1. Introduction
Energy is generally produced by combustion of fossil fuels, such as coal, oil, and natural gases. However, these fuels have their limitations. One of the problems is their decaying storage on earth, which is expected to be running out within a century [1]. Another problem is the release of greenhouses gases (GHCs) and other pollutants during burning, all of which are climate-changers. In terms of human future, new technologies need to be applied for developing clean, new and sustainable energy, or, the renewable resources.
Common renewable resources especially natural ones like solar, wind and waves energy, are abundant within visible human future, however energy storage is required. To store “energy”, they are transformed into other forms of energy, such as mechanical, chemical, thermal, electrical, and electro-chemical. Among all of these classification, electro-chemical storage, such as secondary batteries and flow batteries, is attracting more research interest due to its performance, convenience and cost potential, with high energy exchanging efficiency to transform energy into electricity [2]. Up to 2019, the energy densities of batteries have been developed to hundreds of watt hours per kilogram, making the batteries applicable in electric vehicles, factories and portable electronics.
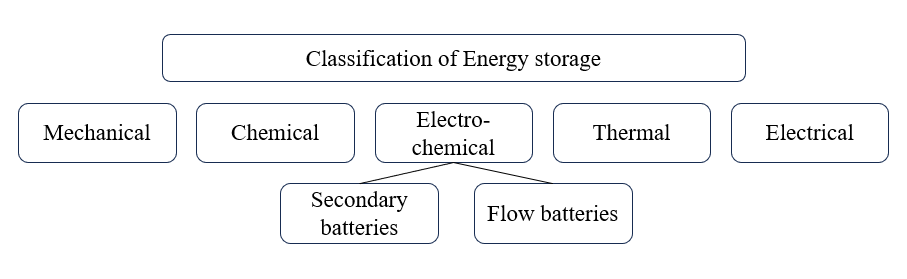
Figure 1. Classification of batteries (Picture credit: Original).
As shown in Figure 1, one of the electro-chemical storages is flow batteries, mainly including verbal fluency battery (VFB), zinc-bromine flow battery (ZBFB) and iron-chromium flow battery (FCFB). They are safe enough, environmental-friendly, having high output-efficiency and high energy density. However, this kind of material still have their limits when being advocated to market. For ZBFB, short circuit may occur to system as a result of the formation of dendrites. The diffusion of the bromide is corrosive as well, leading to the decay of irreversible capacity. Unlike ZBFB (which is usually two-phase system), the FCBC system only contains liquid phase, with easy access of hydrogen evolution and cross-contamination. As for VFB, the system with well-rounded commercial development, the low dissolution of vanadium ions set its boundary to the energy density of this battery. The high expense of this system also limits their broader application in industries.
Secondary battery, including lithium-ion batteries (LIBs) and lithium-sulfur (Li-S) batteries, have more times of reversible charging process, making it low-cost and environmental-friendly [3]. By developing this kind of battery may be essential for portable electronic devices and electric vehicles, even to large-scale energy storage plants [4]. For example, LIBs are widely used for their high energy density and stable performance, reaching an energy of 2.34 MJ/kg. Nevertheless, fatal danger may occur if operated unproperly, accidents have taken places in Samsung Note 7 explosion and Tesla electric car battery fire. One of the possible reasons is that the flammable electrolyte in LIBs is sensitive to temperature’s rise when reaction occurs, leading to a higher reaction rate thus making it possible to burn or explode. Another reason is that toxic and flammable electrolyte as well as organic solvent is contained in batteries [5]. Under crushing, overcharging or short circuiting, gases will generate, leading to a difference of pressure for both internal and external side, thus resulting fire or explosion.
Based on these defects, nanotechnology has been applied to batteries under a better understanding on materials and for a better performance with desired properties. When the particle size is lowered below 100 nm, dramatic changes may occur in its properties. Therefore, materials might be “nanostructured” in order to provide or change new properties. For example, graphitic tubular network could be fabricated to improve the conductivity and completion of structure on the electrodes [6]. Research has also proved that by using silicon nanowire material as anode of lithium battery, the specific capacity will be raised 10 times higher that of batteries with graphite anode. Other batteries with nanotechnology applied can have a longer cycle life, higher energy and safer properties could be invented.
