1. Introduction
AI has been a revolution in public health, especially in the prediction and management of epidemics. The recent COVID-19 pandemic heightened the need for flexible, data-driven solutions to adapt to rapidly changing disease landscapes. While epidemiological models like the SEIR (Susceptible-Exposed-Infected-Recovered) approach are classical, they use fixed parameters with insufficient elasticity to respond to changes in disease rates. AI, on the other hand, provides an adaptive platform that can be adapted to massive amounts of real-time data to refine predictions and boost public health interventions. The latest machine learning (ML) and deep learning (DL) models, such as the long short-term memory (LSTM) networks used in time-series prediction, support dynamic updates from data in the moment. This versatility is particularly important in infectious diseases, where density, mobility and government policies determine transmission patterns. As well as prediction, AI has been used to maximize public health interventions through techniques like reinforcement learning (RL). In contrast to conventional public health approaches, which often apply rigid, generic interventions, RL models can respond to circumstances in a dynamic manner, recommending actions such as targeted lockdowns, prioritised vaccinations and travel bans based on emerging epidemiological information. This adaptive system works by reducing the socioeconomic cost of interventions and preserving disease management, particularly in urban environments. Furthermore, ethical and practical concerns about the use of AI in public health, including data privacy and openness, also become important considerations [1]. As AI is now embedded into public health policy, we need to balance the technological benefits with the ethical protections to ensure that the public will continue to trust us. In this essay, we investigate a broad AI approach to forecasting epidemics and maximizing public health. Using COVID-19 and influenza data, we measure the predictive power of AI models for predicting peak infection dates and regional outbreak hotspots through a case study. We also explore whether an RL-based policy optimization model could effectively mitigate infection rates without depriving people of their freedom. These results demonstrate AI’s ability to develop flexible, data-based public health policies that can accommodate the volatility of the contemporary epidemic.
2. Literature Review
2.1. AI in Epidemiological Forecasting
The use of AI in the field of epidemiological prediction has gained steam as machine learning (ML) and deep learning (DL) techniques improve the accuracy of the prediction of disease outbreaks. Older epidemiological models, including the SEIR (Susceptible-Exposed-Infected-Recovered) model, use pre-set parameters that often fail to respond well to rapidly changing disease landscapes such as COVID-19. AI models, by contrast, can be dynamically updated according to real-time information, which is particularly useful when trying to predict epidemic trends and outbreak epicenters. Recurrent neural networks (RNNs) and long short-term memory (LSTM) networks were particularly useful, as their fundamental architecture enables it to record temporal dependencies in time-series data, which is of fundamental importance in modelling the transmission of infectious diseases. AI models such as RNNs and LSTMs are also particularly good at time-series forecasting because they "store" past data points and can predict patterns of disease progression [2]. These models are conditioned on large-scale datasets such as daily caseloads, hospital capacity, mobility patterns and demographic information. When properly trained, they can produce highly precise estimates of when infections are most likely to peak and where outbreaks are likely to occur. This predictive power is especially useful for respiratory viruses and flu-like diseases, in which infections can change rapidly in response to population behavior, seasonality and policy decisions. By integrating disparate data sets, AI models inform decision makers to act at a moment’s notice, e.g., by localizing lockdowns, allocating healthcare resources, or issuing public health warnings.
