1. Introduction
Nowadays, the use of fossil fuels causes serious environmental issues, such as climate change, with global temperatures rising by about 1 degree since the start of industrial development [1]. Daily transportation also consumes large amounts of fossil fuels, making the development of alternative green fuels increasingly significant, for example, biodiesel [2]. There are four main pathways for synthesizing biodiesel: using and blending raw oils directly, micro-emulsions, pyrolysis, and transesterification [3]. However, when considering production difficulties and costs, transesterification is the most commonly used and the most efficient method for producing biodiesel. Transesterification involves synthesizing esters and glycerol through a reaction between oil and alcohol [4]. For the synthesis of Biodiesel, the triglycerides from the waste vegetable oil are used with methanol or other alcohols within the presence of catalysts [5]. Then the triglycerides form three fatty acids and undergo deoxygenation to get the fuel-like hydrocarbons which is biodiesel [2]. The main effect of the efficiency of transesterification is the use of catalysts. Since catalytic reactions are more economical, mild reaction conditions are preferred. Also, the main three types of catalysts are heterogeneous, homogeneous catalysts, and biocatalysts. The comparison between these catalysts is required and some developments in the synthesis , such as ultrasonication can help make the process even more efficient [5],[6].
2. Homogenous catalyst
2.1. Homogeneous catalysts reaction mechanisms
Homogenous catalysts are those that exist in the same phase as the reaction system; for biodiesel synthesis, they are typically in an aqueous form [7]. This type of catalyst contains both acidic and alkaline catalysts, and the mechanism for the synthesizing biodiesel from vegetable oil with methanol is illustrated in Figures 1 and 2 [8].
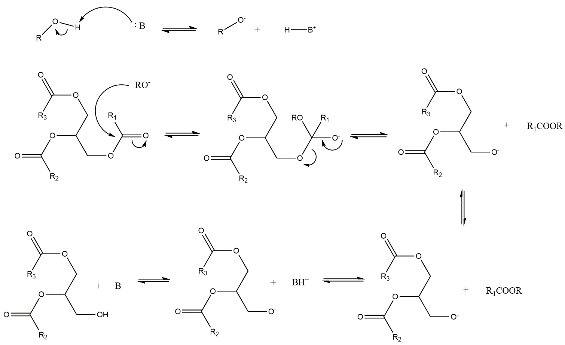
Figure 1: From ChemDraw, the reaction mechanism of the base-catalyzed transesterification of vegetable oil with methanol [1].
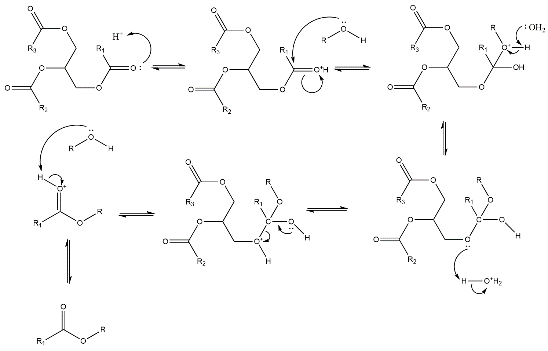
Figure 2: From ChemDraw, the acid catalysis reaction mechanism of the transesterification of vegetable oil with methanol and the R represents the methyl group [2].
In an alkaline-catalyzed reaction, the base(such as NaOH, KOH) first deprotonates the methanol to produce the catalytically active species \( {RO^{-}} \) group [8]. This is followed by a nucleophilic attack by \( {RO^{-}} \) on the partially positive carbonyl group. In acid-catalyzed reactions, the vegetable oil acts as the base to attacks the proton from acids, usually Brønsted acids, such as sulfuric acids or hydrochloric acid [7]. The rest of the steps are similar to the traditional acid-catalyzed reaction. Both acid and base-catalyzed can produce a high yield (about 99.7%) of biodiesel [8]. Between acid and base-catalyzed reactions, base-catalyzed reaction has fewer steps and milder conditions which leads to a kinetic product. So, the use of base catalysts is more common in industrial production. Although the alkaline catalysts exhibit high catalytic activity, resulting in faster reaction speeds, they have drawbacks, such as difficulties in catalyst recovery [3]. Moreover, the acid homogeneous catalysts have slower reaction rates, but can produce higher yields [7]. On the other hand, the alkaline homogeneous catalysts like sodium hydroxide are more preferred and widely used, though saponification might occur between the formed fatty acids and water[5] [9]. As after the three fatty acids are separated from the triglycerides, glycerol is formed, and the separation between the glycerol and the fatty acid can be hindered by the sponification[7]. The main advantage of using homogeneous catalysts is in conversations from low free fatty acid(less than 2%) with high water-containing feedstock to biodiesel [7],[9]. In addition, the last step for the formation of is deoxygenation of fatty acid. At this step, the use of transition metal homogeneous catalysts to synthesize the biodiesel is an emerging pathway [2].
2.2. Radical-based reactions and transition metal-catalyzed reactions
The radical-based reaction shows great activity in the decarboxylation process. It uses the transition metal with carboxylic acid to undergo oxidative decarboxylation. For example, in radical-based reaction involving \( {AgNO_{3}} \) and \( {Na_{2}}{S_{2}}{O_{8}} \) , the oxidation state of Ag changes from +1 to +2, while \( {{S_{2}}{O_{8}}^{2-}} \) is converted to \( {{SO_{4}}^{2-}} \) . This reaction only need 20 minutes with only 78 degrees to give a high mole ratio product [2]. Due to the presence of side reactions, the final yield for the produce is around 10% to 50%, indicating room for improvement.2 Transition metal homogeneous catalysts are highly effective in the decarbonylation step and are usually used as the industrial homogeneous catalysts [2]. With their excellent catalytic activity and selectivity, these reactions typically achieve very high yields (around 90%) [2].
3. Heterogeneous catalysts
Heterogeneous catalysts exist in a different phase than the reactants. Their mechanism is similar to that of homogeneous catalysts, and they include both acid and base catalysts, as well as metal-based catalysts. The main advantage of using these heterogeneous catalysts is that they are easy to separate and recycle, and the purification process is quite easy [7]. Also, they can help to prevent saponification and allow the reaction to occur under mild conditions [7]. One of the key benefits of this type of catalyst is the availability of metal-based catalysts, which are commonly used in transesterification and can be sourced from everyday waste. For example, eggshells and marble waste powder can provide \( CaO \) , one of the most efficient alkaline catalysts. As a result, developments for the use of this daily waste can be a great improvement in sustainable life.
3.1. Eggshell, marble waste powder, and doped metals catalysts
The preparation of eggshell catalysts with high catalytic efficiency involves using milled, water-washed green free-range eggshells, ground to a size of 0.063mm, followed by thermal treatment for 4 hours at 700 degrees. The catalysts are then characterized by X-ray diffraction (XRD) [4],[10]. Thermal treatment is required because it increases the surface area of the oxides forms [10]. However, the total surface area has only a small impact on catalytic efficiency; the main effect is the basicity of the basic sites, which is different among eggshells —hence the choice of green eggshells [10]. These catalysts exhibit similar conditions to standard alkaline heterogeneous catalysts, with a catalyst loading of 3% wt, a methanol: oil molar ratio 9:1 and a reaction time of 2 hours at 65 degrees [8],[10].
The use of marble waste powder as the raw feedstock is similar to the eggshells as the main content of both of them are calcium oxide. The main difference is that the marble waste powder contains \( MgO \) and \( {SiO_{2}} \) , which are acidic impurities that reduce the activity of catalyst by producing water and leading to the saponification [6]. This highlights the importance of CaO's basic sites in determining catalyst activity.
Doped metals catalysts represent another advanced type of alkaline heterogeneous catalysts for synthesis of biodiesel. Their activity depends on the types of metal doping, the mole ratio of the components, and the calcination temperature. For example, the activity of Li/NaY zeolite catalyst is influenced by the mole ratio of \( {LiCO_{3}} \) and \( NaY \) . Increasing the \( {LiCO_{3}} \) content enhances the overall basicity of the catalysts, but this effect plateaus when the \( {LiCO_{3}} \) to \( NaY \) mole ratio reaches 3:1 , possibly due to excessive lithium doping which covers the specific surface area [11]. The optimal reaction time for Li/NaY zeolite catalyst is 2 hours, with a mole ratio of 1:1, as indicated by the FAEE yield [11]. Also, this illustrates the key difference between metal oxide catalysts and doped metal catalysts.
3.2. Discussion about other factors
To optimize biodiesel synthesis, several factors need to be considered, including the alcohol-to-oil ratio, catalyst concentration, reaction time, reaction temperature, reaction kinetics, types of oils, and types of catalysts. Data from response surface plots and contour plots show that higher temperatures and alcohol-to-oil ratio intensify the reaction. However, temperatures higher than 68 degrees cause methanol to evaporate, and if the ratio is higher than 1:11 the saponification will occur. On the other hand, a ratio that's too low slows the reaction. Since transesterification is reversible, a higher ratio is needed for efficiency [4],[11]. So, the best temperature for the reaction is 65 degrees with an alcohol-to-oil ratio of 1:8 [4]. Additionally, with a fixed temperature and methanol-to-oil ratio, catalyst concentration and reaction time are inversely proportional [4]. However, longer reactions increase the risk of side reactions. As a result, the optimal reaction time is 90 minutes, and the catalysts concentration should be at least 1 mg/L for economic efficiency [4]. Also, for the preparation of metal oxide catalysts, the calcination temperature is very important as it affects their morphology and is proportional to the yield of biodiesel as the temperature change also has effect on the total number of basic sites which is related to the activity of the alkaline catalysts [4],[11]. This is due to the decomposition of \( {CaCO_{3}} \) to \( {CO_{2}} \) and \( CaO \) , with only the \( CaO \) acting as the catalyst. The amount of the usable \( CaO \) determines the activity of the catalyst , which can be detected through X-Ray diffraction (XRD) analysis and \( {CO_{2}} \) temperature programmed desorption( \( {CO_{2}} \) -TPD) [6],[10]. For example, the yield is 90% at 700 degrees, the yield is 91% at 800 degrees, and the yield is 93% at 900 degrees [4],[10]. However, if the temperature is too high, some basic sites might be inactivated so that with the consideration of economic efficiency for the industrial production, 700 degrees is preferred [11]. In terms of reaction kinetics, alkaline-catalyzed reactions are faster than the metal oxide-catalyzed reaction, though both of them are effective for catalyzing the production of biodiesel [4]. The final yield for the product can also be affected by the type of waste cooking oils used. After comparison, the soybean and sunflower oils lead to a higher yield due to their higher percentage of palmitic acid and lower amounts of palmitoleic acid [10]. When choosing suitable catalysts for the reaction, a comprehensive comparison is required. Although homogeneous catalysts have a serious problem in the separation step, it can be solved by conducting the reaction in a biphasic medium, which means the products are in the organic layer and the catalyst is in the aqueous layer [2]. Also, saponification can be solved not only by controlling the oil to alcohol ratio, but also be solved by warming the triglycerides which can reduce the water contained [4]. Furthermore, using ultrasonication instead of traditional stirring for \( CaO \) catalyzed reaction can reduce energy by 1.5 times while maintaining production of biodiesel [6]. It is because the ultrasonication method can increase the rate of catalytic reaction between methanol and \( CaO \) which enhances the production of \( {CH_{3}}{O^{-}} \) ions [6]. Also for other catalysts, conditions may vary. For example, with a Li/NaY zeolite catalyst, the best reaction time is 2 hours, but the overall relationships among factors remain consistent, which means the best alcohol-to-oil ratio increases to 1:18, and the catalyst concentration should be 3 wt% [11].
4. Conclusion
In conclusion, even the homogeneous catalysts can produce a high yield, they are less convenient than heterogeneous catalysts because they require additional separation and purification steps, as they can dissolve in glycerol and biodiesel [5][7]. This presents a challenge that needs to be addressed. As a result, discovering new homogeneous catalysts is quite significant. Moreover, in terms of convenience and raw material availability, heterogeneous catalysts are often preferred as their feedstocks are easily to obtain. Both two types have their specific advantages and drawbacks. So, developing new catalytic materials — beyond just heterogeneous and homogeneous catalysts, but also biocatalysts and nanocatalysts — will be crucial in the future, as many other factors have already been optimized [7]. Additionally, efforts to develop metal oxide catalysts from common waste, which perform as efficiently as laboratory-grade catalysts, are ongoing. So, developments for other methods to remove the impurities in the raw feedstocks are also desirable.
Highly active and selective heterogeneous catalysts
Finding new doped metal catalysts, developing new homogeneous alkaline catalysts, or using other types of daily waste as raw materials for catalysts could contribute to sustainable development. Moreover, identifying highly active and selective heterogeneous catalysts that offer both high efficiency and cost-effectiveness for industrial production is another area that requires further research [7]. Discovering methods like ultrasonication and cavitation, which can support the synthesis of biodiesel is also very significant [6].
References
[1]. Gani, A. Fossil fuel energy and environmental performance in an extended STIRPAT model. Journal of Cleaner Production 2021, 297, 126526.
[2]. Tabandeh, M.; Cheng, C. K.; Centi, G.; Show, P. L.; Chen, W.-H.; Ling, T. C.; Ong, H. C.; Ng, E.-P.; Juan, J. C.; Lam, S. S. Recent advancement in deoxygenation of fatty acids via homogeneous catalysis for biofuel production. Molecular Catalysis 2022, 523, 111207.
[3]. Nayab, R.; Imran, M.; Ramzan, M.; Tariq, M.; Taj, M. B.; Akhtar, M. N.; Iqbal, H. M. Sustainable biodiesel production via catalytic and non-catalytic transesterification of feedstock materials–A review. Fuel 2022, 328, 125254.
[4]. Sree, J. V.; Chowdary, B. A.; Kumar, K. S.; Anbazhagan, M. P.; Subramanian, S. Optimization of the biodiesel production from waste cooking oil using homogeneous catalyst and heterogeneous catalysts. Materials Today: Proceedings 2021, 46, 4900-4908.
[5]. Wang, B.; Wang, B.; Shukla, S. K.; Wang, R. Enabling catalysts for biodiesel production via transesterification. Catalysts 2023, 13 (4), 740.
[6]. Bargole, S. S.; Singh, P. K.; George, S.; Saharan, V. K. Valorisation of low fatty acid content waste cooking oil into biodiesel through transesterification using a basic heterogeneous calcium-based catalyst. Biomass and Bioenergy 2021, 146, 105984.
[7]. Rizwanul Fattah, I.; Ong, H.; Mahlia, T.; Mofijur, M.; Silitonga, A.; Rahman, S. A.; Ahmad, A. State of the art of catalysts for biodiesel production. Frontiers in Energy Research 2020, 8, 101.
[8]. Maleki, B.; Talesh, S. A.; Mansouri, M. Comparison of catalysts types performance in the generation of sustainable biodiesel via transesterification of various oil sources: A review study. Materials Today Sustainability 2022, 18, 100157.
[9]. Okechukwu, O. D.; Joseph, E.; Nonso, U. C.; Kenechi, N.-O. Improving heterogeneous catalysis for biodiesel production process. Cleaner Chemical Engineering 2022, 3, 100038.
[10]. Graziottin, P. L.; Rosset, M.; dos Santos Lima, D.; Perez-Lopez, O. W. Transesterification of different vegetable oils using eggshells from various sources as catalyst. Vibrational Spectroscopy 2020, 109, 103087.
[11]. Li, Z.; Ding, S.; Chen, C.; Qu, S.; Du, L.; Lu, J.; Ding, J. Recyclable Li/NaY zeolite as a heterogeneous alkaline catalyst for biodiesel production: Process optimization and kinetics study. Energy Conversion and Management 2019, 192, 335-345.
Cite this article
Yang,S. (2025). Comparison among Main Factors for Biodiesel via Transesterification of Vegetable Oil. Applied and Computational Engineering,123,54-58.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Gani, A. Fossil fuel energy and environmental performance in an extended STIRPAT model. Journal of Cleaner Production 2021, 297, 126526.
[2]. Tabandeh, M.; Cheng, C. K.; Centi, G.; Show, P. L.; Chen, W.-H.; Ling, T. C.; Ong, H. C.; Ng, E.-P.; Juan, J. C.; Lam, S. S. Recent advancement in deoxygenation of fatty acids via homogeneous catalysis for biofuel production. Molecular Catalysis 2022, 523, 111207.
[3]. Nayab, R.; Imran, M.; Ramzan, M.; Tariq, M.; Taj, M. B.; Akhtar, M. N.; Iqbal, H. M. Sustainable biodiesel production via catalytic and non-catalytic transesterification of feedstock materials–A review. Fuel 2022, 328, 125254.
[4]. Sree, J. V.; Chowdary, B. A.; Kumar, K. S.; Anbazhagan, M. P.; Subramanian, S. Optimization of the biodiesel production from waste cooking oil using homogeneous catalyst and heterogeneous catalysts. Materials Today: Proceedings 2021, 46, 4900-4908.
[5]. Wang, B.; Wang, B.; Shukla, S. K.; Wang, R. Enabling catalysts for biodiesel production via transesterification. Catalysts 2023, 13 (4), 740.
[6]. Bargole, S. S.; Singh, P. K.; George, S.; Saharan, V. K. Valorisation of low fatty acid content waste cooking oil into biodiesel through transesterification using a basic heterogeneous calcium-based catalyst. Biomass and Bioenergy 2021, 146, 105984.
[7]. Rizwanul Fattah, I.; Ong, H.; Mahlia, T.; Mofijur, M.; Silitonga, A.; Rahman, S. A.; Ahmad, A. State of the art of catalysts for biodiesel production. Frontiers in Energy Research 2020, 8, 101.
[8]. Maleki, B.; Talesh, S. A.; Mansouri, M. Comparison of catalysts types performance in the generation of sustainable biodiesel via transesterification of various oil sources: A review study. Materials Today Sustainability 2022, 18, 100157.
[9]. Okechukwu, O. D.; Joseph, E.; Nonso, U. C.; Kenechi, N.-O. Improving heterogeneous catalysis for biodiesel production process. Cleaner Chemical Engineering 2022, 3, 100038.
[10]. Graziottin, P. L.; Rosset, M.; dos Santos Lima, D.; Perez-Lopez, O. W. Transesterification of different vegetable oils using eggshells from various sources as catalyst. Vibrational Spectroscopy 2020, 109, 103087.
[11]. Li, Z.; Ding, S.; Chen, C.; Qu, S.; Du, L.; Lu, J.; Ding, J. Recyclable Li/NaY zeolite as a heterogeneous alkaline catalyst for biodiesel production: Process optimization and kinetics study. Energy Conversion and Management 2019, 192, 335-345.