This paper will discuss the performance of nanotechnology applied in Li-based battery materials for electric vehicle. Firstly, the role of nanotechnology in cathode materials of LiBs will be reviewed, including LiFePO4 (LFP), LiMn2O4 (LMO) and lithium-rich layered oxides (LLO). Then, the improvements made by nanomaterials in anode materials, including Si-based and carbon-based composites, which may be of great help of energy density, will be summarized.
2. LiBs cathode materials
Research about LiBs cathode materials have been focusing on LiCoO2 (LCO), LiNixCoyMnzO2 (Ni-based), LiNixMn2-xO4 (Mn-based) and other layer-distributed materials like Li(LixMnyNizCo1-x-y-z)O2 [7]. Among which, LCO is the most applied material. After its first commercialization in 1991, it has been dominating the cathode materials of battery market for nearly 30 years. However, failures including capacity loss and fragmentation of electrodes exist in LCO and even other cathode materials, which limit their further application. In this section, how nanotechnology will improve the drawbacks of cathode materials will be discussed, including LiFePO4 (LFP), LiMn2O4 (LMO) and lithium-rich layered oxides (LLO).
2.1. Improvements for conductivity/diffusion coefficient of LiFePO4
LiFePO4 was successfully commercialized since its application in Sony in 1991. It has a discharge platform of 3.4 V and can reach a high theoretical discharge capacity of 170 mAh/g, with good cyclability as well as thermal stability. Its crystal structure explains that the stable covalent bond between oxygen atom and phosphorus atom makes the oxygen atom stable when electron flows go through [8]. Despite the fact that it has enough safety, this material is limited due to its low electronic conductivity and low diffusion coefficient, with only 10-9~10-19 S/cm and Di (Li+) < 10-14 cm2/S separately.
Its limitation in electronic conductivity is the consequence of long transport length of electrons with in the structure. To improve this property, LiFePO4 with nanostructure has been studied in early research, adding conductive medium to increase its performance. By controlling cation non-stoichiometry to combine with solid-solution (which was doped by metals super-valent to Li+ in advance), the electronic conductivity is raised to 108 times higher than before, reaching approximately 10-2 S/cm at room temperature. Other conductive materials such as carbon, conductive polymer as well as conductive metal can also be applied to enhance conductivity. This discovery reveals that this material is capable of working under high current of 6000 mAh/g, and may underwent full progress of charging/recharging within several minutes while remain significant capacity.
The other drawback is the low diffusion coefficient [9]. Based on one-dimensional channel, the Li+ ion diffuses along the edge-shared LiO6 units, thus making it easy to be affected by impurities or point defects in the diffusion path. Meanwhile, the existence of Fe ions occupied the anti-site defects in Li sites, preventing Li+ ion from crossing the crystal structure, thus resulting in low capability and high potential polarization. The reduction of particle size to certain extent can have a positive influence on the diffusion coefficient as well as lowering the amount of blocked Li+ particles. Therefore, LiFePO4 with nanostructure has been applied to electric vehicles for short range or hybrid electric vehicles [10].
Even though smaller size of particles can have better performance for LiFePO4 it is noticeable that the energy content of cells goes up as the percentage taken by nanoparticles declines [11]. Since the volume of the battery is as important as the weight of battery, further study need to be focusing on balancing the size and energy density.
2.2. Improvements for dissolution of LMO
In 1983, John Goodenough conducted research of LMO based on its previous study of LiM[M2]O4 (M=Fe or Mn), indicating that the spinel frame could provide a three-dimensional structure to allow Li+ to diffuse without restrain. The research also discovered that the framework remained stable even after the insertion of lithium [12]. In recent years, LMO has become one of the dominant oxide electrodes materials being applied in LIBs, and has been accepted in electric vehicles like Chevy Volt and Nissan Leaf. Under normal circumstances, LMO is usually operated at around 4 V, undergoing reversible lithium extraction reaction. Despite that working voltage could be lowered to 3 V by inserting Li, a crystal distortion named Jahn-Teller may occur as a result of the reduction of Mn, thus leading to battery failure. In addition, when working voltage is above 3 V, the dissolution of Mn will be suppressed and capacity decline will be prevented.