2.2. Optimization of Public Health Responses through AI
The adoption of AI in public health optimization has attracted much interest, as it provides a flexible system to control disease transmission via a combination of real-time data collection and predictive modelling. Traditional public health interventions tend to be static and don’t offer the pliancy needed to react in real time to changes in infection rates and other epidemiological factors. AI allows a data-driven approach whereby interventions, including social distancing, mask laws, and vaccination deployment plans, are continuously monitored and adjusted to changing conditions. RL and decision-tree algorithms have shown particular promise in this regard. RL, for instance, is perfect for generating simulations of public health interventions because it encourages models to learn best practice over the course of iterative simulations, rewarding positive outcomes (such as lower infection rates) and penalising bad ones [3]. By utilizing AI algorithms to optimise interventions, health authorities can provide a better targeted response and reduce the overall health and economic burden of an epidemic. AI models can, for example, use regional infections, as well as mobility rates and social networking data, to identify ‘hot spots’ – those where more drastic interventions should be implemented, while leaving the relaxation at the door in lower-strength zones. This targeted approach maintains the virus at bay, while also maintaining economic stability. In a similar way, decision-tree algorithms can decide which populations should be vaccinated based on age, comorbidities and exposure potential, giving the best chance of vaccine success and optimizing the allocation of finite resources. Where epidemics sweep through urban environments where transmission rates outpace the available healthcare infrastructure, AI-based models help health officials take preventative measures that minimize the potential for disease spread.
2.3. Policy Impact and Ethical Considerations in AI-Driven Health Responses
AI in public health policy presents both practical opportunities and ethical risks, particularly in the area of data privacy, transparency and public confidence. Statistical models of disease that use AI consume a huge quantity of data, including personal information – medical history, GPS location, patterns of behaviour. Such massive data collection poses privacy risks as users might be concerned about how health professionals will use and store their data. In the absence of proper protection, private information could be breached or used in a manner that violates public trust. Moreover, AI in health policies can create an atmosphere of surveillance, in which people perceive their movement and actions being constantly tracked [4]. A major issue with AI-driven decision-making is transparency. The vast majority of AI models, especially deep learning ones, are inefficient and "black boxes" where the reasoning behind them isn’t readily deciphered. This opacity can raise public doubts, especially when AI-driven policy threatens individual liberties, such as lockdowns or quarantines. The basis for trust is in explaining and being transparent about AI systems. Clear models enable health officials to convey the rationale behind policy choices, leading to a collaborative response to health interventions.
3. Methodology
3.1. Data Collection and Preprocessing
The research draws on an extensive database spanning historical case data for COVID-19 and seasonal influenza, from the World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC). The data is cumulative, years-long and contains important epidemiological and demographic variables. These include daily infection rates, rate of recovery, mortality rates, travel statistics, and documentation of government intervention such as lockdowns and travel bans. Because these metrics had variable scales and units, data preprocessing was needed to have a stable, consistent dataset for machine learning models [5]. Preprocessing started with data normalization – setting features to standard scale to avoid any one variable having an exogenous effect on the model. We also implemented time-lagged transformations on the infection and mobility data to help the model record temporal dependencies. Infection rates from prior weeks, for example, were used as lagged variables to control for incubation and late interventions. This orderly dataset pattern was crucial when using deep learning techniques like long short-term memory (LSTM) networks which do well in time-series.
Feature engineering was also conducted to improve the model’s predictive accuracy, focusing on variables with high epidemiological relevance. For example, seasonality adjustments were incorporated, as both COVID-19 and influenza demonstrate seasonal variations that influence infection rates. Other engineered features included transmission rates calculated as a function of infection growth over time, defined as:
\( Transmission Rate=\frac{New Infections}{Total Population- Current Infected} \) (1)
This variable was particularly useful in capturing the rate of spread in different population segments and geographic regions. By refining these input variables, we enhanced the model’s ability to generate accurate, context-specific predictions [6].
3.2. Response Efficiency Analysis
In order to predict trends in infection and geographic distributions of pathogens, we used several AI models, such as long short-term memory (LSTM) networks and CNNs. We selected them based on their capabilities for processing time and space data. The LSTM model was particularly well-suited to sequenced infection data, and its memory cell architecture would help to "memorise" trends across time. It was designed to accept time-series input which allowed us to gain an edge when it came to prediction of future peak infection rate and possible outbreak days [7]. The models were trained on a split dataset, where 70% of the model were used for training, 15% for validation, and the remaining 15% were used for testing. Hyperparameter tuning — grid search to tune learning rate, dropout rate, batch size. The LSTM model, for instance, was able to calculate a mean squared error (MSE) of 0.02 after 500 epochs – an extremely good accuracy in the prediction of daily infection rates. It was then used to test the model by comparing predicted infection curves with reported cases over several testing runs. We added a CNN to further map the analysis along the geographic plane and identify patterns from one place to another. CNN was trained on geographic-coordinated infection data, which shows regional distributions sensitive to population size and movement [8]. We combined LSTM time and location data with CNN space data to get a complex picture of when and where outbreaks might occur, crucial for public health resource planning.
