1. Introduction
The reliance on fossil fuels is attributed to severe climate challenges, notably on air pollution, environmental degradation in mining areas, and also excessive greenhouse gas release of carbon dioxide, which is believed to be the criminal for the global warming. So scientists are turning to renewable energy, like solar, wind, and hydro power. Biomass, recognized as one of the most primitive and extensively studied forms of renewable energy, serves as a readily available feedstock that garners global interest, particularly with biodiesel being a preferred option. [1]. Although this reaction can happen without catalysts, still to be viable in industrial practice and the core part of the reaction, catalysts, common bases and acids can be applied in this reaction, like sulfuric acid, hydrochloric acid, sodium hydroxide, and potassium hydroxide. While various base and acid compounds can also be very effective. Heterogeneous catalysts, modified structures will also be applied in the lab.
Three different types of catalysts, enzymatic catalysts, which are not used due to their high cost and high sensitivity to temperature, acid catalysts and basic catalysts, popular throughout history, will be introduced. New advances like modified structures, MOFs, and even some ubiquitous materials which can be used also will be discussed later. And prospects for the future of this reaction will also be included.
2. Reaction without catalysts
The reaction is highly kinetically decisive, and catalysts are favored. However, scientists have found that this reaction can also occur without catalysts. Several conditions, such as microwave, ultrasonic are reported. [2]The reaction tends to go much faster than under ambient conditions, and the product is excellent . But still, it has yet to be widely adopted and is still in the lab phase. Reaction under supercritical conditions is also studied. It is quite unsensitive to Free Fatty Acid(FFA) and water. Thus, the application range is extensive. It is also very easy to control the reaction condition. However, it still needs more industrial practice for high temperatures and pressure are required in supercritical conditions, leading to higher costs. Still, it is an unknown technique. Things like the choice of solvents and the production of the reactors remained questionable and challenging, engineers just reluctant to take these risks, and this technique still can’t compete with traditional ways of producing biodiesel . There were some success however. One US company managed to build a supercritical plant with a very good capacity, after some but research had to stop due to lack of fund. One research group successfully launched a pilot biodiesel project and did good experiments on it. But it can be too far away from commercial production.[2]
3. Reaction with catalysts
3.1. Enzymic catalysts
Enzymatic catalysts has captivated the curiosity of researchers in recent years. Compared with other catalysts, they can be more eco-friendly. These catalysts tend to be very reactive, have superb selectivity, the product is of high purity and unlike homogeneous catalysts, just a minimum amount of wastewater is produced. Less purification is needed and energy is saved due to its rather mild temperature, However, drawback for this kind of catalysts can be fatal, for the production of these enzymes can be extraordinarily expensive, and bad stability makes it nearly impossible for commercial use. [3]. Scientists have made great efforts to cut the cost, as they are trying different ways to make and modify the enzyme. Fortunately enough, there are some even commercially available enzymes, but, it can’t match with other catalysts in a viable way in general.
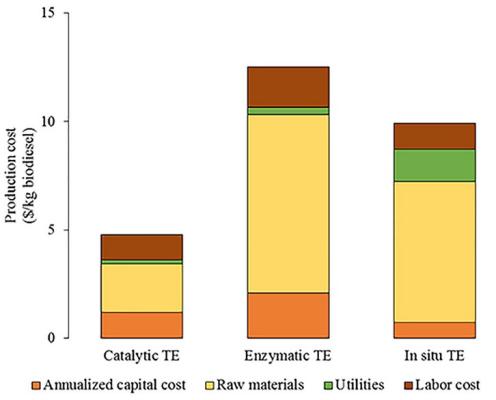
Figure 1: Cost of catalytic practice.
This paper provides a comprehensive analysis of the expenses associated with biodiesel. However, there is an error regarding the specific figure mentioned, as the current price of gasoline is approximately 70 USD per barrel, equating to 0.5 USD per liter. Actually, the comparative assessment of these three values is accurate.
The most noteworthy aspect of these catalysts is that they are very expensive compared with the latter homogeneous and heterogenous catalysts,[4] which makes them nearly impossible to use pratically in the real world.
3.2. Homogeneous catalysts
Homogeneous catalysts are applied widely in industrial practice. These catalysts are economically favored, easy to obtain, and yield good. Both acidic catalysts and basic catalysts are common in practice.
3.2.1. Basic catalysts
Sodium hydroxide, Potassium hydroxide are among the most popular basic catalysts. The reaction can undergo ambient pressure as well as mild temperature, so they have broad application in industry . For different reactants, the yield also varies accordingly, generally stay at a yield of 80%-97%, with most stayed at more than 90%, which is quite high.[5]
As for numerous reactants, basic catalysts always function well.