There are two different angles for improving the performance of LMO battery. On the one hand, the influence of Jahn-Teller effect could be mitigated. Several ways can achieve this improvement, including introducing Al atoms, stabilizing the octahedral sites to suppress crystal distortion, designing porous structure to ensure high crystallinity, and introducing tubular structure to buffer the strain and stress from Jahn-Teller effects [13]. On the other hand, to avoid Mn dissolution, nanocoatings are commonly used to resist dissolution and capacity loss. Nano-coatings (10-20 nm thick) introduced with various kinds of oxides or fluorides, including SiO2, ZrO2, TiO2, Al2O3, and AlF3, are proved to have protective function against dissolution by maintaining the average valence state of Mn [14]. However, a direct comparison of the protective effect of different nanocoatings is not widely known. With the differential of thickness of coatings, coverage of surface, smoothness and methods, it is even harder to have well-rounded research of the conclusion.
2.3. Improvements for voltage drops and low initial coulombic efficiency of LLO
LLO is usually in the form of Li1+xM1-xO2 or xLi2MnO3-(1-x)LiMO2 (M=Ni, Co, Mn), gaining increasing interest in recent years for its high working potential of 3.7 V (vs. Li+/Li). It also has a maximum capacity of around 280 to 310 mAh/g and an incredible energy density of around 900 Wh/kg [15]. However, several drawbacks are setting limits for its commercialization. The first problem is its drop-in voltage. By studying Li-layered Li1.2Ni0.15Co0.1Mn0.55O2, Mn sites are discovered to have poorer reaction kinetics on Li2MnO3, compared to Ni and Co, which are only affected by the irreversible structure change of Li2MnO3 in a negative way [16]. It is also discovered that Mn3+/Mn4+ redox ions, along Co2+/Co3+ redox ions, have direct connections with voltage decline as well as voltage profiles, proving that voltage fade may not only occur as a result of transition metals migration, but also a consequence of valence drop process.
To mitigate the effect of voltage fade, there are several ways achieving this. One of them is via surface modification, which can protect the material while improving the electronic conductivities. Liu et al. proposed a coating layer of Mg2+ pillar mixed with Li-Mg-PO4 which results showed an enhanced performance on capacity and stabilization of average discharge voltage [17]. Another widely applied approach is via lattice doping, a method which can be applied to improve voltage fading through the blocking of transition metal migration and the stabilization of LLO structures.
Another problem is its low initial columbic efficiency (ICE), generally causing a capacity loss of 60-120 mAh/g, implying an ICE of 65% to 83%. Research have also reported an ICE of 25% for Li1.2Ni0.2Mn0.6O2, and an ICE of 81.3% for 0.3Li2MnO3-0.7LiMn0.5Ni0.5O2. This also indicates a change may occur in ICE when composition varies [18]. From the angle of mechanism, it is believed that irreversible Li and O loss from the LLO lattice as well as the decomposition pf electrolyte under high voltage are the reasons for ICE. To mitigate this property, researchers have provided several methods for improvements, and one of them is using nanoparticles with high surface areas to apply in modification. Research have shown that the particle size can have a great impact on LLO’s structure and properties, that by comparing nanomaterials with different size, conclusion is made that large-particle LLO behave a two-phase reaction mechanism while the small-particle LLO behave no phase separation when working voltage is above 4.5 V. However, it is noticeable that the capacity as well as the completeness of the reactions are sacrificed in order to enhance the ICE, pointing out a potential way for future development [19].
3. LIBs anode materials
For anode materials in LIBs, graphite is the most commercially used for its layer-distributed structure. The insertion of Li+ could create an interstice between two layers, thus preventing cathode from structure changing during working process. However, inevitable energy losses exist during intercalation/de-intercalation cycle. One of the major reasons is the creation of solid electrolyte interface (SEI). Despite the fact that SEI ensures the penetration of lithium ions, the formation itself consumes lithium ions, thus leading to an irreversible capacity loss [20]. In this section, how nanotechnology will improve the drawbacks of anode materials will be discussed, including Si-based materials and carbon-based materials.