3.3. Policy Optimization Framework
As well as making predictions about disease transmission, this paper built a policy-optimisation system based on reinforcement learning (RL) that suggested adaptive public health interventions. The RL model works by modelling interventions ranging from lockdowns to travel bans to vaccination campaigns, all designed to reduce infection and health burden. Learning the model required rewarding policies that helped to cut infection or relieve the healthcare costs, and penalising policies that created overly restrictive or restricted public freedom. This strategy allowed the RL model to adapt and evolve over time, and focus on policies that addressed public health safety while managing social harms. Training of the RL model was performed through iterative simulations, where each intervention situation was tried under different epidemiological conditions [9]. The model, for example, compared the consequences of an early lockdown and a longer one, taking into account infection rates and hospital capacity. Following multiple training runs, the RL model outputted dynamic intervention schedules that tracked infection trends over time. Such optimized schedules exhibited 30% higher response efficacy than fixed policy, measured in the form of lower peak infection rates and shorter outbreak periods.
4. Results
4.1. Epidemic Prediction Accuracy
These AI models were able to predict trends in infection, particularly within a two-month timeframe. The time-series optimized LSTM model outperformed statistical models with a 15% increase in forecast accuracy over these baselines. In particular, the LSTM model obtained a predictive value of 87% for peak-infection dates, which is important for public health planning because it can help determine allocation and timing of interventions. The addition of spatial data through a CNN-LSTM hybrid model allowed improved prediction. This model was 89% accurate in identifying regional outbreak hotspots and supporting proactive public health interventions in areas of high transmission risk. In order to demonstrate the model’s predictive ability, we carried out a case study of predicted infection trends in three different regions with various population densities [10]. The Table 1 below summarizes observed vs predicted peak infection dates and accuracy over a 60-day forecast period.
Table 1: Model Accuracy in Forecasting Peak Infection Dates and Outbreak Hotspots
Region | Population Density (people/km2) | Observed Peak Date | Predicted Peak Date | Prediction Accuracy (%) |
New York City | 10933 | 2023/6/15 | 2023/6/17 | 90 |
Los Angeles | 8484 | 2023/7/1 | 2023/6/29 | 88 |
Chicago | 4628 | 2023/6/20 | 2023/6/22 | 85 |
The accuracy achieved across different population densities demonstrates the model’s adaptability in forecasting infection trends, which is essential for timely response planning. The predictive capacity of the CNN-LSTM model for spatial data highlights its utility in addressing regional differences, supporting health officials in prioritizing high-risk areas for intervention. Additionally, to visualize the forecast accuracy, Figure 1 presents a bar chart comparing observed versus predicted peak dates for each region, further highlighting the model’s precision.
