1. Introduction
With the rapid industrial development, the energy crisis and environmental pollution problems are becoming more and more serious. This requires us to develop low-cost, high-energy-density batteries. In this situation, metal air batteries attract much attention because of their low price, safety and reliability, high energy and capacity density, and green environmental protection [1]. As the highest metal element in the earth’s crust, aluminum has high hopes in the field of air batteries [2]. In terms of aluminum-air battery, its application can be traced back to 1960, Zaromb first proposed the application of aluminum metal anode and proved the feasibility of its technology [3]; in 1962, Holzer F studied metal aluminum-air battery in his experiments [4]. Later, after continuous development; in 2016, an aluminum-air battery weighing 100 kg was introduced, increasing the range of electric cars to more than 3,000 kilometers [5]. The aluminum-air battery has a simple structure, a wide range of raw materials, can be changed according to different use environments and requirements, and has strong adaptability. It can be used as both a power battery and a signal battery with long life and high specific energy. Another advantage of aluminum-air battery is that aluminum can be recycled. Aluminum anode is used as fuel to generate aluminum hydroxide, which can be economically recovered and regenerates into metallurgical grade alumina, and returns to the aluminum electrolytic cell to obtain aluminum fuel again. The whole fuel cycle process of aluminum-air battery is green, pollution-free and zero-emission of greenhouse gases. The chemical reaction of aluminum-air battery is similar to that of zinc-air battery. Aluminum-air battery takes high purity Al (including aluminum 99.99%) as the anode, oxygen as the cathode, and takes potassium hydroxide (KOH) or sodium hydroxide (NaOH) aqueous solution as the electrolyte. Aluminum takes oxygen in the air and produces a chemical reaction when the battery discharges, aluminum and oxygen react to form alumina. Oxygen reduction reaction is particularly important in the background of today’s new energy development, but oxygen reduction reaction is a slow reaction. Due to the limited process, the slow kinetic reaction rate leads to low power density, which seriously affects its application in practical application. At this time, appropriate catalysts are needed to accelerate the reaction. It is found that nano carbon materials have outstanding structural and electrical properties, and nano carbon materials doped with transition metal Fe and heteroatoms N are comparable to Pt-based catalyst in oxygen reduction catalytic activity and stability, which is considered as the most potential substitute for precious metal catalyst. However, due to the small reaction area of the single cathode, also can lead to a slow reaction rate. Therefore, this paper aims at these technical problems to carry out technical research. Experimental studies have found that the use of double-cathode structure can increase the reaction area and accelerate the chemical reaction rate, so as to improve the electricity generation performance of the battery.
2. Preparation of cathode
The preparation of catalyst is divided into two steps. The first step is to synthesize hollow nano carbon materials by non-template method, and the second step is the doping of Fe and N elements. The above synthesized nano carbon catalyst is mixed with the membrane solution and anhydrous ethanol to form a slurry, and then the slurry is evenly sprayed on the other side of the carbon cloth with a waterproof layer. so that the catalyst load is 1 mg cm-2, and then fully dry to get the desired cathode. In this study, the structure of aluminum-air battery with two non-precious metal cathodes was designed. The cost of the nano carbon catalyst used in this study is 1/22 of the precious metal Pt, as shown in Figure 1, which greatly improves the electricity generation performance of the battery under the premise of low cost.