3.1. Improvements for volume change of Si-based material
Si is considered to be one of the promising materials for LIBs anode, having a capacity up to 3579 mAh/g (Li15Si4) at room temperature. The voltage platform for Si (0.4 V vs. Li+/Li) is also higher than graphite electrode (0.125 V vs. Li+/Li), making it possible to avoid the formation of dendritic lithium as well as lithium electro-plating. Its abundant storage in earth and the safety performance also attracts great research interest. However, the drawbacks of Si are still neglected. The core challenge is decline of its great capacity loss due to vast volume fluctuation during working process. For example, a reduction of volume in Li15Si4 could reach to 280%, while an increase of volume is shown in Li22Si5, with approximately 310% under 415 Celsius. With such a high-volume change, the decline of capacity as well as cycle performance are exposed. Besides, low ICE is also shown in Si-based anode material, as reactions are processed between lithium ions and silicon oxide on the surface of anode, even when Si is nanosized [20].
With the help of nanotechnology, it is concluded that nanoparticles may prevent the occur of particle fracture and pulverization. It is proved that the size of nanoparticles with less than 150 nm are less likely to have fracturing, and the value would be 300 nm when it turns to Si nanowires [21]. Previous research has also proved that the energy stored from electro-chemical process is not enough to cause cracking when the particle size of nano-Si is smaller than 150 nm. Kim et al studied the connection between the size effect and electro-chemical performance, finding that nano-Si with a size of lower than 5 nm have lower specific capacities, compared to particles with 10 nm size [22]. Meanwhile, modifying Si to porous Si can enhance battery performance as well. The working principle is similar to the model of hollow core-shell, allowing the expansion of silicon during lithiation, thus limiting the effect of volume change while maintain the particle size at stable. Furthermore, a stable size of particle is helpful to the formation of SEI layer, which keeps the working particles in contact with efficiency. Zhao et al studied the 3D morphological evolution of nano-Si anode in LIBs, comparing the morphological changes under different cycling capacities. It is noticed that nano-Si were not well-rounded lithiated during the initial cycling stage and a heterogeneous distribution of thickness was shown, leading to the great loss of capacity. However, by introducing nano-porous Si with enough porosity and appropriate particle size, structural integrity of electrode is maintained, thus alleviating delamination caused by uneven distribution [23].
3.2. Improvements for low capacity of graphene
Carbon-based material is widely applied in anodes of commercial LIBs for its outstanding electronic conductivity, cheap expense and easy access for Li+ insertion. However, its low theoretical capacity and negative capability have prevented its further development in market. For example, graphite only reaches a capacity of 372 mAh/g. The uncertainty of safety also prompted further research of improving the performance of this carbon-based anode material.
However, graphene, a material which is merely graphite sheet, shows twice the capacity of graphite, reaching 744 mAh/g. Besides its large surface area and their relative high conductivity, its mechanical flexibility expands its application in portable devices, which gains great research interest. Nevertheless, its drawback of capacity decline occurs as a result of restacking of graphene layers. Another defect for this material is that the intense van der Waals interaction and π-π stacking, graphene shows a tendency to Li+ aggregate. As a result of its high aspect ratio, Li+ has longer transferring length, thus leads to a lower capacity and higher energy loss.
Currently, efforts have been made via nanotechnology. Graphene nanosheet is a potential research area for it provides a stable conducting platform for electro-active materials like Fe2O3, MnO2, SnO2, etc. It can also suppress the delamination of layered materials which are active, showing a higher rate capability and longer cycling stability, as shown in Figure 2. For example, adding Co3O4 to graphene nanosheet is of great improvement for structural integrity and cycle stability, having an increased capacity of 1134.4 mAh/g 2.25 C. This hybrid material also has a good capacity retention rate, which is 92.1% for 2000 under the same condition [24]. Besides, doping graphene with heteroatom has been proved to be effective in overcoming the disadvantages. With the introduction of S, N or B, functionalized graphene materials could be fabricated [20].
Figure 2. Illustration showing the structure of graphene sheet (Picture credit: Original).
4. Conclusion
This paper has discussed the performance of nanotechnology applied in Li-based batteries materials. By studying different kinds of approaches applied in cathode materials including LFP, LMO and LLO, it is noticeable that nanotechnology enhances or improves the defects in atom-scale, thus bring great changes to macroscopical behaviour like capacity and conductivity. Then, the improvements of nanomaterials in anode of Li-ion batteries, including Si-based and graphene composites, which may be of great help of energy density are also reviewed, that from which can be known that even in commercialized materials like graphene can still gain reinforced properties. These attempts of modification for materials are aiming at providing batteries with longer cycle life, higher discharge capacity and higher ICE. It is clear that with the progress of battery research, Si-based materials are gaining more research interests than before as a result of its high capacity and safety, but it is also clear that scientific research is not always a smooth sailing when balancing the performance and particle size.