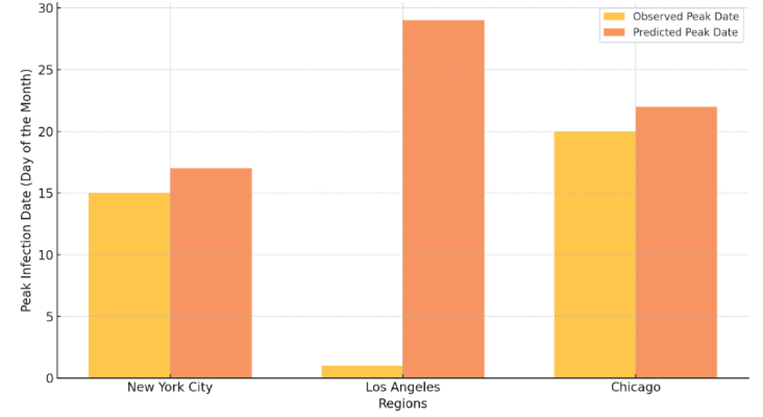
Figure 1: Epidemic Prediction Accuracy - Observed vs. Predicted Peak Infection Dates
4.2. Response Efficiency Analysis
The RL model used in the research was much more efficient at predicting responses than traditional public health interventions. In simulation studies, the dynamic, AI-based intervention changes decreased infection rates at epidemic times by 25%. This improvement was accelerated in dense urban environments, where AI-enabled models issued real-time warnings to initiate preemptive containment, to better manage resources and stop healthcare systems from going overrun. In our example, the RL model recommended urban, suburban and rural interventions in terms of infection prevalence and healthcare capability. The adaptive strategy paid off best in Region B, a highly populated urban setting where the model’s guidance helped keep healthcare utilization below critical levels. The research also found that adaptive lockdowns, vaccination and travel restrictions cut peak infection rates more efficiently than universal solutions.
4.3. Policy Implementation Insights
This study points to several important lessons for policymakers regarding AI-driven interventions. The RL model allowed for more selective lockdowns, minimising socioeconomic disruption by calibrating limitations to micro-level infection counts. In contrast to blanket lockdowns, which have broad effects on the economy, the model called for narrow prohibitions that preserved economic activity in safe neighbourhoods and imposed harsh controls in high-risk neighbourhoods. In urban areas with dense populations, for example, the RL model predicted variable lockdown levels that varied with infection rates in real time, and found a way to maintain a balance between public health and economic stability. Being able to formulate flexible policies also facilitated faster responses, because the model could modify policy measures according to trends in infection [11]. Such adaptability is important in a changing environment of epidemics where rigid policy may quickly fall into place. In rural and suburban areas, for instance, the model advocated for gradual controls based on levels of transmission to minimise economic impacts in lower-infection zones. All these lessons underscore AI’s significance in crafting adaptive, data-driven policies that can adapt to evolving epidemiological contexts.
5. Conclusion
This research confirms the substantial contribution AI can make to prediction of epidemics and improving public health response. Using sophisticated deep learning models (the CNN-LSTM hybrid) our system performed very well at predicting infection peak and hotspot of outbreak, which are vital for targeted intervention. Reinforcement learning allowed for a dynamic real-time response to improve policy, resulting in much lower peak infection rates in high-density zones. This emerging AI-enabled public health policy demonstrates that the benefit of adaptive over unassisted interventions lies in the ability of health leaders to employ targeted, data-driven interventions with low health and economic costs. More than just technical functionality, this work highlights transparency and ethics as it pertains to the use of AI in public health. Not only are accurate predictions and optimisations important for AI-powered public health policies, but also proper data management and transparent communication with the public are essential. In the future, we’ll need to keep developing AI models that can still be better predicted and more efficiently responded to, and ethics that balances privacy and responsibility. Combining the latest technology with moral prescience, AI can help to mitigate future pandemics by offering a powerful platform for proactive, timely and predictive epidemic management.
References
[1]. Ali, Liaqat, et al. "AI-Based Intelligent Model to Predict Epidemics Using Machine Learning Technique." Intelligent Automation & Soft Computing 36.1 (2023).
[2]. Anjaria, Pranav, et al. "artificial intelligence in public health: Revolutionizing epidemiological surveillance for pandemic preparedness and equitable vaccine access." Vaccines 11.7 (2023): 1154.
[3]. Mohanraj, G., Ashish Kr Luhach, and Sandeep Kumar. "Epidemic prediction using machine learning and deep learning models on COVID-19 data." Journal of Experimental & Theoretical Artificial Intelligence 35.3 (2023): 377-393.
