1. Introduction
With the progress and development of society, the global energy demand continues to grow. The contradiction between the limited nature of fossil fuels and the environmental impact is becoming increasingly prominent, which promotes the exploration and utilization of clean and renewable energy in society. Energy storage technology plays a significant role in the development of renewable energy, which can mitigate the output fluctuations of renewable energy sources, enhance the peak load capacity and energy utilization efficiency of power grids, and facilitate the transition towards a more sustainable energy structure [1].
Among many energy storage technologies, flow batteries, as an emerging energy storage technology, has become a research hotspot. It has the advantages of no pollution, high efficiency, long cycle life, etc. Compared with other energy storage technologies, flow battery technology has broad development prospects [2]. Iron-chromium flow battery (ICFB) is considered as a large-scale energy storage technology with great potential due to its advantages of wide application range, low cost, low environmental impact and high flexibility [2]. However, Fe-Cr flow batteries still face some technical challenges in practical applications, including the stability of the electrolyte, the activity of the electrode material, the selectivity of the ion conductive film, and the overall efficiency of the battery system. To overcome these challenges, researchers have conducted a large amount of basic and applied research to improve battery performance and reduce costs.
In this paper, the basic working principle, key technologies, application fields, current challenges and future development direction of iron-chromium flow batteries are reviewed. By analyzing the latest research findings and market dynamics, this paper aims to provide readers with a comprehensive perspective on the role and potential of iron-chromium flow batteries in modern energy systems.
2. Basic Overview of Iron-chromium Flow Batteries
2.1. History and Development of Iron-chromium Flow Batteries
The history of the iron-chromium flow battery (ICFB) can be traced back to the 1970s, when NASA initiated research into this innovative battery technology to address the need for efficient and reliable energy storage systems in space exploration. [3] In the 1980s, Sumitomo Electric Company of Japan developed a 10 kW iron-chromium flow battery system prototype based on NASA technology, and conducted system integration and scale-up studies [4]. In the 1990s, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, began to conduct in-depth research on Fe-Cr flow batteries and successfully developed a 270-W small Fe-Cr flow battery stack [5]. However, with the increase in global demand for renewable energy and energy storage technologies, the research of iron-chromium flow batteries has received a new impetus, and researchers have begun to explore new electrolyte formulations, electrode materials, and battery designs to improve battery performance and reduce costs [6]. In the 2020s, advancements in technology and growing concerns about environmental impact have led to a renewed focus on research into iron-chromium flow batteries. Enterprises such as China State Power Investment Group have successfully developed megawatt iron-chromium flow battery energy storage systems, demonstrating their potential for practical applications [7].
The development of iron-chromium flow batteries reflects the process from proof of concept to technology maturity, driven by continuous technological innovation and market demand. Despite the technical challenges, its low cost and environmentally friendly characteristics make it an important candidate for future energy storage technologies. With further research and development, iron-chromium flow batteries are expected to play a key role in energy transition and sustainable development.
2.2. The Working Principle and Components of Iron-chromium Flow Battery
Iron-chromium flow batteries store and release energy based on the conversion of active substances between different oxidation states. As shown in Figure 1, the battery consists of two half cells, each containing an electrolyte of iron and chromium. During the charging process, iron ions are oxidized from Fe2+ to Fe3+, while chromium ions are reduced from Cr3+ to Cr2+. The discharge process is reversed, Fe3+ gains electrons and is reduced to Fe2+, while Cr2+ loses electrons and is oxidized to Cr3+. The Fe-Cr flow battery consists of an ion-conducting membrane, an electrode, a bipolar plate, a flow frame, and other components. The electrolyte is a solution containing Fe2+/Fe3+ and Cr2+/Cr3+, which is the key medium for battery energy storage. Electrodes are usually made of highly conductive and catalytically active materials that facilitate the REDOX reaction. An ion exchange membrane separates the two half cells, allowing ions to pass through while preventing the electrolyte from mixing. The bipolar plate serves as the support structure of the electrode and distributes the electrolyte flowing through the electrode.