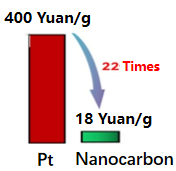
Figure 1. Cost comparison of nano carbon used in this study versus precious metal Pt
3. Battery structure design
The cathode part of conventional aluminum-air battery usually uses precious metal catalyst, which is expensive, so the single-sided cathode design is adopted. The cathode reaction area is small, resulting in unsatisfactory battery performance. Due to the synthesis of non-precious metal cathode catalysts in this study, the preparation process is simple, the materials are easy to obtain and the price is low. Therefore, this study innovatively proposed to use bilateral-cathode structure to increase the cathode reaction area to enhance the electricity generation performance of the battery. The designed battery structure with bilateral cathodes is shown in Figure 2:
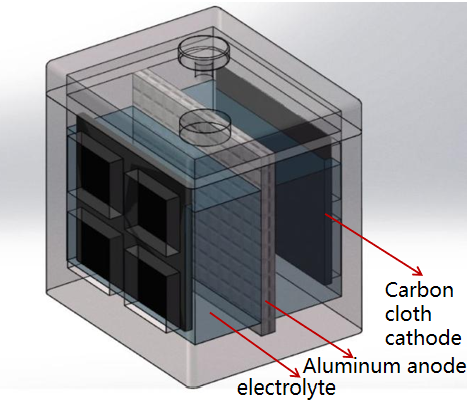
Figure 2. Structural design diagram of aluminum-air battery with bilateral non-precious metal cathodes
The overall structure of the battery is of a cubic type, Figure 3 shows the battery structure cutaway drawing, in which, the shell and cover materials are both acrylic plates with an area of 5×5 cm2, the bilateral cathodes are waterproof carbon cloth with catalysts, a single reaction area is 3×3 cm2, the reaction contact area is 2 times that of the single cathode, the anode is a mesh aluminum sheet, electrolyte is alkaline, in order to realize the battery ion conduction.
The holes on the “cover plate” can be used to add electrolytic solution, at the same time, a reference electrode can be inserted to test the potentials of cathode and anode to judge the cathode performance limitations or anode performance limitations;
The “battery hull” is a symmetrical cavity to wrap around the whole, and because the cathode material is a carbon cloth with a catalyst, therefore, in order to increase the overall mechanical bearing strength of the battery, the cathode has a cross window design, which can achieve both oxygen transmission and structural support;
“Bilateral porous oxygen electrodes” are attached to the cross window of the two side shells, one side of the cathode faces the electrolyte and the other side faces the air. (There is a hydrophobic air-permeable membrane on one side of the air, which is used to let the air spread smoothly and prevent the electrolyte from spilling over. The layer is usually made of activated carbon polyene or PTFE by pressing);
The “Anode mesh aluminum electrode plate” serves as the battery anode, in order to increase the anode reaction specific surface area, mesh aluminum is used instead of the solid aluminum plate. The anode is inserted into the middle groove and immersed in the electrolyte, after the discharge, the aluminum plate can be replaced to achieve continuous discharge;
The “electrolyte chamber” contains the electrolyte, usually an alkaline sodium hydroxide solution or a potassium hydroxide solution.
The design of the structure is made of lightweight materials, the overall battery weight is light, simple structure, and low price.
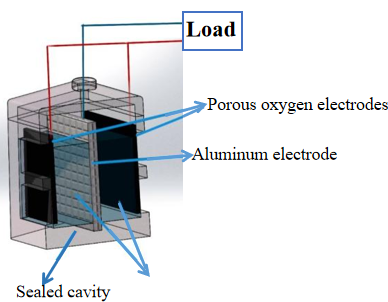
Figure 3. The cutaway drawing of aluminum-air battery with bilateral non-precious metal cathodes
4. The working principle of the device
Aluminum-air battery is composed of aluminum alloy anode (negative), air cathode (positive), neutral or alkaline electrolyte and battery shell. The chamber contains the KOH electrolyte. Two electrodes are connected with an external circuit and a load to form a loop discharge [6] .The reaction principle is shown in Figure 4. During discharge, metal aluminum anode produces electrons and is oxidized to Al3+, then Al3+ ions combine with OH- ions in the electrolyte to generate Al(OH)3. The cathode is a porous gas diffusion electrode. Oxygen enters the air electrode to obtain the electrons and is reduced to OH-. In this process, the chemical energy of the aluminum metal is converted into electricity. When the battery works, electrons flow from the anode to the cathode through the external circuit to generate current. The OH- ions flow from the cathode to the anode through the internal electrolyte, thus forming a current loop. The electrode reaction formula is as follows [7]:
Anodic reaction: 4Al-12e- → 4Al³+ (2-1)
Cathodic reaction: 3O2 + 6H2O + 12e- → 2 OH- (2-2)
Total battery reaction: 4Al + 3O2 + 6H2O → 4Al (OH)3 (2-3)
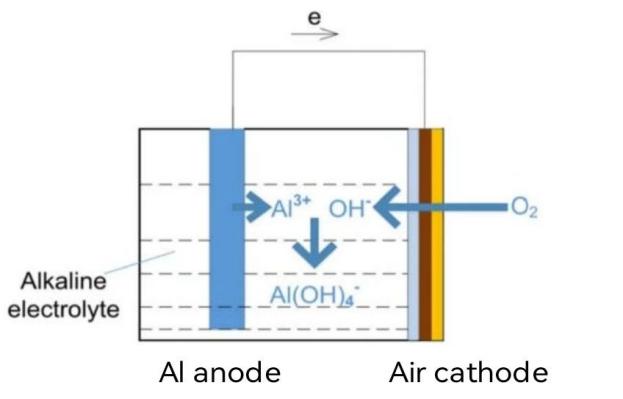
Figure 4. Schematic reaction diagram of aluminum-air battery
5. Results and discussion
After the battery assembly was installed, we tested the discharge curve and power density curve of the battery at 22 degrees Celsius through the electrochemical workstation.