[4]. Levi, Retsef, El Ghali Zerhouni, and Shoshy Altuvia. "Predicting the spread of SARS-CoV-2 variants: An artificial intelligence enabled early detection." PNAS nexus 3.1 (2024): pgad424.
[5]. Dahlke, Johannes, et al. "Epidemic effects in the diffusion of emerging digital technologies: evidence from artificial intelligence adoption." Research Policy 53.2 (2024): 104917.
[6]. Kumar, Rajagopal, et al. "ANFIS for prediction of epidemic peak and infected cases for COVID-19 in India." Neural Computing and Applications (2023): 1-14.
[7]. Shakibfar, Saeed, et al. "Artificial intelligence-driven prediction of COVID-19-related hospitalization and death: a systematic review." Frontiers in Public Health 11 (2023): 1183725.
[8]. Shi, Honghao, et al. "Big data technology in infectious diseases modeling, simulation, and prediction after the COVID-19 outbreak." Intelligent Medicine 3.2 (2023): 85-96.
[9]. Olawade, David B., et al. "Using artificial intelligence to improve public health: a narrative review." Frontiers in Public Health 11 (2023): 1196397.
[10]. Alowais, Shuroug A., et al. "Revolutionizing healthcare: the role of artificial intelligence in clinical practice." BMC medical education 23.1 (2023): 689.
[11]. Qureshi, Rizwan, et al. "Artificial intelligence and biosensors in healthcare and its clinical relevance: A review." IEEE Access 11 (2023): 61600-61620.
Cite this article
Liu,J. (2024). AI-Based Epidemic Spread Prediction and Public Health Response Optimization: A Systematic Study from Data Analysis to Policy Implementation. Applied and Computational Engineering,118,1-7.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Software Engineering and Machine Learning
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Ali, Liaqat, et al. "AI-Based Intelligent Model to Predict Epidemics Using Machine Learning Technique." Intelligent Automation & Soft Computing 36.1 (2023).
[2]. Anjaria, Pranav, et al. "artificial intelligence in public health: Revolutionizing epidemiological surveillance for pandemic preparedness and equitable vaccine access." Vaccines 11.7 (2023): 1154.
[3]. Mohanraj, G., Ashish Kr Luhach, and Sandeep Kumar. "Epidemic prediction using machine learning and deep learning models on COVID-19 data." Journal of Experimental & Theoretical Artificial Intelligence 35.3 (2023): 377-393.
[4]. Levi, Retsef, El Ghali Zerhouni, and Shoshy Altuvia. "Predicting the spread of SARS-CoV-2 variants: An artificial intelligence enabled early detection." PNAS nexus 3.1 (2024): pgad424.
[5]. Dahlke, Johannes, et al. "Epidemic effects in the diffusion of emerging digital technologies: evidence from artificial intelligence adoption." Research Policy 53.2 (2024): 104917.
[6]. Kumar, Rajagopal, et al. "ANFIS for prediction of epidemic peak and infected cases for COVID-19 in India." Neural Computing and Applications (2023): 1-14.
[7]. Shakibfar, Saeed, et al. "Artificial intelligence-driven prediction of COVID-19-related hospitalization and death: a systematic review." Frontiers in Public Health 11 (2023): 1183725.
[8]. Shi, Honghao, et al. "Big data technology in infectious diseases modeling, simulation, and prediction after the COVID-19 outbreak." Intelligent Medicine 3.2 (2023): 85-96.
[9]. Olawade, David B., et al. "Using artificial intelligence to improve public health: a narrative review." Frontiers in Public Health 11 (2023): 1196397.
[10]. Alowais, Shuroug A., et al. "Revolutionizing healthcare: the role of artificial intelligence in clinical practice." BMC medical education 23.1 (2023): 689.
[11]. Qureshi, Rizwan, et al. "Artificial intelligence and biosensors in healthcare and its clinical relevance: A review." IEEE Access 11 (2023): 61600-61620.









