1. Introduction
One of the most important bulk commodity chemicals, acetic acid is now produced via methanol carbonylation reactions using homogenous organometallic complexes of rhodium or iridium and halide-containing promoters called BP CativaTM homogeneous processes or Monsanto homogeneous processes, respectively [1]. Although these classic methods have high efficiency which was demonstrated industrially, it is still difficult to separate production and cause a high loss of noble metal catalysts in homogeneous ways. The benefits of heterogeneous catalysts, which are derived from research on homogeneous catalyst systems, include easy product separation and vapor phase operation, which can reduce catalytic losses [2].
The paper reviews research about traditional methanol carbonylation methods including anionic rhodium and iridium carbonylation recent research in more efficient methods for methanol carbonylation. The paper makes a detailed introduction of methanol carbonylation for producing acetic acid, looks to the future, and comes up with new ideas and new initiatives of heterogeneous catalysis methanol carbonylation system for jointly promoting acetic acid production.
2. Acetic acid
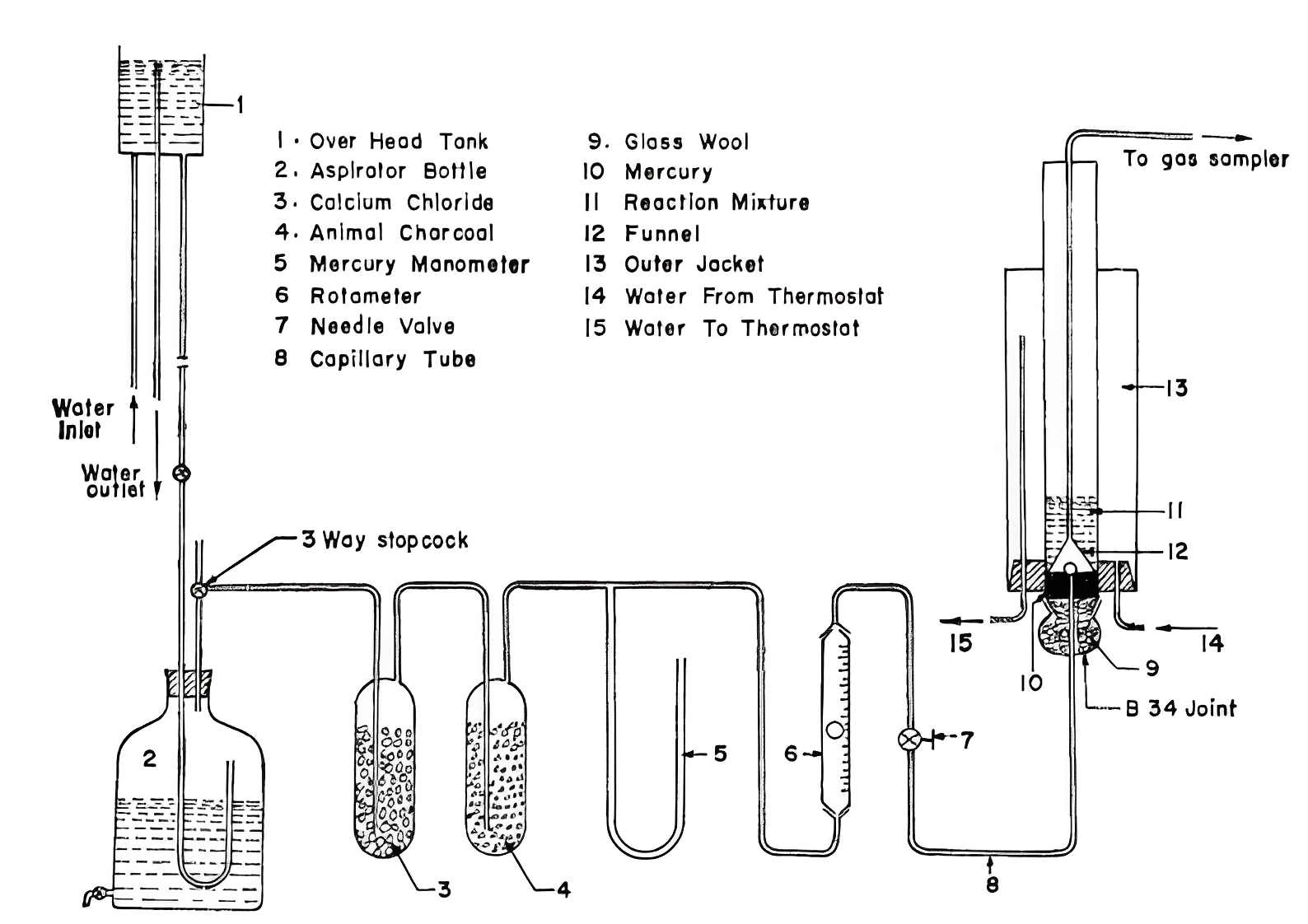
Figure 1. Experimental setup for studies of oxidation of acetaldehyde to acetic acid [3].