In order to characterize the differences in catalytic activity between double and single cathode structures , the oxygen reduction current response of the air cathode at different potentials was tested by LSV, thus reflecting the catalytic activity of the cathode. The reference electrode used in this experiment was Hg/HgO electrode, and the linear sweep voltammetry (LSV) test of air cathode was studied in 0.1mol/L KOH electrolyte. The potential range was -0.4 to 0.1 V vs. Hg/HgO with a scanning rate of 10 mV/s. Figure 5 shows the LSV curves between single cathode and double-cathode structures. As can be seen in Figure 5, the initial potentials of single cathode and double -cathode structures are close. But in the potential range of -0.4 to -0.2 V vs. Hg/HgO, the double-cathode structure has a higher current response than the single cathode. Take the -0.3 V vs. Hg/HgO potential as an example, the current is 508.1mA, 296.1mA for the double and single cathode batteries, respectively. The above test results show that the double-cathode structure has better electrical production performance than the single cathode structure.
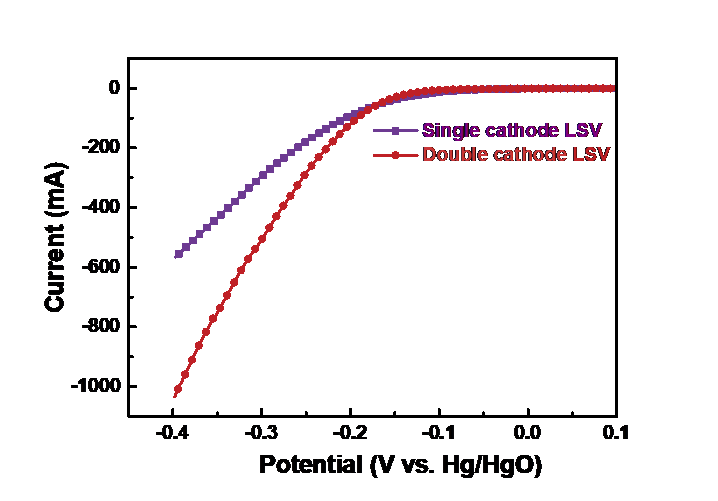
Figure 5. LSV curves of single cathode and double cathode batteries in 0.1mol/LKOH electrolyte and 10mV/s scanning speed
Next, in this experiment, the chronoamperometry was used for double-cathode battery. A constant potential of -0.2V was applied to the working electrode to monitor the current output stability of the electrode and obtained the curve of electrode current changing with time under the potential step as shown in Figure 6. As can be seen from the figure 6, the current curve is extremely stable, and the current is basically maintained at 400 mA. This indicates that the bilateral-cathode structure designed in this study can achieve a uniform distribution of the current and has good stability.