Positive reaction as: Fe2+-e- ⇌Fe3+
Negative reaction as: Cr3++e- ⇌Cr2+
Total reaction of Fe-Cr flow cell as [8]: Fe2++Cr3+⇌Fe3++Cr2+
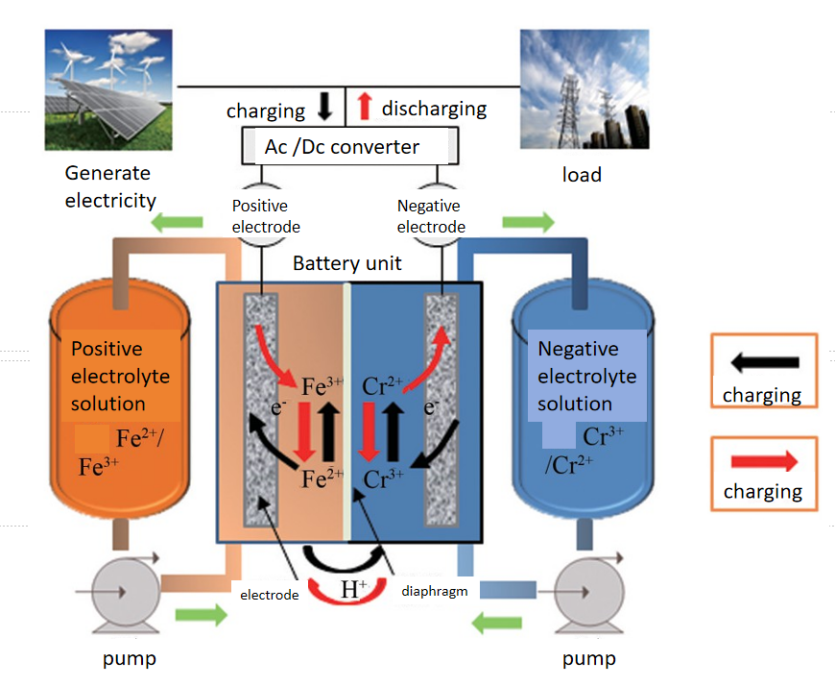
Figure 1: Working principle of iron-chromium flow battery[7]
3. The Key Technology of Iron-chromium Flow Battery
3.1. Electrode
The electrodes of iron-chromium flow batteries play a crucial role in this technology, as they provide the necessary active sites for REDOX reactions to occur with the active substances present in the electrolyte. The ideal electrode material should possess the characteristics of high electronic conductivity, high activity, high stability, high wettability and high specific surface area.
Commonly used carbon electrode materials include a variety of forms, mainly carbon felt, graphite felt, carbon cloth and carbon paper. On the one hand, carbon and graphite felt are made through a textile process, and they have a porous three-dimensional matrix of fibers, which provides a rich channel for the penetration and flow of electrolytes. Carbon paper, on the other hand, is made by mixing short-cut carbon fiber with an organic binder and subsequently undergoing a carbonization and curing process. Its surface is relatively flat, its thickness is similar to that of paper, and its internal structure is compact and porous, which gives it uniform electrolyte diffusion characteristics in electrode applications [9].
Ren et al. designed a novel electrode that uses cobalt oxide for surface modification of graphite felt [10]. The cobalt oxide is uniformly dispersed and attached to the fiber structure of the graphite felt, which significantly increases the number of oxygen-containing functional groups on the surface of the graphite felt and introduces numerous electrochemically active sites. Thanks to these improvements, the overall performance of the electrode has been significantly improved. When tested at a current density of 140 mA/cm², the modified electrode demonstrated an 85% higher charging capacity than untreated graphite felt. In addition, Ahn et al. successfully developed a composite catalyst composed of Kochen carbon (KB) and bismuth nanoparticles, which has excellent bifunctional catalytic performance[11]. In experiments, the catalyst achieved an energy efficiency of 86.4% when the current density was set to 80 mA/cm². This study confirms that the combined use of bismuth metal with carbon-based materials can effectively improve the overall performance of iron-chromium flow batteries. In addition, Zhou Yang et al. successfully prepared a bismuth-based organic skeleton (Bi-MOF/CC) with Bi-MOF as a precursor on carbon cloth substrate by using hydrothermal synthesis technology, and then produced bismuth-based carbon cloth (C-Bi/CC) electrode [12]. The impact of adding metal salt on electrode performance was thoroughly investigated, leading to the optimization of the electrode's performance. The experimental results indicate that when the amount of metal salt is 90 mg, the polarization resistance of the electrode is reduced to 1.069Ω, which is 8.5% lower than that of the original carbon cloth, and the reduction overpotential of Cr3+ is reduced to 0.25V.
3.2. Electrolyte
The electrolyte of the iron-chromium flow battery is a crucial component of this technology, which contains a solution of iron and chromium ions, which directly affects the performance of the battery.