References
[1]. Md M, Abayomi O, Eskinder G and Amit K 2020 J. Energy Conversion and Management 223 113295
[2]. Guangmin Z, Lin X, Guangwu H, Liqiang M and Yi C 2019 J. Chemical Reviews 119 20
[3]. Wenjing L, Zhizhang Y, Yuyue Z, Hongzhang Z, Huamin Z and Xianfeng L 2017 J. Chemical Society Reviews 46 2199-2236
[4]. Kai L, Yayuan L, Dingchang L, Allen P and Yi C 2018 J. Science Advances 4 6
[5]. Shichen C, Zhirong W, Jinghong W, Xuan T and Wei Y 2020 J. Journal of Loss Prevention in the Process Industries 63 103992
[6]. Xiaoyan L, Weichen C, Qingrong Q, Haitao H, Yuming C, Ziqiang W, Qinghua C, Jing Y, Ju L and Yiu-Wing M 2020 J. Advanced Energy Materials 11 2
[7]. Sanghan L, Wooyoung J, Su H, Se H, Gyutae N, Pilgun O, Young-Ki K, Sang K, and Jaephil C 2019 J. Angewandte Chemie International Edition 58 10478-85
[8]. Huanhuan Z, Zhengguang Z, Shuchao Z, Jie L and Shenglin Z 2020 J. International Journal of Electrochemical Science 15 12041-67
[9]. Taehoon K, Wentao S, Dae-Yong S, Lusi K.O and Yabing Q 2019 J. Journal of Materials Chemistry A 7 2942-64
[10]. Jun L, Zonghai C, Zifeng M, Feng P, Larry A.C and Khalil A 2016 J. Nature Nanotechnology 11 1031-38
[11]. Rahul M, Damian B, Martin B and Gerbrand C 2010 J. Nano Letters 10 4123-7
[12]. Michael M.T and Khalil A 2021 J. Nature Energy 6 566
[13]. Zhuoxin L, Yan H, Yang H, Qi Y, Xinliang L, Zhaodong H and Chunyi Z 2020 J. Chemical Society Reviews 49 180-232
[14]. Marcel J.H, Daniel E, and Jurgen J 2021 J. Batteries & Supercaps 4 1003-17
[15]. Sijiang H, Anoop.S P, Gemeng L, Wei K.P, Hongqiang W, Qingyu L and Zaiping G 2019 J. Electrochemical Energy Reviews 2 277-311
[16]. Xiqian Y, Yingchun L, Lin G, Huiming W, Seong-Min B, Yongning Z, Khalil A, Steven N.E, Hong L, Kyung-Wan N and Xiao-Qing Y 2013 J. Advanced Energy Materials 4
[17]. Wen L, Pilgun O, Xien L, Seungjun M, Woongrae C and Jaephil C 2015 J. Advanced Energy Materials 5
[18]. Jing X, Meiling S, Ruimin Q, Sara E.R, Lu M, Tianpin W, Sooyeon H, Dennis N, Dong S, Khalil A, Jun L, Bryan D.M, Wanli Y and Wei T 2018 J. Nature 9
[19]. Sijiang H, Anoop S.P, Gemeng L, Wei K.P, Hongqiang W, Qingyu L and Zaiping G 2019 J. Electrochemical Energu Reviews 2 277-311
[20]. P. U N, A. D O, F. I E, E. I I and A.C.N 2022 J. Applied Surface Science Advances 9 1002
[21]. Xiuyun Z and Vesa-Pekka L 2020 J. Nanotechnology 32 042002
[22]. Volker S, Joerg V W, Stephan S and Ulrich G 2009 J. Advanced Materials 21 2681-702
[23]. Chonghang Z, Takeshi W, Vinceng D A, Doga G, Hidemi K and Yu-chen K C 2018 J. Nano Energu 52 381-390
[24]. Hongya G, Yan P, Liangti Q, Haijiao Z and Minghong W 2020 J. Advanced Energy Materials 10
Cite this article
Liu,Y. (2024). What does nanotechnology have to offer for lithium-ion batteries materials?. Applied and Computational Engineering,85,10-16.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Md M, Abayomi O, Eskinder G and Amit K 2020 J. Energy Conversion and Management 223 113295
[2]. Guangmin Z, Lin X, Guangwu H, Liqiang M and Yi C 2019 J. Chemical Reviews 119 20
[3]. Wenjing L, Zhizhang Y, Yuyue Z, Hongzhang Z, Huamin Z and Xianfeng L 2017 J. Chemical Society Reviews 46 2199-2236
[4]. Kai L, Yayuan L, Dingchang L, Allen P and Yi C 2018 J. Science Advances 4 6
[5]. Shichen C, Zhirong W, Jinghong W, Xuan T and Wei Y 2020 J. Journal of Loss Prevention in the Process Industries 63 103992
[6]. Xiaoyan L, Weichen C, Qingrong Q, Haitao H, Yuming C, Ziqiang W, Qinghua C, Jing Y, Ju L and Yiu-Wing M 2020 J. Advanced Energy Materials 11 2
[7]. Sanghan L, Wooyoung J, Su H, Se H, Gyutae N, Pilgun O, Young-Ki K, Sang K, and Jaephil C 2019 J. Angewandte Chemie International Edition 58 10478-85
[8]. Huanhuan Z, Zhengguang Z, Shuchao Z, Jie L and Shenglin Z 2020 J. International Journal of Electrochemical Science 15 12041-67
[9]. Taehoon K, Wentao S, Dae-Yong S, Lusi K.O and Yabing Q 2019 J. Journal of Materials Chemistry A 7 2942-64
[10]. Jun L, Zonghai C, Zifeng M, Feng P, Larry A.C and Khalil A 2016 J. Nature Nanotechnology 11 1031-38
[11]. Rahul M, Damian B, Martin B and Gerbrand C 2010 J. Nano Letters 10 4123-7
[12]. Michael M.T and Khalil A 2021 J. Nature Energy 6 566
[13]. Zhuoxin L, Yan H, Yang H, Qi Y, Xinliang L, Zhaodong H and Chunyi Z 2020 J. Chemical Society Reviews 49 180-232
[14]. Marcel J.H, Daniel E, and Jurgen J 2021 J. Batteries & Supercaps 4 1003-17
[15]. Sijiang H, Anoop.S P, Gemeng L, Wei K.P, Hongqiang W, Qingyu L and Zaiping G 2019 J. Electrochemical Energy Reviews 2 277-311
[16]. Xiqian Y, Yingchun L, Lin G, Huiming W, Seong-Min B, Yongning Z, Khalil A, Steven N.E, Hong L, Kyung-Wan N and Xiao-Qing Y 2013 J. Advanced Energy Materials 4
[17]. Wen L, Pilgun O, Xien L, Seungjun M, Woongrae C and Jaephil C 2015 J. Advanced Energy Materials 5
[18]. Jing X, Meiling S, Ruimin Q, Sara E.R, Lu M, Tianpin W, Sooyeon H, Dennis N, Dong S, Khalil A, Jun L, Bryan D.M, Wanli Y and Wei T 2018 J. Nature 9
[19]. Sijiang H, Anoop S.P, Gemeng L, Wei K.P, Hongqiang W, Qingyu L and Zaiping G 2019 J. Electrochemical Energu Reviews 2 277-311
[20]. P. U N, A. D O, F. I E, E. I I and A.C.N 2022 J. Applied Surface Science Advances 9 1002
[21]. Xiuyun Z and Vesa-Pekka L 2020 J. Nanotechnology 32 042002
[22]. Volker S, Joerg V W, Stephan S and Ulrich G 2009 J. Advanced Materials 21 2681-702
[23]. Chonghang Z, Takeshi W, Vinceng D A, Doga G, Hidemi K and Yu-chen K C 2018 J. Nano Energu 52 381-390
[24]. Hongya G, Yan P, Liangti Q, Haijiao Z and Minghong W 2020 J. Advanced Energy Materials 10