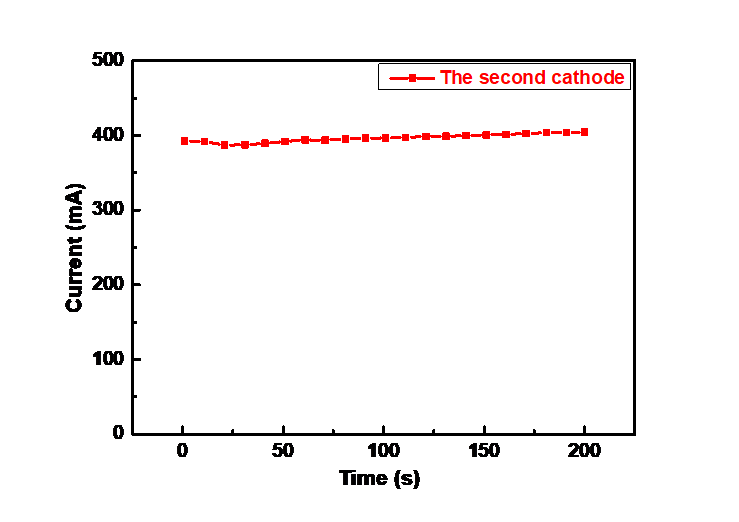
Figure 6. The chronoamperometry test of the second cathode
In the next step, this experiment monitored the open-circuit voltage of the double-cathode battery. As shown in Figure 7, the battery open-circuit voltage is stable at 1.38V, which can provide an ideal energy output.
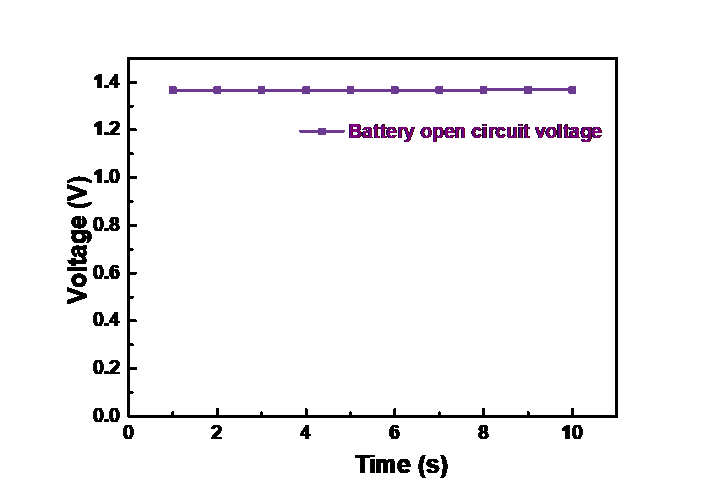
Figure 7. Open-circuit voltage of the double-cathode structure
Then, in this experiment, the battery performance of single cathode and double-cathode structure was studied by step discharge method, and the curves of discharge voltage changing with current as shown in Figure 8 were obtained. The figure shows that the discharge voltage of both structures gradually decrease with the increase of discharge current. In the initial stage, the open-circuit voltage is roughly equal, both around 1.3V. But at the same current, the voltage of the double-cathode battery is significantly higher than that of the single cathode battery. For example, when the current is 164 mA, the voltage of the double-cathode battery is 0.94V, while the voltage of the single cathode is only 0.8V, indicating that the double-cathode structure has better electrical production performance. With the increase of the current, both of the discharge voltage is decreased. However, the slope of the curve of the double-cathode structure is gentle and decays slowly than the single cathode. When the current increases to 225 mA, the discharge voltage of the single cathode battery suddenly decreases to 0.3V. However, the discharge voltage of the double-cathode battery is more than 0.7V, which still has a good discharge capacity. This shows that the discharge performance of the double-cathode battery designed in this study is better than the conventional single cathode.