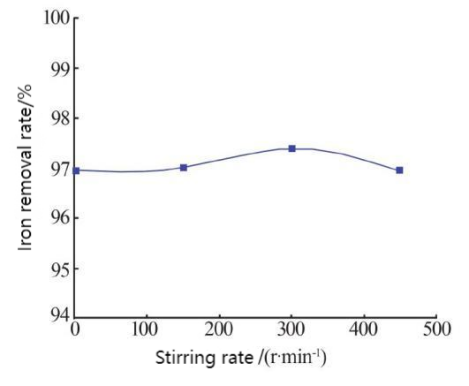
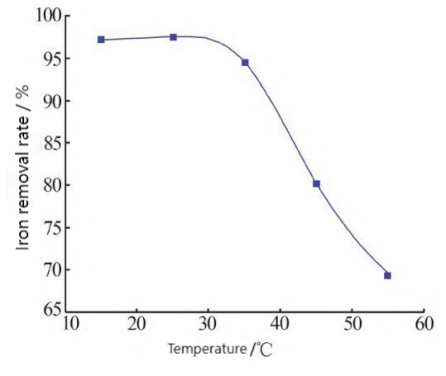
In order to comprehensively utilize the electrolyte of iron-chromium flow battery, Li Shuqi et al. studied the use of oxalic acid precipitation to remove iron ions in a trivalent chromium system, and analyzed the effects of oxalic acid addition amount, solution pH value, reaction temperature, stirring speed, etc., on the iron removal rate [13]. Finally, when the amount of oxalic acid added is 1.2 times the theoretical value, the solution pH value is 3.0, and the reaction temperature is 25 degrees Celsius (Figure 2b), the iron removal rate can reach more than 97%, as shown in Figure 2a. The stirring rate has little effect on the iron removal rate, indicating that the oxalic acid precipitation method is an effective way to comprehensively utilize the electrolyte.
(a) |
(b) |
Figure 2: The effect of iron removal rate; (a) Iron removal rate and stirring rate; (b) Iron removal rate and temperature
Wang et al. conducted experimental research on the utilization of the same electrolyte for both the positive and negative electrode materials in iron-chromium flow batteries [14]. The study investigated the impact of varying concentrations of iron ions, chromium ions, and hydrochloric acid on the conductivity, viscosity, and electrochemical behavior of the electrolyte. After a series of experimental analyses, they found that the overall performance of the electrolyte was optimal when the electrolyte was composed of 1.0 moles per liter of ferrous chloride (FeCl), 1.0 moles per liter of chromium chloride (CrCl) and 3.0 moles per liter of hydrochloric acid (HCl). At this specific ratio, the electrolyte exhibits the highest conductivity, the best electrochemical activity and the best transmission performance, and the best synergistic effect of performance is achieved.
3.3. Ion Exchange Membrane
The ion-exchange membrane in iron-chromium flow batteries is a key component to realize the isolation of the positive and negative electrolytes of the battery, which allows the passage of carriers such as protons (H+) to maintain the closed circuit of the battery while preventing cross-contamination of the active substances in the electrolyte. The ideal ion exchange membrane should have several key characteristics: it needs to be able to efficiently screen different ions to ensure isolation between electrolytes, while ensuring rapid ion transport to maintain the high conductivity of the battery [15]. In addition, the film should also possess strong mechanical durability to withstand various physical stresses during the use of the battery, and should also demonstrate good chemical stability to resist the erosion of chemical substances in the electrolyte to ensure long-term reliability [16]. In short, an excellent ion exchange membrane is a key factor in battery performance and longevity.
Ion-exchange membranes exhibit varying properties based on their thickness. Scientists have carried out performance tests on ion-exchange membranes of various thicknesses and have identified the characteristics and properties associated with each thickness. Sun et al. conducted a series of performance tests for Nafion series ion exchange membranes of different thicknesses, specifically Nafion212 (50 microns), Nafion115 (126 microns) and Nafion117 (178 microns) [17]. These tests include key parameters such as ion exchange capacity, proton conductivity and ion permeability. Their goal was to assess the specific effects of these membranes on the performance of iron-chromium flow batteries. Their research revealed that thicker membranes, while more effective at preventing the diffusion of ions and reducing the mixing of active substances, also resulted in decreased voltage efficiency due to increased membrane resistance. In contrast, the thinner Nafion212 film exhibits better voltage and energy efficiency at current densities between 40 and 120 mA/cm2 due to its lower resistance. Therefore, from the dual point of view of cost and performance, the Nafion212 membrane is considered to be the most suitable option for iron chromium flow batteries among the three membranes.
4. Application of iron-chromium flow battery
For power systems, there are many application scenarios for energy storage, which can be specifically divided into power generation measurement energy storage, grid side energy storage and user side energy storage, but the most suitable energy storage mode should be selected in practical application [18].
Specifically, taking the iron-chromium flow battery energy storage demonstration project of State Power Investment Group as an example. As shown in Table 1, the project has been put into trial operation in Zhanshigou photovoltaic power Station, Guyuan County, Zhangjiakou City, Hebei Province, demonstrating the advantages of iron-chromium flow batteries in long-term energy storage scenarios, and their ability to effectively adapt to adverse effects of high temperature and cold weather. Furthermore, it exhibits significant safety performance [7].
Table 1: Major energy storage application scenarios for power systems
Scope of Application | Type of application | Frequency/year | Charging and discharging time (per application) |
Generation-side | Thermal power unit peaking | 300 | 4 - 8h |
Thermal power unit frequency regulation | >5,000 | 15min - 2h | |
New Energy Smoothing | >5,000 | 15min - 1h | |
New energy power quality improvement | 50 | S class - 15min | |
New energy follow group plan output | 300 | 0.5 - 0.4h | |
New Energy Peaking | 300 | 0.5 - 0.4h | |
Grid-side | Grid-side FM | >5,000 | 15min - 2h |
Grid-side peaking | 100 | 4 - 16h | |
Delayed capacity expansion of transmission and distribution equipment | 300 | 4 - 8h | |
Black start | 1-10 | 1h | |
User-side | Peak and valley arbitrage and capacity management | 300 | 4 - 8h |
Power quality improvement | 100 | S class - 30min | |
Backup power | 1-10 | 4 - 8h |
In addition, the large-scale application of iron-chromium flow battery technology is of great significance for promoting the green transformation of energy, ensuring energy security, promoting the high-quality development of clean energy, and combating climate change. Finally, iron-chromium flow batteries can also be used in industrial energy storage, optical storage systems, and commercial and residential energy storage [18-19].
5. Conclusion
Iron-chromium flow batteries, with their advantages of low cost, long life and high safety, show great potential in promoting global energy transformation and upgrading. Despite technical challenges such as electrolyte stability, electrode performance and ion conductive film selectivity, recent advances in material modification and battery design have laid a solid foundation for improving performance and reducing costs. From renewable energy connected to smart microgrids, from peak-valley price arbitrage to backup power systems, iron-chromium flow batteries have broad application prospects and are expected to occupy an important position in the future energy storage market. Continuous research, policy support and market drive will further promote its commercialization and industrialization, and provide strategic support for achieving carbon reduction targets and optimizing the energy structure. For the optimization of electrode materials, the performance of electrode materials can be improved through surface modification, the use of suitable catalysts, and hydrothermal synthesis technology. In terms of the improvement of the electrolyte, scientists greatly improved the comprehensive utilization efficiency of the electrolyte by using the oxalic acid precipitation method, and obtained the best ratio by changing the different ratios of the electrolyte, so that the conductivity, activity and transmission efficiency of the electrolyte reached the best. However, there are still some limitations in this paper, such as the lack of empirical research on the performance and efficiency of iron-chromium flow batteries in practical applications, which may affect the comprehensive evaluation of the practical feasibility of the technology. Although the key technical parameters of the Fe-Cr flow battery are given in this paper, it may not cover all the factors that affect the performance of the battery, such as environmental adaptability and long-term stability. Therefore, in order to overcome these limitations, future research can include more extensive empirical research, more comprehensive technical parameter analysis, and specific strategies to solve technical challenges, so as to enhance their research capabilities and make greater contributions to the development and application of iron-chromium flow battery technology.
With the continuous progress of technology, iron-chromium flow batteries are expected to play a more critical role in the global energy transition, serving as an important bridge connecting clean energy and future power systems.
References
[1]. Gong L., Yang Y., Zhao X., Xie Y., & Jiang H. Analysis and Outlook of Global Electrochemical Energy Storage Market. Water Power. 2024, 1-5.
[2]. Yang, P. & Zeng, Y. (2024).Progress in the Optimization of Electrode Modification for Ferrochromium Liquid Flow Batteries. Refrigeration and Air Conditioning, 2024, 38(03), 351-359+393.
[3]. THALLER L. Electrically rechargeable REDOX flow cell[M].(NASA TM X-71540)1974.
[4]. Lin, Z, Jiang, Z. Research progress of Fe-Cr redox-flow battery in Japan- 1. Development progress ofFe-Cr redox-flow battery[J]. Chinese Journal of Power Sources, 1991 (2):32-39.
[5]. Yi B., Liang B., Zhang E. & Wu L. IRON/CHROMIUM REDOX FLOW CELL SYSTEM[J].CIESC Journal, 1992, (03):330-336.
[6]. Gui, Z., Wang, X., Chen, B. & Zhang, S. Research Status of Electrochemical Energy Storage Technology. Journal of Luoyang Institute of Science and Technology (Natural Science Edition) 2024, (01), 1-6+91.
[7]. Yang L., Wang H., Li X., Zhao Z., Zuo Y., Liu Y., & Liu Y.Introduction and engineering case analysis of 250 kW/1.5 MW·h iron-chromium redox flow batteries energy storage demonstrationpower station[J]. Energy Storage Science and Technology, 2020, 9(03):751-756. DOI:10.19799/j.cnki.2095-4239.2020.0046.
[8]. Zhu, H., Xie, X, Bai, E., Xu, C., & Wu, S. Study of iron-chromium redox flow battery and modification of its electrode[J]. Chinese Journal of Power Sources, 2023, 47(11):1394-1398.
[9]. Zhang, H., Zeng, Y., Yuan, Y. & Zhou, J. Research Progress on Key Materials of the Iron-chromium Flow Battery. Refrigeration & Air Conditioning. 2023, (01), 129-136.
[10]. Jiang Y. Cheng, G., Li, Y., He, Z., Zhu, J., Meng, W., Dai, L., & Wang, L.. Promoting vanadium redox flow battery performance by ultra-uniform ZrO2@C from metal-organic framework. Chemical Engineering Journal, 2021, 415.
[11]. Ahn Yeonjoo et al. (2020). High-performance bifunctional electrocatalyst for iron-chromium redox flow batteries. Chemical Engineering Journal, 421(P2), pp. 127855-.
[12]. Zhou, Y., Han, P., Niu, Y., , Xu, C. &Xu, Q. Fabrication of metal-organic framework-derived C-Bi/CC electrode materials and their electrochemical properties in ICRFB. Energy Storage Science and Technology, 2024, (02), 381-389.
[13]. Li, S., Li, X., Feng, H., Li, B., Niu, Z., & Dong, Y. Study on separation of iron from chromium trichloride solution.Inorganic Chemicals Industry, 2023, (08), 91-94+101. doi:10.19964/j.issn.1006-4990.2022-0614.
[14]. WANG S L, XU Z Y, WU X L, et al.Analyses and optimization of electrolyte concentration on the electrochemical performance of iron-chromium flow battery[J].Applied Energy, 2020, 271:115252.
[15]. https://www.westlake.edu.cn/academics/School_of_Science/newsevents/news/202403/t20240324_38091.shtml
[16]. Liu, X., Xie, N., Xue, J., Li, M., Zheng, C., Zhang, J., Qin, Y., Yin, Y., Dekel, D. R. & Guiver, M. D. (2022). Magnetic-field-oriented mixed-valence-stabilized ferrocenium anion-exchange membranes for fuel cells. Nature Energy, 7(4), 329–339. https://doi.org/10.1038/s41560-022-00978-y
[17]. Sun C.Y., Zhang H. Investigation of Nafion series membranes on the performance of iron-chromium redox flow battery[J]. International Journal of Energy Research, 2019, 43(14) :8739-8752.
[18]. Song, Z., Zhang, B., Tong, B., Zhong, Y. & Kang, M. Commercialization progress of flow battery and its application prospects in electric power system. Thermal Power Generatio, 2022, 51(03), 9-20. doi:10.19666/j.rlfd.202111209.
[19]. Guo, X., Yu, H., Zheng, X., Liu, Y., Zuo, Y., & Zhang, M. Optimal configuration of liquid flow battery energy storage in photovoltaic system, Energy Storage Science and Technology, 2023, 12(04):1158-1167. DOI:10.19799/j.cnki.2095-4239.2022.0707.
Cite this article
Huang,M. (2025). Application and Future Development of Iron-chromium Flow Batteries. Applied and Computational Engineering,123,24-30.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Gong L., Yang Y., Zhao X., Xie Y., & Jiang H. Analysis and Outlook of Global Electrochemical Energy Storage Market. Water Power. 2024, 1-5.
[2]. Yang, P. & Zeng, Y. (2024).Progress in the Optimization of Electrode Modification for Ferrochromium Liquid Flow Batteries. Refrigeration and Air Conditioning, 2024, 38(03), 351-359+393.
[3]. THALLER L. Electrically rechargeable REDOX flow cell[M].(NASA TM X-71540)1974.
[4]. Lin, Z, Jiang, Z. Research progress of Fe-Cr redox-flow battery in Japan- 1. Development progress ofFe-Cr redox-flow battery[J]. Chinese Journal of Power Sources, 1991 (2):32-39.
[5]. Yi B., Liang B., Zhang E. & Wu L. IRON/CHROMIUM REDOX FLOW CELL SYSTEM[J].CIESC Journal, 1992, (03):330-336.
[6]. Gui, Z., Wang, X., Chen, B. & Zhang, S. Research Status of Electrochemical Energy Storage Technology. Journal of Luoyang Institute of Science and Technology (Natural Science Edition) 2024, (01), 1-6+91.
[7]. Yang L., Wang H., Li X., Zhao Z., Zuo Y., Liu Y., & Liu Y.Introduction and engineering case analysis of 250 kW/1.5 MW·h iron-chromium redox flow batteries energy storage demonstrationpower station[J]. Energy Storage Science and Technology, 2020, 9(03):751-756. DOI:10.19799/j.cnki.2095-4239.2020.0046.
[8]. Zhu, H., Xie, X, Bai, E., Xu, C., & Wu, S. Study of iron-chromium redox flow battery and modification of its electrode[J]. Chinese Journal of Power Sources, 2023, 47(11):1394-1398.
[9]. Zhang, H., Zeng, Y., Yuan, Y. & Zhou, J. Research Progress on Key Materials of the Iron-chromium Flow Battery. Refrigeration & Air Conditioning. 2023, (01), 129-136.
[10]. Jiang Y. Cheng, G., Li, Y., He, Z., Zhu, J., Meng, W., Dai, L., & Wang, L.. Promoting vanadium redox flow battery performance by ultra-uniform ZrO2@C from metal-organic framework. Chemical Engineering Journal, 2021, 415.
[11]. Ahn Yeonjoo et al. (2020). High-performance bifunctional electrocatalyst for iron-chromium redox flow batteries. Chemical Engineering Journal, 421(P2), pp. 127855-.
[12]. Zhou, Y., Han, P., Niu, Y., , Xu, C. &Xu, Q. Fabrication of metal-organic framework-derived C-Bi/CC electrode materials and their electrochemical properties in ICRFB. Energy Storage Science and Technology, 2024, (02), 381-389.
[13]. Li, S., Li, X., Feng, H., Li, B., Niu, Z., & Dong, Y. Study on separation of iron from chromium trichloride solution.Inorganic Chemicals Industry, 2023, (08), 91-94+101. doi:10.19964/j.issn.1006-4990.2022-0614.
[14]. WANG S L, XU Z Y, WU X L, et al.Analyses and optimization of electrolyte concentration on the electrochemical performance of iron-chromium flow battery[J].Applied Energy, 2020, 271:115252.
[15]. https://www.westlake.edu.cn/academics/School_of_Science/newsevents/news/202403/t20240324_38091.shtml
[16]. Liu, X., Xie, N., Xue, J., Li, M., Zheng, C., Zhang, J., Qin, Y., Yin, Y., Dekel, D. R. & Guiver, M. D. (2022). Magnetic-field-oriented mixed-valence-stabilized ferrocenium anion-exchange membranes for fuel cells. Nature Energy, 7(4), 329–339. https://doi.org/10.1038/s41560-022-00978-y
[17]. Sun C.Y., Zhang H. Investigation of Nafion series membranes on the performance of iron-chromium redox flow battery[J]. International Journal of Energy Research, 2019, 43(14) :8739-8752.
[18]. Song, Z., Zhang, B., Tong, B., Zhong, Y. & Kang, M. Commercialization progress of flow battery and its application prospects in electric power system. Thermal Power Generatio, 2022, 51(03), 9-20. doi:10.19666/j.rlfd.202111209.
[19]. Guo, X., Yu, H., Zheng, X., Liu, Y., Zuo, Y., & Zhang, M. Optimal configuration of liquid flow battery energy storage in photovoltaic system, Energy Storage Science and Technology, 2023, 12(04):1158-1167. DOI:10.19799/j.cnki.2095-4239.2022.0707.