Due to its transformation into vinyl acetate monomer, ethyl, propyl, n-butyl, and isobutyl acetate, acetic anhydride, and as a solvent in the manufacture of terephthalic acid, acetic acid finds extensive application in the chemical industry. At first in the early 1970s, people chose to produce acetic acid by the direct oxidation of acetaldehyde. Figure 1 shows the experimental setup for research on the oxidation of acetaldehyde to acetic acid. Researchers found that the oxidation of acetaldehyde to acetic acid in a sparger reactor using manganese acetate as the catalyst has a high reaction rate and can be neglected for the rate equation of a sparger reactor [3].
Because of methanol’s abundant production per year and its low cost, methanol carbonylation replaced acetaldehyde oxidation and became the capital pathway in acetic acid production. Today, methanol carbonylation produces 85% of the acetic acid. Since 2015, its production has increased dramatically, from 13 million tons to 18 million tons in 2020, or an annual growth of almost 5% [4].
The process of methanol carbonylation (Eq. (1)) to acetic acid shows a high selectivity and conservation rate.
\( {CH_{3}}OH+CO→CHCOOH\ \ \ (1) \)
3. Main ways of methanol carbonylation to acetic acid
3.1. Anionic rhodium carbonylation
At first, during the 1910s, scientists found that acidic corrosive could be incorporated from methanol and carbon monoxide under unforgiving states of tensions surpassing 100 bar and temperatures surpassing 300 ℃ [5-7]. Combinations of CO and methanol work under the activity of different metal impetuses. It is worth focusing on that BASF and later Celanese UK professed to utilize fundamentally low-dynamic iron, as well as cobalt and nickel carbonyls for high strain and high-temperature carbonylation responses within the sight of iodine or iodized salts [8].
In the 1970s, Monsanto Corporation invented a rhodium complex as the catalyst in methanol carbonylation. Researchers designed a novel catalyst system that consists of rhodium complexes and iodide. The reaction (Eq. (1)) can set off in a very mild condition. CO and methanol can operate at pressures of 3-6 MPa and temperatures of 150-200 ℃. Especially compared to the previous method catalyzed with nickel complexes which need 900℃ and high pressure. And it has excellent selectivity and conservation rate [9-11].
The catalyst cycle example has been shown in Figure 2. 1a reacts with methiodide as an oxidative addition from 2a. 2a molecule goes through a migratory insertion of CO and the reductive elimination of 4a formed 1a and iodoacetaldehyde which will react with H2O to produce acetic acid. The rate-determined step is the migratory insertion of 2a [12].