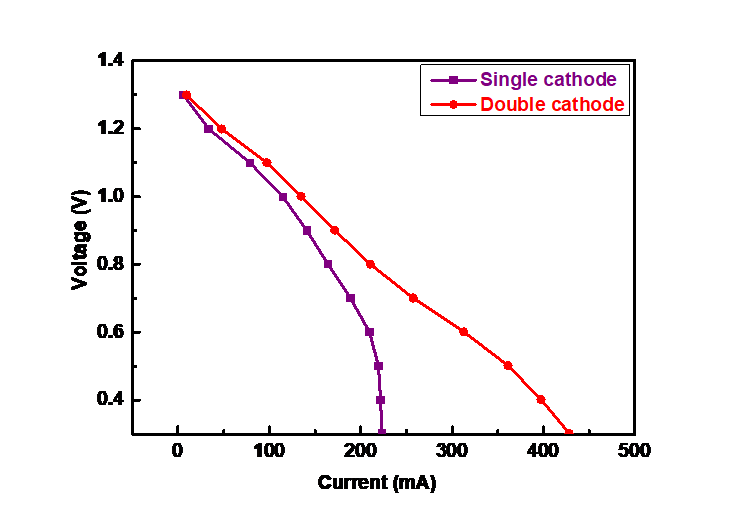
Figure 8. Variations of discharge voltage of single cathode and double cathode batteries with current
Power is also an important indicator of evaluating battery performance. Finally, the power curves of the double-cathode battery designed in this study and the traditional single cathode battery were tested by step discharge method, as shown in Figure 9. As can be seen from the figure, within a certain range, the power of both increases first and then decreases. The peak power of single cathode battery is 132.2 mW, while that of double-cathode battery is 188.1 mW, which is 1.42 times that of traditional single cathode. This shows that the electricity generation performance of the double-cathode battery designed in this study is better than that of conventional single cathode battery, which can obtain good application and effectively improve the battery performance.
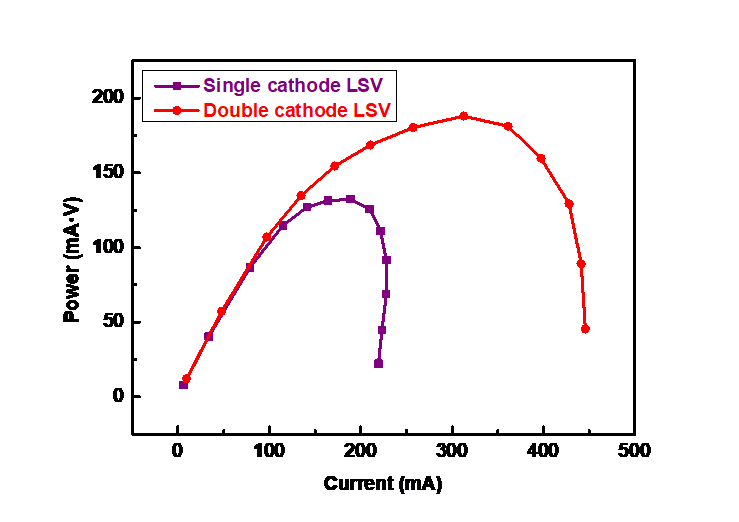
Figure 9. Power variations with current of single cathode and double-cathode structures
6. Conclusion
The world is currently facing the increasingly serious problem of global environmental pollution and energy shortage. Energy conservation and environmental protection have become the most important challenges for all countries in the world. Saving resources and developing new energy power generation technology have become an indispensable research hotspot [8]. Therefore, this study innovatively used cheap non-noble metal nano-carbon catalysts for the cathode of aluminum-air battery and designed the aluminum-air battery structure with two non-precious metal cathodes, which increased the reaction contact area and greatly improved the cathode electrical production performance. The performance of the battery was tested by experimental method, and the results show that the open circuit voltage of the battery is up to 1.38 V. The peak power of the double-cathode battery is 188.1 mW, which is 1.42 times that of the single cathode battery, which proves that the performance of the battery with double-cathode structure designed in this study is better than that of the traditional single cathode, and it had good stability.
It greatly improves the power generation performance of the battery at low cost and has strong feasibility. At the same time, aluminum-air batteries have obvious advantages in long service life, convenient replacement of fuel, high current discharge, high specific energy and environmental protection. In conclusion, the experimental results demonstrate the feasibility of the aluminum-air battery designed in this study, which provides a new strategy for battery energy saving in the future.
Acknowledgments
This work was financially supported by the Hunan Students’ Platform for innovation and entrepreneurship training program(D202305161520574220).
References
[1]. Gröger, O. , Gasteiger, H. A. , & Suchsland, J. P. (2015). Electromobility: Batteries or fuel cells?. Journal of The Electrochemical Society, 162(14), A2605.
