 kg battery pack [1]. Because of its smaller atomic radius and higher electrode potential against standard hydrogen potential (at -3.04 V) it has, the ultimate goal of the LIBs is to achieve a 500 Wh kg-1 energy density [1]. The operating mechanism of a typical LIB comes from ion-intercalation, where a layered structure is on a stable host material and Li-ion, often referred to the guest material, occupies the vacancy inside that of the host material [1]. Free energy of lithium travels in-between cathode and anode due to their electrochemical potential difference, which then creates a flow of current. The equation below describes the relationship between different factors affecting LIB’s current density, i.
\( i=k(-\frac{d∅}{dx})\ \ \ (1) \)
where k describes the electrical conductivity, ∅ describes the potential, i refers to the current density, and x describes the cell thickness [4].
For any electrochemical battery, the cathode is the electrode that absorbs the electrons, with a positive charge. In contrast to anode material, lower cathode material capacity greatly hinders the development of the battery itself, further highlighting the need to investigate on different commercialized cathode material and how the application of nanotechnology can help boost the battery performance overall.
One layered oxide classes widely applied among EVs that carries most significance are the NMC (Li𝑥Ni𝑦Mn𝑧Co1−𝑦−𝑧O2) that has specific capacity greater than 200mAhg-1 and capacity density greater than 750 Whkg-1 NCA (LiNi𝑥Co𝑦Al1−𝑥−𝑦) cathodes, 811 and 622 are the ratio os transition metals (xyz), as Figure 1 shown below [1].
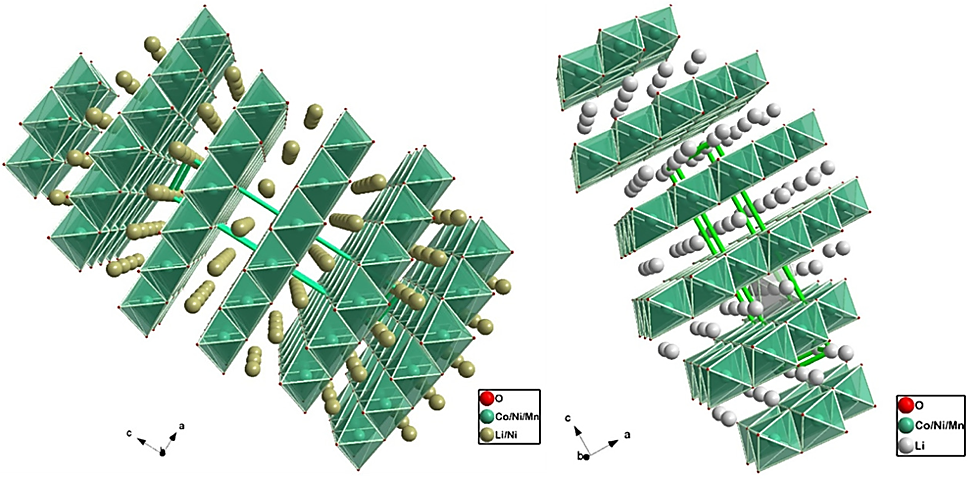
Figure 1. NMC 811 rhombohedral (layered) structure; R-3m space group and NMC 622 rhombohedral (layered) structure; R-3m space group [1].
NCA and NMC are used in cylindrical cells for their high energy density. One of the concerns of using these types of batteries is that over time with absolute cobalt demand on the rise, and the growing production of batteries is presumed to outpace the per-cell basis cobalt reduction overall rate [5]. But reduced cobalt content and increased nickel content in NMC and NCA can improve the range and cut the cost. Nevertheless, the electrical conductivity and structural stability that Co provides ensures electrochemical performance in a long run. Therefore, it poses a dilemma among EV manufacturers like Tesla as removing cobalt can lead to safety issues and reduced longevity of the batteries. The chemical instability of Ni-based cathodes can be mitigated by adding Mn, which has a higher stability than NiO2 for NMC [1]. This reduces the irreversibility caused by the chemical instability of Ni-based cathodes. Nickel is also pivotal for securing high specific capacity [1]. In contrast, NCA cathodes contain Aluminium that is more chemically stable, which reduces the mixing of Li+/Ni2+ to some extent and enhances the electrochemical performance of cathode side [2]. But all these two types lead to potential decay, poor rate performance, and capacity fading.
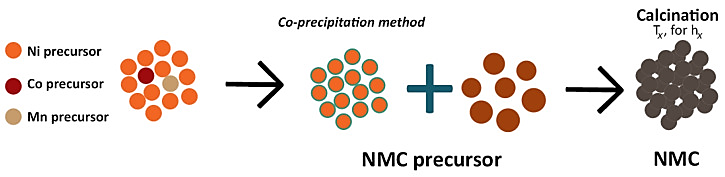
Figure 2. NMC precursor secondary particles are produced during the manufacture of NMC cathodes by coprecipitating transition metal precursors and calcining for hx hours at constant temperature Tx in NMC cathodes synthesis process [1].
Figure 2 illustrates the Ni-rich cathodes’ key degradation mechanisms on both micrometer and atomic scale. Preparation and first cycles have high strain, leading to secondary particles undergo microcracking, and on the other hand, substantial change in volume with high strain states occurrence during delithiation/lithiation [3]. Several NMC cathodes studied by Ryu et. Al., (0.6 ≤ x ≤ 0.95) found that the capacity degradation happened to increase significantly when x>0.8 (x+y+z=1) [3]. This phenomenon-anisotropy--both in discharge expansion and delithiation shrinkage during phase transitions. Lower nickel concentration prevents the phase transition, which leads to enhanced structural stability over time.
Additionally, charge/discharge cycles' chemical instability caused a reduction in the amount of active substances and the formation of the transition metal ions Ni4+, Mn3+/4+, and Co4+ [1]. As a result, layers will ultimately form that resemble spinel-like and eventually a rock-salt phase, which aids in the chemical activity of the electrode and electrolyte [1]. During the charging and discharging cycles, transition metals will fill Li layers after significant change in volume and larger Li layers vacancies by high capacity of Ni oxides. Those filled Li layers would have octahedral sites in transition metals with initial layered transitional oxide to create a rock-salt phase, which eventually inhibit Li ions diffusion process that plays a vital role in battery’s overall performance [1]. Side reaction, on the other hand, can essentially increase the cathode electrolyte interphase (CEI) thickness, thus decreasing ionic conductivity by Ni4+ ions that formed at highly deintercalated states that can erode the cathodes surface, resulting in transforming from active Nickel phases to inactive Nickel Oxides rock-salt phase [6]. According to L. Zhu et al., other reasons short life cycle behind Ni-rich cathodes might come solely from the fabrication process –co-precipitation- that about 1/3 of NMC811 cathode material crack at the micrometer scale [3]. This proves that the manufacturing process is also important for maintaining the structural integrity of NMC cathodes, even though the precise defect rate is highly dependent on the manufacturer [3]. The performance fading of Ni-rich cathodes is also explained by the mixing of Li+/Ni2+ cations. In the course of the delithiation/lithiation, Ni2+ ions travel to Li layers. Compared to NCA cathodes, bare NMC cathodes with a high Ni content degrade more quickly. And major degradation mechanisms taken into account among NMC and NCA are as follows in Table 1.
Table 1. Comparison of degradation mechanisms for bare NMC and NCA cathodes [1].
Name | NMC | NCA |
Electrolyte oxidation | Higher, Increased rate of Mn dissolution; More susceptible for irreversible layered structure of Mn cations for dissolution and disproportionation | Lower, Al dissociation |
Particle pulverization/separation of primary particles | Less severe, higher efficacy of Mn for volume changes during cycling | More severe, Al is less effective at mitigating volume from the introduction of anisotropic lattice changes |
Therefore, with an urge to mitigate capacity fading and low ionic conductivity of Ni-rich cathodes, inner surface doping and surface coating have proved to be more plausible solutions proposed of all.
Ni-rich cathodes can be replaced partially by doping elements, which result in decreased Ni2+/Li+ disorder, providing superior resistance to microcracks initiation and structural stability [1]. More specifically, Nb5+ has been applied for improving capacity retention, and Ti4+ can improve electrochemical properties at low temperature [1].
It is also proved that 95% of Ni content in NCA cathodes were doped with W that can deliver a starting 242 mAh·g-1 discharge capacity at a rate of 0.1 C [1]. After 1000 cycles, the level maintained at 77.4% of the original capacity, compared to the controlled group (non-doped) of 14.5% of its original capacity [1]. The lattice structure of LiNi0.80Mn0.05Co0.15O2 has also been found to have a lower level of anisotropy during delithiation/lithiation in expansion and shrinkage as a result of Al-doping in 1.2 M LiPF6 electrolyte dissolved in EC/DMC (1:1) solvent, as studied by Jeong et al. [1]. After 20 cycles, there’s a distinct difference of discharge capacity compared to that of non-doped electrode; Furthermore, the Al-doped NMC changed from its layered structure to a spinel-like structure, whereas the non-doped NMC underwent additional metamorphosis into the rock-salt phase [1]. However, according to Xie, Li, and Zhou et al., higher Al content can result in decreased initial discharge capacity and Initial Coulombic Efficiency (ICE), and the formation of LiAlO2 and Li5AlO4 cause lowered account of active Li+ inside the cathode [1]. This is indicative of a maximum dopant value at 5.6% of Al content that might obstruct specific capacity (157 mAh·g-1) of cathodes otherwise as Al is electrically inactive. Therefore, the particle size and density play a role on electrochemical performance, as energy and theoretical specific capacity can only be enhanced to a certain level through decreased level of porosity for any Ni-rich cathodes. Therefore, an optimal porosity level at 40% is the most desired for obtaining maximum capacity retention. Referring back to figure 2, NMC cathodes synthesis, controlling the primary and secondary particle size aids in the final obtained porosity level, so the low particle size is crucial for a decent rate performance for a highly porous electrode with a high Nickel content through electrolyte-electrode contact [1].
Surface coating is defined by the deposition of a nanometer coating film on a cathode surface or through creation of composite layered materials. Al-based coatings is particularly remarkable at increasing structural stability, and carbon coatings can improve ionic, electron conductivity and structural stability to a great extent as well. Chen et al. studied on a synthesized carbon-coated NMC811 cathodes with PVDF applied at 0.2 C as a binder with different wt% [1]. The result, having initial discharge capacity at around 190–195 mAh·g−1 and Coulombic efficiency with no impact by carbon content, shows that the capacity decay increased from 6.85% to 1.26% (2.5% PVDF) after 1000 cycles [1]. Any effective coating layer should strike a balance between chemical stability and conductivity; these layers may help the coated cathodes' conductivity. However, to prevent direct electrolyte contact, a minimum thickness is required. For the nano LiFePO4 coated LiNi0.82Co0.12Mn0.06O2 (nano LiFePO4 coated) composite prepared through mechanical fusion by Zhong et al, the result shows that a higher specific capacity is achieved where material systems are electrochemically active [1].
Nano-Al2O3 is another type of coating for less contact between the electrolyte and electrode for preventing generation of Ni oxide type rock-salt phase and prevent the transition metal ion dissolution, further enhancing the electrochemical performance of NCA. AlF3 and LiPO2F2, an electrolyte additive that helps developing uniform SEI film for improvements on the cycle life stability, are formed from Al2O3 reacting with LiPF6 [1]. The issue associated with Al2O3, however, is that its poor Li+ diffusion ability. With LiAlO2 (α-LiAlO2 ), the diffusion problem will be alleviated [1]. This cathode metal oxide coating is in good lattice match with NCA as it belongs structurally to R-3m group. In order to reduce mechanical shedding, our improved high-nickel materials coating method strengthens the bonding force in between the coating and the host material. It also improves stability of the electrode/electrolyte interface, and ensures high-temperature cycle stability. Therefore, it evoked the application of NCA in severe working environments. Overall, surface coating protects the cathode surface from exposure to the electrolyte, slowing down the kinetics of side reactions and phase transformation inversibility (electrolyte/ Ni4+) [1].
2.2. Anode: Graphite vs. Nano-Si/Carbon Composite Anodes
Graphite is usually regarded as the most commonly used for commercialized batteries. The graphite anode has low chemical potential at about 0.1 eV, good electrochemical stability, widely available worldwide and safer than lithium when overheated [7]. As anode degradation is attributive to full cell degradation mostly, the current studies remain in improving anode at its component level. Since particle sizes has a negative effect on delithiation/lithiation, 20 nm particle size was found as the most ideal [7]. Single walled carbon nanotubes (SWCNT) technology is able to reduce charging and discharging time by at least 30 times [7]. Carbon nanotubes and graphene remain a competent alternative, but their limited use is concerned for cost arising during manufacturing. Silicon remains another subject of interest in replacement of graphite for their abundancy on Earth. It has high specific capacity for a theoretical capacity ~4200 mAh·g-1 for Li22Si5 and 3579 mAh·g−1 for Li15Si4 phases and working potential, ~0.4 V superior to commercialized graphite, thus proving it a safe option during charge/discharge cycles [7]. Si-composite materials like SiNW, and the main challenge for industrial development however, is its degradation mechanism and/or high manufacturing cost [8]. Nevertheless, they can increase ionic conductivity and have porous structures for huge volume variations.
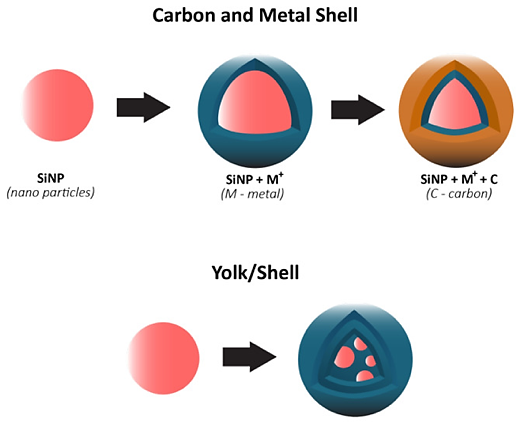
Figure 3. Yolk/Shell design techniques and carbon shell design for composite Si anodes [1].
Another possible choice is the composite material structure, which is made up of Si-based shell-core/shell or yolk/shell designs [9]. Via void-filling, improved capacity retention, and SEI stability, this design enables growing volume. As seen in Figure 3, the bottom depicts a void area between two substances, which allows for higher volume expansions but adversely affects ionic and electrical conductivity [9]. The top depicts a dual layer design that interacts between the outer shell and the inner core. Other metallic elements may also be used into Si/C composite anodes for potential future commercialization [10].
2.3. Electrolyte (ASSBs vs. liquid)
The electrolyte also carries significance on the speed lithium ions travels, and faster movement among lithium-ion contributes to higher battery output and shorter battery charging times. The conventional lithium-ion batteries use a liquid organic solvent as its electrolyte which suffers high chemical deterioration of the electrolyte under high temperatures, and the flammability of organic solvents can evoke safety issues like ignition. All ASSBs, on the other hand, is not volatile or flammable, thus referred to as a safer option having fast-charging performance and having high operating temperature limits; more combination of the material can be proposed for their solid nature [10]. Solid state electrolytes (SSEs) can create a battier to lithium dendrites, allow use of metal lithium anode. Ideally, the redox reaction of Lithium ion occurs only at the anode, and the plating and stripping are presumably be homogeneous in ASSBs, shortening the charging time and resulting in lower costs [10]. Due to side interfacial reactions' instability, poor physical contact at the cathode interface, low ionic conductivity, and dendritic development at lithium metal anode interface, it is still difficult to manufacture such good ASSBs [10]. There are dendrites forming at the lithium metal anode interface, and the cathode interface has poor physical contact and low ionic conductivity. Specific materials must be carefully chosen to meet the above conditions; cell design also needs to have surface pressures catered to have conditions for uniform mixing in the manufacturing process.
For interfacial reactions, the stability of the electrolyte is determined by the chemical potential of the lowest unoccupied molecular orbital (LUMO) and the highest occupied molecular orbital (HOMO) [10]. The most ideal situation is that the chemical potential lies between the LUMO and HOMO [10]. If Li anode chemical potential exceeds that of the LUMO and that of the cathode is likewise less than for the HOMO, another interlayer will otherwise emerge at the cathode and anode contact at this thermodynamically stable interface [10]. For the battery's performance over time, the development of kinetically and thermodynamically stable interfaces may be beneficial. In reality, the majority of solid electrolytes are unstable thermodynamically, and the anode often has a mixed-conductive and kinetically stable interface.
For ionic conductivity: another problem relates to low ionic conductivity. More energy barriers are needed to overcome by the mobile ion’s diffusion through nonuniform environment, including electrostatic forces, interactions with the inherent lattice, motional energy, and that influence the ionic conductivity by a great extent [10]. Coordination polyhedral and spatial arrangements in its crystalline solid materials enables lots of vacancies of transportation of ions. Due to the limited electrochemical potential window of the solid electrolyte and the inevitable interface chemical reactivity, the interface between the solid electrolyte and the cathode is thermodynamically unstable [10]. A reduction in ionic conductivity will incur when the voltage is high, of which the electrolyte will decompose. Therefore, for lithium-ion batteries specifically, the formation of space charge layer (SCL) is necessary into careful design of ASSBs, as they hinder the lithium-ion transportation at the surface when there’s electrochemical potential difference of the solid-state electrolyte and cathode [10].
3. Conclusion
After analyzing with the major trends of cathode, anode, and electrolyte materials in the Li-ion batteries, Si/C composite material can be applied to replace traditional graphite material with superior electrochemical stability to mitigate cell degradation; doping and surface coating, additionally, can be applied to alleviate capacity fading and low ionic conductivity of Ni-rich cathodes. However, as the rising concerns of sourcing cobalt and nickel for batteries for NMC and NCA cells, lithium-ion phosphate (LFP) is used for Tesla across all its standard models as its less energy-dense, less expensive, even though they are not perfect for long range vehicles. This pricing flexibility and sourcing availability has made EVs players like Tesla to invest in more of it possibly in the future. In the end, choosing whether to employ LFP batteries in EVs is difficult and depends on a number of factors, including battery cost, required range, and accessibility of charging infrastructure. Additionally, more research interest has been shifting towards investigating ASSBs. This type of batteries will open up a new whole era apart from conventional lithium-ion batteries as it uses solid electrolyte instead of liquid that has lower temperature limits.
The current obstacle in research still remains reaching the theoretical limits energy density via maximized compaction with nanomaterials or having adequate porosity for reaching high ionic conduction for Ni-rich cathode development discussed in this paper, which will pave the way for future research for refining the current understanding of solid-state electrochemistry. The afterlife in terms of recycling of batteries might need more attention as well. With the practical usage of nanomaterials on EVs, the cycle doesn’t end without cycling EV batteries as the toxic chemicals inside can lead to contamination of water and soil. The repurposing of used batteries still remains as a lingering question to solve as well, as most commercialized EV batteries will be left with up to 70% of capacity left for other energy storage needs, though LFP could be a viable option, with much less profitability generated compare to that of NMC or NCA with nickel and cobalt content so that less motivation for companies to pursue. The future development of battery components into commercialization has to take sustainability into consideration for future endeavours.
References
[1]. Salgado RM, Danzi F, Oliveira JE, El-Azab A, Camanho PP, Braga MH. The Latest Trends in Electric Vehicles Batteries. 2021 Molecules 26(11) 3188
[2]. Bubulinca C, Kazantseva NE, Pechancova V, Joseph N, Fei H, Venher M, et al. Development of All-Solid-State Li-Ion Batteries: From Key Technical Areas to Commercial Use. 2023 Batteries 9(3) 157
[3]. Zhao G, Wang X, Negnevitsky M. Connecting Battery Technologies for Electric Vehicles from Battery Materials to Management. 2022 IScience 25(2) 103744
[4]. Liu HY, Lin MF, Wu JY. Essential Electronic Properties of Silicon Nanotubes. 2021 Nanomaterials 11(10) 2475
[5]. Wang F, Deng Y, Yuan C. Comparative Life Cycle Assessment of Silicon Nanowire and Silicon Nanotube Based Lithium-Ion Batteries for Electric Vehicles. 2019 Procedia CIRP 80 310–5
[6]. Bi CX, Zhao M, Hou LP, Chen Z, Zhang XQ, Yuan H, et al. Anode Material Options toward 500 Wh kg−1Lithium–Sulfur Batteries. 2021 Advanced Science 16 9(2) 2103910–0
[7]. Sarkar S, Halim Z, El-Halwagi MM, Khan F. Electrochemical models: Methods and Applications for Safer lithium-ion Battery Operation. 2022 Journal of the Electrochemical Society. 169(10) 100501–1
[8]. Shan W, Huang S, Zhang H, Hou X. Surface Coating for high-nickel Cathode Materials to Achieve Excellent Cycle Performance at Elevated Temperatures. 2021 Journal of Alloys and Compounds. 862 158022
[9]. Gat S, Karnataka I. Nanotechnology in Development of Batteries for Electric Vehicles. 2021 IJARCCE International Journal of Advanced Research in Computer and Communication Engineering 10(10)
[10]. Sivaramkrishnan M, Agenya R, Santhi Mary Antony A, Baskar R, Prakash RBR, Ramya D, et al. A Certain Investigation of Nanomaterial-Based Li-Ion Batteries for Electrical Vehicles. 2022 Journal of Nanomaterials e2700050
Cite this article
Zhao,S. (2024). Application of nanotechnology-based battery performance improvement in the field of electric vehicles. Applied and Computational Engineering,59,14-21.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Salgado RM, Danzi F, Oliveira JE, El-Azab A, Camanho PP, Braga MH. The Latest Trends in Electric Vehicles Batteries. 2021 Molecules 26(11) 3188
[2]. Bubulinca C, Kazantseva NE, Pechancova V, Joseph N, Fei H, Venher M, et al. Development of All-Solid-State Li-Ion Batteries: From Key Technical Areas to Commercial Use. 2023 Batteries 9(3) 157
[3]. Zhao G, Wang X, Negnevitsky M. Connecting Battery Technologies for Electric Vehicles from Battery Materials to Management. 2022 IScience 25(2) 103744
[4]. Liu HY, Lin MF, Wu JY. Essential Electronic Properties of Silicon Nanotubes. 2021 Nanomaterials 11(10) 2475
[5]. Wang F, Deng Y, Yuan C. Comparative Life Cycle Assessment of Silicon Nanowire and Silicon Nanotube Based Lithium-Ion Batteries for Electric Vehicles. 2019 Procedia CIRP 80 310–5
[6]. Bi CX, Zhao M, Hou LP, Chen Z, Zhang XQ, Yuan H, et al. Anode Material Options toward 500 Wh kg−1Lithium–Sulfur Batteries. 2021 Advanced Science 16 9(2) 2103910–0
[7]. Sarkar S, Halim Z, El-Halwagi MM, Khan F. Electrochemical models: Methods and Applications for Safer lithium-ion Battery Operation. 2022 Journal of the Electrochemical Society. 169(10) 100501–1
[8]. Shan W, Huang S, Zhang H, Hou X. Surface Coating for high-nickel Cathode Materials to Achieve Excellent Cycle Performance at Elevated Temperatures. 2021 Journal of Alloys and Compounds. 862 158022
[9]. Gat S, Karnataka I. Nanotechnology in Development of Batteries for Electric Vehicles. 2021 IJARCCE International Journal of Advanced Research in Computer and Communication Engineering 10(10)
[10]. Sivaramkrishnan M, Agenya R, Santhi Mary Antony A, Baskar R, Prakash RBR, Ramya D, et al. A Certain Investigation of Nanomaterial-Based Li-Ion Batteries for Electrical Vehicles. 2022 Journal of Nanomaterials e2700050