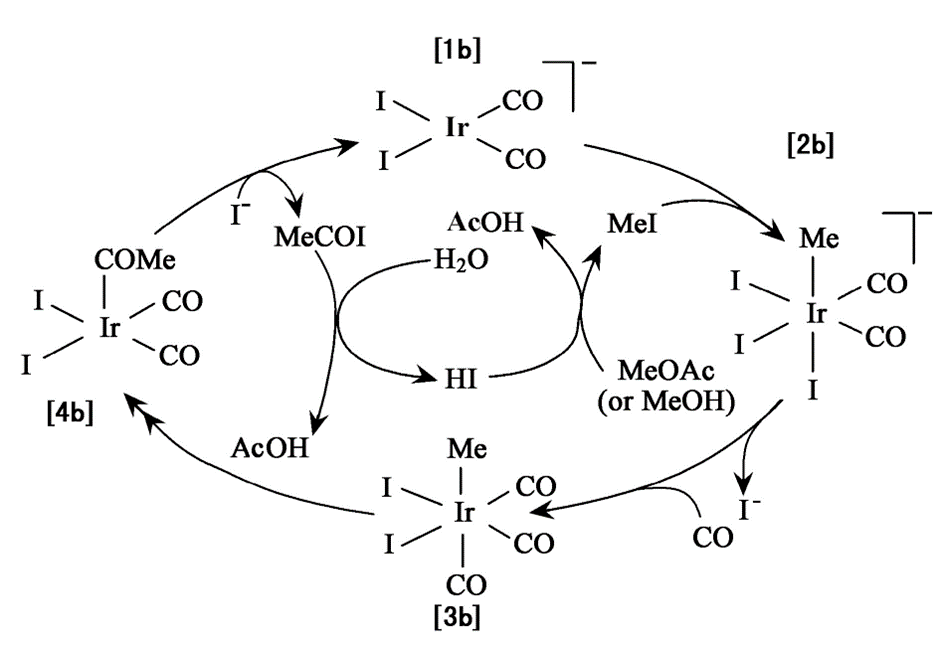
Figure 2. Anionic rhodium carbonylation catalyst cycle [13].
Though Monsanto’s process does not require demanding conditions and has high product purity, it requires water(14-15 wt%) which makes it difficult to separate acetic acid from the reaction system [12].
A heterogeneous catalyst system could be a better pathway for methanol carbonylation to acetic acid.
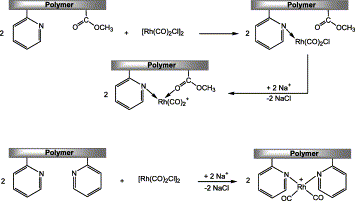
Chiyoda and Universal Oil Products released research about the novel supporting system for rhodium complex in methanol carbonylation. Figure 3 demonstrates the supporting system derived from vinyl pyridine and vinyl acetate. This new supporting system not only keeps the high activity of rhodium catalyst but also has no significant rhodium loss which cuts down the cost. Moreover, the supporting system makes the reaction require mild conditions as well but needs low water concentration [14-16].
Figure 3. Supporting the rhodium catalyst on copolymer derived from vinyl pyridine and vinyl acetate [12].
3.2. Anionic iridium carbonylation
The iridium catalyst cycle is shown in Figure 4. The catalytic cycle mainly consists of three basic steps: oxidative addition, migratory insertion, and reductive elimination. It is similar to the rhodium catalyst cycle. In the 20th century, compared to rhodium, iridium catalysts had an evident price advantage.
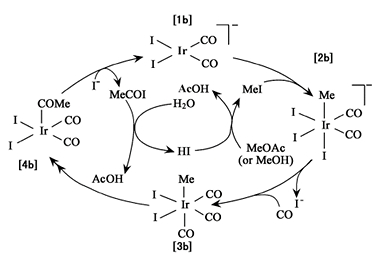
Figure 4. Anionic iridium catalyst cycle [13].
Based on the Monsanto process, BP invented the CativaTM process using iridium complex [Ir(CO)2 I4] – as a catalyst. CativaTM successfully cut down the water concentration during the reaction, the catalyst can operate at reduced water levels (<8 wt.%). Consequently, there is a reduction in by-product production, an increase in carbon monoxide efficiency, and a decrease in steam usage [17].
Single-site heterogeneous catalysts are a perfect substitute for their homogeneous counterparts. Researchers Feng et al. from Dalian National Laboratory for Clean Energy looked into the effectiveness and stability of vapor methanol carbonylation using heterogeneous single-site catalysts (HSSCs). To improve the interaction between noble metal ions or atoms with supporting materials and take advantage of HSSCs, they designed the system constituted by an Ir–La binuclear complex on activated carbon (Ir1-La1/AC). In Figure 5, the potential mechanism is displayed. The bonding order of LaO-AC in La1/AC and Ir-O-AC in Ir1/AC are depicted in Figures 5 (a) and (b); Figure 5(c) illustrates the bonding order of Ir–La in the binuclear complex; The Ir1/AC catalyst in Figure 5(d) and the Ir1-La1/AC catalyst in Figure 5(e) simplified reaction process models along with the related energy barriers (kJ/mol) for heterogeneous methanol carbonylation. The steps ①, ②, and ③ are the oxidative addition of the starting species with CH3I, the migratory insertion of CO, and the reductive removal of acetyl iodide. It has been demonstrated that these new HSSCs can reduce the energy barrier of the rate-determining phase, which in turn reduces the amount of carbon that deposits on the catalyst [18].
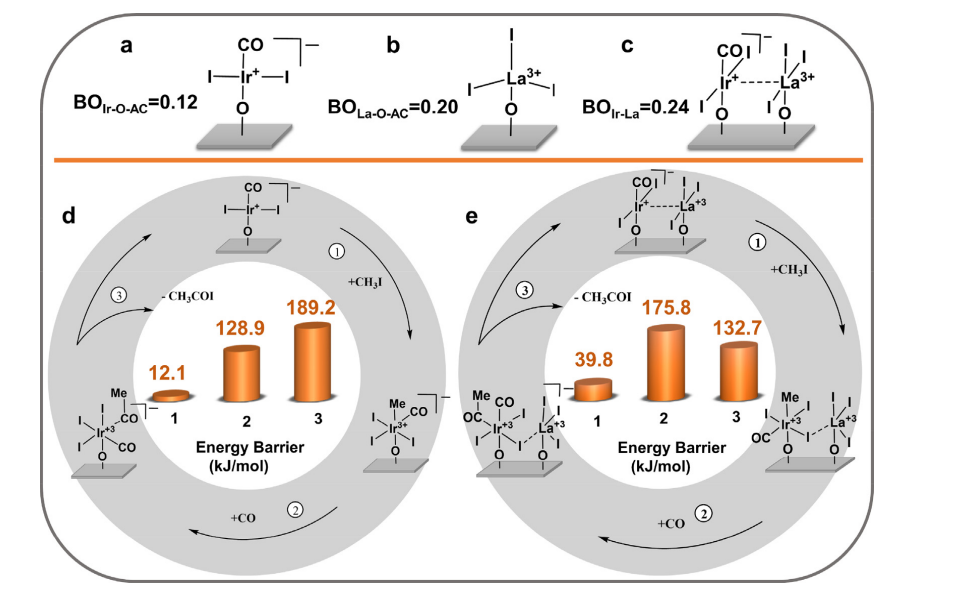
Figure 5. The mechanisms for methanol heterogeneous carbonylation on Ir1/AC and Ir1-La1/AC [18].
4. Discussion
Thus far, iridium or rhodium catalyst-based CativaTM or Monsanto procedures account for more than 80% of the acetic acid’s capacity [19].
But both these classic Rhodium or Iridium homogeneous catalyst systems still cannot solve problems like complicated formation separation, the corrosion of instruments from iodide, and the loss of noble metal catalysts. Several research-improved catalysts have emerged, especially the addition of ligands. At the same time, a heterogeneous catalyst system could be developed as the solution and supplement of homogeneous catalysts in acetic acid production. Researchers reported techniques supporting the metal catalysts by copolymer and binuclear complex to guarantee the stability of the heterogeneous system and enhance the interaction of noble metal atoms or ions with support materials. A synthetical heterogeneous system composed of multiple materials may be the mainstream catalyst system in the future.
5. Conclusion
The history of the carbonylation of methanol to produce acetic acid is reviewed in the report. Mainly introduces the process of methanol carbonylation with homogeneous catalyst and heterogenous catalysts and by literature review of previous research. The report shows the advantages and disadvantages of both two ways and develops the idea that the research of heterogenous catalysis systems to produce acetic acid should be encouraged in the future.
One limitation is the report lacks experimental receipts as a strong argument and the research will continue in the future setting synthesis of a more efficient, clean heterogeneous catalyst system with lower cost to produce acetic acid as an object.
References
[1]. Ni, Y., Shi, L., Liu, H., Zhang, W., Liu, Y., Zhu, W., & Liu, Z. (2017). A green route for methanol carbonylation. Catalysis Science & Technology, 7(20), 4818-4822.
[2]. Kalck, P., Le Berre, C., & Serp, P. (2020). Recent advances in the methanol carbonylation reaction into acetic acid. Coordination Chemistry Reviews, 402, 213078.
[3]. Oxidation of Acetaldehyde to Acetic Acid in a Sparger Reactor. Balakrishnan Venugopal, Rojinder Kumar, and N. R. Kuloor. Industrial & Engineering Chemistry Process Design and Development 1967 6 (1), 139-146.
[4]. Shah, K., Acetic acid: overview and market outlook. Indian Petrochem Conference. 2014.
[5]. BASF, Verfahren zur Darstellung von Kohlenwasserstoffen und deren Derivaten. DE 293787 Patent 1913 (8 March), granted on 23 Aug 1916.
[6]. A. Mittasch, C. Schneider, Producing compounds containing carbon and hydrogen. US Patent 1,201,850 to BASF (19 May 1970) 1914 (Feb. 11), granted on 17 October 1916. F. Fischer, Liquids fuels from water gas, Ind. Eng. Chem. 17 (1925) 574–576.
[7]. N. von Kutepow, W. Himmele, H. Hohenschutz, Die Synthese von Essigsäureaus Methanol und Kohlenoxyd, Chem. Ing. Technol. 37 (1965) 383–388.
[8]. A. Mullen, Carbonylations catalyzed by metal carbonyls – Reppe reactions, in J. Falbe (Ed.), New Syntheses with Carbon Monoxide, Springer Verlag, 1980, pp. 243–308.
[9]. F.E. Paulik, A. Hershman, G.R. Knox, J.F. Roth, Monocarboxylic acids by carbonylation of alcohols with new catalysts. DE Patent 1941449 to Monsanto (April 5, 1969) was granted on June 1, 1972.
[10]. F.E. Paulik, J.F. Roth, Novel catalysts for the low-pressure carbonylation of methanol to acetic acid, Chem. Commun. 1578 (1968).
[11]. F.E. Paulik, A. Hershman, J.F. Roth, J.H. Craddock, D. Forster, Carboxylic acids and esters from alcohols. GB Patent 1234641 to Monsanto (April 5, 1967) was granted on June 9, 1971.
[12]. Ligand effects in the rhodium-catalyzed carbonylation of methanol, Coordination Chemistry Reviews, Volume 243, Issues 1–2,2003, Pages 125-142,
[13]. Wang Yuhe, He Dehua, Xu Baiqing. Methanol carbonylation to acetic acid. Advances in Chemistry, 2003(03):215-221.N. Yoneda, Y. Shiroto, K. Hamato, S. Asaoka, T. Maejima, US Patent to Chiyoda (1994) 5334755.
[14]. T. Minami, K. Shimokawa, K. Hamato, Y. Shiroto, N. Yoneda, US Patent to Chiyoda (1994) 5364963.
[15]. T. Minami, K. Hamato, K. Shimokawa, Y. Shiroto, US Patent to Chiyoda (1996) 5576458.
[16]. The Cativa™ process for the manufacture of acetic acid. Platinum Metals Review, 2000, 44(3): 94-105.
[17]. Insight into the stability of binuclear Ir–La catalysts for efficient heterogeneous methanol carbonylation, Journal of Catalysis, Volume 377,2019, Pages 400-408.
[18]. Chemical Process Economics Program Technical and Economic Analyses of Acetic Acid, IHS (2016).
Cite this article
Yan,Q. (2024). A review of process of methanol carbonylation to produce acetic acid. Applied and Computational Engineering,61,216-221.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Ni, Y., Shi, L., Liu, H., Zhang, W., Liu, Y., Zhu, W., & Liu, Z. (2017). A green route for methanol carbonylation. Catalysis Science & Technology, 7(20), 4818-4822.
[2]. Kalck, P., Le Berre, C., & Serp, P. (2020). Recent advances in the methanol carbonylation reaction into acetic acid. Coordination Chemistry Reviews, 402, 213078.
[3]. Oxidation of Acetaldehyde to Acetic Acid in a Sparger Reactor. Balakrishnan Venugopal, Rojinder Kumar, and N. R. Kuloor. Industrial & Engineering Chemistry Process Design and Development 1967 6 (1), 139-146.
[4]. Shah, K., Acetic acid: overview and market outlook. Indian Petrochem Conference. 2014.
[5]. BASF, Verfahren zur Darstellung von Kohlenwasserstoffen und deren Derivaten. DE 293787 Patent 1913 (8 March), granted on 23 Aug 1916.
[6]. A. Mittasch, C. Schneider, Producing compounds containing carbon and hydrogen. US Patent 1,201,850 to BASF (19 May 1970) 1914 (Feb. 11), granted on 17 October 1916. F. Fischer, Liquids fuels from water gas, Ind. Eng. Chem. 17 (1925) 574–576.
[7]. N. von Kutepow, W. Himmele, H. Hohenschutz, Die Synthese von Essigsäureaus Methanol und Kohlenoxyd, Chem. Ing. Technol. 37 (1965) 383–388.
[8]. A. Mullen, Carbonylations catalyzed by metal carbonyls – Reppe reactions, in J. Falbe (Ed.), New Syntheses with Carbon Monoxide, Springer Verlag, 1980, pp. 243–308.
[9]. F.E. Paulik, A. Hershman, G.R. Knox, J.F. Roth, Monocarboxylic acids by carbonylation of alcohols with new catalysts. DE Patent 1941449 to Monsanto (April 5, 1969) was granted on June 1, 1972.
[10]. F.E. Paulik, J.F. Roth, Novel catalysts for the low-pressure carbonylation of methanol to acetic acid, Chem. Commun. 1578 (1968).
[11]. F.E. Paulik, A. Hershman, J.F. Roth, J.H. Craddock, D. Forster, Carboxylic acids and esters from alcohols. GB Patent 1234641 to Monsanto (April 5, 1967) was granted on June 9, 1971.
[12]. Ligand effects in the rhodium-catalyzed carbonylation of methanol, Coordination Chemistry Reviews, Volume 243, Issues 1–2,2003, Pages 125-142,
[13]. Wang Yuhe, He Dehua, Xu Baiqing. Methanol carbonylation to acetic acid. Advances in Chemistry, 2003(03):215-221.N. Yoneda, Y. Shiroto, K. Hamato, S. Asaoka, T. Maejima, US Patent to Chiyoda (1994) 5334755.
[14]. T. Minami, K. Shimokawa, K. Hamato, Y. Shiroto, N. Yoneda, US Patent to Chiyoda (1994) 5364963.
[15]. T. Minami, K. Hamato, K. Shimokawa, Y. Shiroto, US Patent to Chiyoda (1996) 5576458.
[16]. The Cativa™ process for the manufacture of acetic acid. Platinum Metals Review, 2000, 44(3): 94-105.
[17]. Insight into the stability of binuclear Ir–La catalysts for efficient heterogeneous methanol carbonylation, Journal of Catalysis, Volume 377,2019, Pages 400-408.
[18]. Chemical Process Economics Program Technical and Economic Analyses of Acetic Acid, IHS (2016).