[2]. Egan, D. R. , De León, C. P. , Wood, R. J. K. , Jones, R. L. , Stokes, K. R. , & Walsh, F. C. (2013). Developments in electrode materials and electrolytes for aluminium–air batteries. Journal of Power Sources, 236, 293-310.
[3]. Nestoridi, M. , Pletcher, D. , Wood, R. J. , Wang, S. , Jones, R. L. , Stokes, K. R. , & Wilcock, I. (2008). The study of aluminium anodes for high power density Al/air batteries with brine electrolytes. Journal of Power Sources, 178(1), 445-455.
[4]. Holzer, F. , Müller, S. , Desilvestro, J. , & Haas, O. (1993). Aluminium alloys in sulphuric acid Part I: Electrochemical behaviour of rotating and stationary disc electrodes. Journal of applied electrochemistry, 23, 125-134.
[5]. Liu, Y. , Sun, Q. , Li, W. , Adair, K. R. , Li, J. , & Sun, X. (2017). A comprehensive review on recent progress in aluminum–air batteries. Green Energy & Environment, 2(3), 246-277.
[6]. Zhang, Z. , Zuo, C. , Liu, Z. , Yu, Y. , Zuo, Y. , & Song, Y. (2014). All-solid-state Al–air batteries with polymer alkaline gel electrolyte. Journal of Power Sources, 251, 470-475.
[7]. Mokhtar, M. , Talib, M. Z. M. , Majlan, E. H. , Tasirin, S. M. , Ramli, W. M. F. W. , Daud, W. R. W. , & Sahari, J. (2015). Recent developments in materials for aluminum–air batteries: A review. Journal of Industrial and Engineering Chemistry, 32, 1-20.
[8]. Hu, T. , Fang, Y. , Su, L. , & Li, K. (2019). A novel experimental study on discharge characteristics of an aluminum‐air battery. International Journal of Energy Research, 43(5), 1839-1847.
Cite this article
Hu,J. (2024). Design of high performance aluminum-air battery based on cheap non-precious metal cathodes. Applied and Computational Engineering,72,93-100.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Functional Materials and Civil Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Gröger, O. , Gasteiger, H. A. , & Suchsland, J. P. (2015). Electromobility: Batteries or fuel cells?. Journal of The Electrochemical Society, 162(14), A2605.
[2]. Egan, D. R. , De León, C. P. , Wood, R. J. K. , Jones, R. L. , Stokes, K. R. , & Walsh, F. C. (2013). Developments in electrode materials and electrolytes for aluminium–air batteries. Journal of Power Sources, 236, 293-310.
[3]. Nestoridi, M. , Pletcher, D. , Wood, R. J. , Wang, S. , Jones, R. L. , Stokes, K. R. , & Wilcock, I. (2008). The study of aluminium anodes for high power density Al/air batteries with brine electrolytes. Journal of Power Sources, 178(1), 445-455.
[4]. Holzer, F. , Müller, S. , Desilvestro, J. , & Haas, O. (1993). Aluminium alloys in sulphuric acid Part I: Electrochemical behaviour of rotating and stationary disc electrodes. Journal of applied electrochemistry, 23, 125-134.
[5]. Liu, Y. , Sun, Q. , Li, W. , Adair, K. R. , Li, J. , & Sun, X. (2017). A comprehensive review on recent progress in aluminum–air batteries. Green Energy & Environment, 2(3), 246-277.
[6]. Zhang, Z. , Zuo, C. , Liu, Z. , Yu, Y. , Zuo, Y. , & Song, Y. (2014). All-solid-state Al–air batteries with polymer alkaline gel electrolyte. Journal of Power Sources, 251, 470-475.
[7]. Mokhtar, M. , Talib, M. Z. M. , Majlan, E. H. , Tasirin, S. M. , Ramli, W. M. F. W. , Daud, W. R. W. , & Sahari, J. (2015). Recent developments in materials for aluminum–air batteries: A review. Journal of Industrial and Engineering Chemistry, 32, 1-20.
[8]. Hu, T. , Fang, Y. , Su, L. , & Li, K. (2019). A novel experimental study on discharge characteristics of an aluminum‐air battery. International Journal of Energy Research, 43(5), 1839-1847.