Things can go differently. In the case of reactants with a high FFA content[5], side reaction, saponification will occur. Water will also be generated in the reaction, leading to a decline of the yield and further separation and purification. While using CH3OK instead can solve this problem.
The amount of base will also need to be noticed, for the increase of base may cause a dramatic decline in the yield. Still, catalysts are difficult to separate from the products where post-processing like distillation and purification are needed.
3.2.2. Acid catalysts
Acid catalysts are less favored for their lengthy process, slower in reaction(approximately 4000 times compared with base), and a higher molar ratio of alcohol to glycerol is required to react and can even be corrosive to the reactor. Nevertheless, it has some advantages over the base for its neutrality to the reactants. Thus, it does not react with oil with a high content of FFA[6]. This is also used as a way to pretreat the reactants which are hypersensitive to base. Common Acids like sulfuric acid, hydrochloric acid, and sulfonic acid are widely used, but in general, research on acid compared to bases is very limited.
3.2.3. Other homogeneous attempts
Some other research is also reported. Like Iron(III)materials, which in fact is a dinuclear complex[7]can also be catalysts for this reaction. The preparation and ingredients are quite common, and the material is not difficult to prepare. Solvents are no longer needed in this reaction. And the results just showed perfect. The yield can exceed 95%(cat. loading 0.5%mol/mol) and, even 93% with(cat. loading 0.05%mol/mol),which is quite a small amount. Proposed catalytic cycle and mechanism were given. And it is a pioneering systematic work showing that these homogeneous Iron(III) structures can play an important role on these catalysis. These research are very few but hopefully this work will bring a bright future to followers
3.3. Heterogeneous catalysis
Unlike homogeneous catalysts, during which process purification and separation are needed and the lack of reusability, heterogeneous catalysts seem like a perfect answer[5]. The catalysts, which are in the solid state, can easily separate from products and are easy to recycle, leading to fewer by-products in the desired ones and higher purity altogether. Unlike liquid homogeneous catalysts, homogeneous ones have minimum corrosiveness to the containers, which attracts wide attention.
Heterogeneous basic catalysts have been widely studied in the past few decades. Catalysts like alkaline earth catalysts and transitional metal catalysts are intensively studied. [8]For alkaline earth catalysts, calcium oxide is very well studied because it is very easy to get and prepare, cheap, and not toxic. They also have a very strong basicity and are active in the reaction. Though they have these advantages, they are still susceptible to FFA and moisture[5]. As a result, many scientists turned to transition metals like Zn, Zr, and Sn, among which zinc is the most favored. Lots of new catalysts with these elements are reported, showing good yields. Moreover, doped materials can also solve some problems by leaching and sensitivity[6].
Acids, which are low in rate and yield, are often less favored. However, reactants with high FFA and water content show great superiority. Mesoporous silicas, which are abundant in nature and have wide parameters of pore sizes, are reported.[6]. Meanwhile, polymers and resins, and some waste carbon-derived solid acids have also been studied.
3.4. Modified and ubiquitous materials
For this reaction, some modified and even magic materials were also reported. Like heterogeneous MOFs materials, [9] originated from solid catalysts. They are typically alkaline earth-based with good properties, easy to access, and economically viable. Drawbacks are obvious, such as problems caused by leaching, contaminating, and bad reusability. Numerous works were reported in this field to modify it, leading to stronger basicity and activation of the previous material. Concern over FFA contents, also led to the research on acid MOFs materials
Nano-particle catalysts play a huge role in this reaction. [10]Generally, they have a larger surface area and better reactivity. Zinc oxide-based materials and Calcium oxide based materials, Calcium oxide in particular, environmental-friendly and cheap, were used widely.
Materials like, well, even eggshells can be used as catalysts for this reaction[11].
CaO with one profound advantage of high surface area(some can exceed 12.4m2/g). together with SrO’s activity in transesterification, (while a much smaller surface area) (around 1m2/g) form this new material.
So SrO with CaO base was synthesized. And the raw material for CaO was waste eggshell.
Results showed that in the case of transesterification of Jatropha oil, impregnation with Sr(NO3)2 material showed a yield of 99.71%(7mmol/g of SrO, 65, 89.8min).
The advantages are quite obvious: low cost of materials and high yield, which is a very fascinating work.
4. Conclusion
For different conditions in the transesterification reaction. Catalytic ones are generally cheaper, consume less energy, and are viable in real practice compared with non-catalytic conditions like microwave and ultrasonic, which were proved useful at a lab-scale. While supercritical conditions can be implausible for their cost in energy and new reactors and solvents. Generally, basic catalysts have far better performance than the acid ones and are favored by scientists and industry. But still difficulties like leaching, wastewater and highly sensitive to FFA contents, make it difficult for all applications. Some modified structures, like MOFs and nano-particles might bring some new progress to the catalysts. Numerous new materials, like doped materials, materials with impregnation, precipitation, and even pre-treated eggshells, activate the basic/acid sites and improve their basicity/acidity, making the materials a better one that might be used in real practice. The integration of these novel materials into established processes could lead to significant improvements in reaction yields and overall process efficiency. Additionally, the use of computational modeling and machine learning techniques can further optimize catalyst performance by predicting interactions at the molecular level. Such advancements not only aim to reduce energy consumption but also to minimize the environmental impact associated with traditional methods. Continued collaboration between academia and industry will be essential to address existing bottlenecks and to facilitate the transition from laboratory-scale innovations to commercial viability. As research progresses, the focus on sustainability and scalability will remain paramount, prompting the development of economically viable solutions tailored to specific industrial needs.
References
[1]. Ghosh, N., & Halder, G. (2022). Current progress and perspective of heterogeneous nanocatalytic transesterification towards biodiesel production from edible and inedible feedstock: A review. Energy Conversion and Management, 270, 116292.
[2]. Qadeer, M. U., Ayoub, M., Komiyama, M., Daulatzai, M. U. K., Mukhtar, A., Saqib, S., & Bokhari, A. (2021). Review of biodiesel synthesis technologies, current trends, yield influencing factors and economical analysis of supercritical process. Journal of Cleaner Production, 309, 127388.
[3]. Pasha, M. K., Dai, L., Liu, D., Du, W., & Guo, M. (2021). Biodiesel production with enzymatic technology: progress and perspectives. Biofuels, Bioproducts and Biorefining, 15(5), 1526-1548.
[4]. Heo, H. Y., Heo, S., & Lee, J. H. (2019). Comparative techno-economic analysis of transesterification technologies for microalgal biodiesel production. Industrial & Engineering Chemistry Research, 58(40), 18772-18779.
[5]. Changmai, B., Vanlalveni, C., Ingle, A. P., Bhagat, R., & Rokhum, S. L. (2020). Widely used catalysts in biodiesel production: a review. RSC advances, 10(68), 41625-41679.
[6]. Lee, A. F., Bennett, J. A., Manayil, J. C., & Wilson, K. (2014). Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chemical Society Reviews, 43(22), 7887-7916.
[7]. Melchiorre, M., Amendola, R., Benessere, V., Cucciolito, M. E., Ruffo, F., & Esposito, R. (2020). Solvent-free transesterification of methyl levulinate and esterification of levulinic acid catalyzed by a homogeneous iron (III) dimer complex. Molecular Catalysis, 483, 110777.
[8]. Kibar, M. E., Hilal, L., Çapa, B. T., Bahçıvanlar, B., & Abdeljelil, B. B. (2023). Assessment of homogeneous and heterogeneous catalysts in transesterification reaction: a mini review. ChemBioEng Reviews, 10(4), 412-422.
[9]. Ma, X., Liu, F., Helian, Y., Li, C., Wu, Z., Li, H., ... & Zhou, S. (2021). Current application of MOFs based heterogeneous catalysts in catalyzing transesterification/esterification for biodiesel production: A review. Energy conversion and management, 229, 113760.
[10]. Ghosh, N., & Halder, G. (2022). Current progress and perspective of heterogeneous nanocatalytic transesterification towards biodiesel production from edible and inedible feedstock: A review. Energy Conversion and Management, 270, 116292.
[11]. Palitsakun, S., Koonkuer, K., Topool, B., Seubsai, A., & Sudsakorn, K. (2021). Transesterification of Jatropha oil to biodiesel using SrO catalysts modified with CaO from waste eggshell. Catalysis Communications, 149, 106233.
Cite this article
Zhou,X. (2025). A Review of Transesterification Catalysts. Applied and Computational Engineering,122,61-65.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Ghosh, N., & Halder, G. (2022). Current progress and perspective of heterogeneous nanocatalytic transesterification towards biodiesel production from edible and inedible feedstock: A review. Energy Conversion and Management, 270, 116292.
[2]. Qadeer, M. U., Ayoub, M., Komiyama, M., Daulatzai, M. U. K., Mukhtar, A., