1. Introduction
Nowadays, environmental pollution and energy crisis has become the focus of attention and urgent problems to be solved around the world. To deal with these two thorny problems, electric vehicles were appeared. Electric vehicles are environmentally friendly and energy efficient, with no exhaust emissions and reduced consumption of traditional fossil fuels. Battery technology is one of the core technologies of electric vehicles. The battery of an electric vehicle plays a decisive role in the powertrain and safety system of the entire vehicle. The developments of battery status and future trend are of great significance for the wide application of electric vehicles. Currently, the most widely used battery for electric vehicles is the chemical battery. Chemical batteries can be divided into two categories: storage batteries and fuel cells [1].
Hassoun professor studies the sustainable energy storage systems to improve the performance and sustainability of electrochemical energy storage. Dr. Yaxiang Lu proved that layered O3-type NaNi0.12Cu0.12Mg0.12Fe0.15Co0.15Mn0.1Ti0.1Sn0.1Sb0.04O2 have the longer cycling stability and the outstanding rate capability. This material may become the mainstream of battery materials in the future.
2. Electric vehicle battery types and characteristics
2.1. Lead-acid battery
Lead-acid battery is a type of battery with a long history of development, which is low cost, mature technology and high safety. At present, the types of batteries in China are divided according to the positive and negative electrode materials, mainly including VRLA batteries, nickel-cadmium batteries and zinc-manganese batteries, among which VRLABs are widely used in aerospace, communications, transportation and other fields due to the advantages of good sealing, low price, high stability and mature regeneration technology [2]. The lead-acid battery is composed of a positive plate, a negative plate, an electrolyte, a separator, an outer shell, a pole, a tab, a busbar and a safety valve (Figure 1). These materials containing lead elements give them extremely high chemical stability, making them highly safe. However, with the increasing demand for power in electric vehicles, the low energy density of lead-acid batteries restricts the trend of lead-acid batteries to become mainstream batteries.
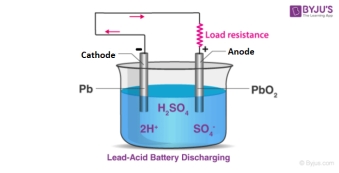
Figure 1. Lead-acid battery discharging.
2.2. Fuel cells
Fuel cell is a power generation device that directly converts the chemical energy of fuel into electrical energy, which has the advantages of high power generation efficiency and less environmental pollution. The main ones that are widely used in the household market are proton exchange membrane fuel cells (PEMFC) and solid oxide fuel batteries (SOFC) [3]. The working principle of fuel cells is the reverse process of water electrolysis, that is, the conversion of fuel and oxidant into electricity, water, and heat through electrodes [4]. Proton exchange membrane fuel cells (PEMFC) are widely recognized for their low operating temperature, fast start-up, high specific power, and easy operation, and are regarded as the preferred energy source for powered vehicles.
Proton exchange membrane fuel cells (PEMFC) are widely recognized for their low operating temperature, fast start-up, high specific power, simple structure, and easy operation, and are regarded as the preferred energy source for powered vehicles. The working principle is shown in Figure 2, hydrogen enters the anode and is decomposed into hydrogen ions and electrons by the anode catalyst on the anode, and then the electrons form an electric current in the external circuit. Hydrogen ions travel through the proton exchange membrane to the cathode. Oxygen enters the cathode and passes through the cathode catalyst on the cathode to form water with the hydrogen ions produced by the anode, while releasing heat. The chemical equation [5] is:
Anode reaction equation \( {H_{2}}→2{H^{+}}+2{e^{-}} \)
The cathode reaction equation is \( \frac{1}{2}{O_{2}}+2{H^{+}}+2{e^{-}}→{H_{2}}O \)
The total reaction equation \( {H_{2}}+\frac{1}{2}{O_{2}}→{H_{2}}O \)
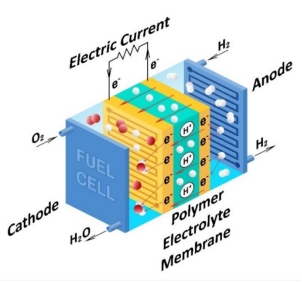
Figure 2. Fuel cell circuit diagram.
2.3. Lithium-ion batteries
Lithium-ion batteries are currently one of the most commonly used battery types in electric vehicles. It has the advantages of high energy density, long service life, and no memory effect, and is widely used in various electronic products and electric vehicles [6]. The reduction potential of lithium metal is extremely low, and the theoretical specific energy can be greater than 3800 mAh/g, far exceeding that of conventional carbon materials. This shows that lithium-ion batteries have a very high ceiling, even though they are currently prone to fire. The main components of lithium-ion batteries are cathode materials, anode materials, exchange membranes, electrolytes, shells, etc [7]. At present, commercial lithium-ion batteries use graphite as the negative electrode, which is difficult to meet the high demand for energy storage characteristics in today's era,so, the key to improving the energy storage of lithium-ion batteries currently lies in finding electrode materials with high energy density.For example, vanadium disulfide (VS2) and silicon germanium composite materials are very suitable negative electrode materials for this requirement. Of course, in addition to alloy materials that can be used as negative electrode materials for lithium-ion batteries, carbon based materials are also very suitable as negative electrode materials. The advantage of this carbon based composite material is minimal pollution during production and low cost. As the largest component in terms of weight and cost in LIBs, positive electrode materials are still one of the main bottlenecks in achieving high performance of LIBs.
2.4. Nickel metal battery
Nickel metal batteries can be divided into nickel-chromium batteries and nickel-metal hydride batteries. The energy density and charging times of nickel-chromium batteries have certain advantages, but their pollution to the environment does not conform to the original design intention of electric vehicles, which restricts its development. Currently, 99% of the market share of hybrid power batteries belongs to nickel-metal hydride batteries , and 99% of the existing market share of hybrid batteries is NiMH power batteries [8]. The anode material of nickel-metal hydride batteries is a hydrogen storage alloy. The types of hydrogen storage alloys include AB5 hydrogen storage alloys, superlattice hydrogen storage alloys, etc. A typical representative of AB5 hydrogen storage alloy is LaNi5 intermetallic compound. The advantages of the AB5 alloy electrode are good cycling stability and rate performance [9]. In pursuit of higher density batteries, AB5 hydrogen storage alloys began to become AB3 unidirectional superlattice alloys. In addition to the AB5 hydrogen storage battery, researchers also studied A2B7 single-phase superlattice alloys, A5B19 and AB4 single-phase superlattice alloys.
3. The future development trend of new energy vehicle batteries
With the continuous progress of electric vehicle technology and decreasing costs, the price of electric vehicle batteries is gradually decreasing, enabling more consumers to buy EVs. Recently, China’s electric vehicle industry has experienced rapid growth due to strong government support and promotion. The number of new energy vehicles has increased from under 10,000 in 2011 to 6.03 million in 2021 [10]. Currently, research and development efforts in new EV battery technology primarily focus on lithium-ion batteries which offer high energy density and low self-discharge rates. In the future, advancements in battery technology will be a key driving force for EV development.
3.1. Increase cruising range
The problem of short cruising range faced by new energy vehicle battery technology is mainly due to the limitation of the energy density of the battery itself and the reduced efficiency under actual use conditions. In other words, increasing the energy density can greatly increase the cruising range. The gap in energy density compared with fossil fuels has significantly limited the range of new energy vehicles.
The weight of the battery has an important impact on the performance and range of new energy vehicles. In new energy vehicles, batteries usually account for a considerable part of the vehicle's weight. Therefore, the development of lightweight battery technology is of great significance for improving the performance and mileage of new energy vehicles. At present, some new lightweight battery technologies are constantly developing, such as: lithium-sulfur batteries, solid-state batteries, etc. These new battery technologies have higher energy density and lower weight, which can further improve the performance and range of new energy vehicles. At the same time, it can not only reduce the internal weight of the battery, but also reduce the weight of the outer packaging of the battery, that is, the battery pack shell.
3.2. Shorten the charging time
Shorter charging times are another important trend in the development of battery technology. In order to accelerate the charging efficiency of the battery, it is adjusted according to the fast charging theory. Fast charging methods can be roughly divided into three categories: segmented constant current charging method, pulse charging method, and intermittent fast charging method [11]. The focus here is on segmented constant current charging. Segmented constant current charging, as the name suggests, refers to the process of charging an electric vehicle by keeping the input current constant in stages. The flowchart is shown below.
Figure 3. Segmented constant current charging process diagram.
Discharge the power battery; Check the terminal voltage of the battery to determine if it is less than V1 (V1=2.55V). If it is less than V1, proceed to the next step of low current (1/10C) pre charging; When the voltage exceeds V1, check if its voltage is less than V2 (V2=3.0V). If it is less than V2, perform segmented constant current charging. Once the terminal voltage of the battery exceeds the set value V3 (V3=3.3V), it is necessary to check whether the value is lower than the actual measured voltage. If this is indeed the case, high current forward and reverse pulse charging should be adopted. If the battery voltage has reached the preset 3.3V, it indicates that the battery is fully charged. To prevent excessive polarization reactions in power batteries, it is necessary to use high current forward pulse charging. Pulse charging is to solve the problem of the maximum charging current that the battery can accept gradually decreasing with time during the charging process. By introducing discharge, efficiently improving the performance of the battery and delaying its effective working life. Intermittent charging is divided into two forms: current intermittent charging and voltage intermittent charging. Taking intermittent current charging as an example, in the initial stage of battery charging, a larger current is usually selected for rapid charging. When the voltage at both ends of the battery reaches the preset value, and then the current value will be reduced repeatedly until charging is completed. The same applies to intermittent voltage charging.
Lacking of charge device is also one of the factors that lead to long charging times. In order to realize the popularization of new energy vehicles, the construction of a large number of charging piles needs to be carried out, and these charging piles need to have high-power charging capacity to meet the user’s fast charging needs.
3.3. Battery recycling and reuse technology
The purpose of new energy vehicles is to protect the environment, which requires electric vehicle batteries to be environmentally friendly. At present, except for fuel cells that can achieve near zero pollution throughout the entire process, all other batteries have a certain impact on the environment. The ore raw materials used to produce these batteries also have significant pollution, indicating an urgent need for battery recycling and reuse technology.
4. Conclusion
In the field of new energy vehicles, the advancement of battery technology is a key factor driving the development of the entire industry. There are still many difficulties in batteries, such as the impact of energy density, battery life, safety, cost, and other aspects. The research and commercial application of new battery technology will also inject new vitality into the development of the new energy vehicle industry. The battery technology of future electric vehicles will inevitably break through the shortcomings of short range, long charging time, and high battery pollution.
References
[1]. Yan Guo. Can the recycling of power batteries open the “Blue Ocean” while the new regulations are officially announced, Resource regenerating, 2018(7): 16-23.
[2]. Chengzhong Kuang, Shifeng Ou. Overview of Valve-Regulated Lead Acid Battery Online Monitoring and Health Assessment Technologies. Mechanical and Electrical Information, 2024(04): 1-11.
[3]. Jiajun Zhao,Peihong Wang. The Present Situation and Trend of Mainstream Fuel Cell Technologies. Shanghai Energy Conservation, 2015(04): 199-203.
[4]. Shengtao, Zhang and Yan, Wen. Development and Application of Fuel Cells. World Science and Technology Research and Development, 2003(3): 57-66
[5]. Bei, Gou, Woon, Ki, Na, and Bill, Diong. Fuel Cell-Modeling, Control, and Application. CRC Press, 2010
[6]. Huiren, Liang. Current Status and Trends of Electric Vehicle Battery Technology Development. Automotive Test Report, 2023(23): 149-51.
[7]. Hengyi Jia Research and application of materials for lithium-ion batteries. power technology, 2011(07): 869-71.
[8]. Sihui, Zeng. Analysis of Power Battery Recycling of New Energy Vehicles from the Perspective of Circular Economy.China Resources Comprehensive Utilization, 2022(12): 94-96.
[9]. Fengxia, Zhang, and Cheng, Tan. Research Progress of Advanced Cathode Electrode Materials for Nickel-Metal Hydride Battery. Materials Reports, 2023(02): 18-26.
[10]. Zhen, Bao. Exploration of Optimization Strategies for New Energy Vehicle Battery Technology. Automotive Test Report, 2023(23): 67-69.
[11]. Tiezhou, Wu, Chengdong, Lu, and Zhuyue, Huang. A method to improve energy utilization of lithium battery pulsecharging. Power technology, 2020(12): 5.
Cite this article
Liao,Z. (2024). Future development trend of batteries for electric vehicles. Applied and Computational Engineering,91,41-45.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Functional Materials and Civil Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Yan Guo. Can the recycling of power batteries open the “Blue Ocean” while the new regulations are officially announced, Resource regenerating, 2018(7): 16-23.
[2]. Chengzhong Kuang, Shifeng Ou. Overview of Valve-Regulated Lead Acid Battery Online Monitoring and Health Assessment Technologies. Mechanical and Electrical Information, 2024(04): 1-11.
[3]. Jiajun Zhao,Peihong Wang. The Present Situation and Trend of Mainstream Fuel Cell Technologies. Shanghai Energy Conservation, 2015(04): 199-203.
[4]. Shengtao, Zhang and Yan, Wen. Development and Application of Fuel Cells. World Science and Technology Research and Development, 2003(3): 57-66
[5]. Bei, Gou, Woon, Ki, Na, and Bill, Diong. Fuel Cell-Modeling, Control, and Application. CRC Press, 2010
[6]. Huiren, Liang. Current Status and Trends of Electric Vehicle Battery Technology Development. Automotive Test Report, 2023(23): 149-51.
[7]. Hengyi Jia Research and application of materials for lithium-ion batteries. power technology, 2011(07): 869-71.
[8]. Sihui, Zeng. Analysis of Power Battery Recycling of New Energy Vehicles from the Perspective of Circular Economy.China Resources Comprehensive Utilization, 2022(12): 94-96.
[9]. Fengxia, Zhang, and Cheng, Tan. Research Progress of Advanced Cathode Electrode Materials for Nickel-Metal Hydride Battery. Materials Reports, 2023(02): 18-26.
[10]. Zhen, Bao. Exploration of Optimization Strategies for New Energy Vehicle Battery Technology. Automotive Test Report, 2023(23): 67-69.
[11]. Tiezhou, Wu, Chengdong, Lu, and Zhuyue, Huang. A method to improve energy utilization of lithium battery pulsecharging. Power technology, 2020(12): 5.









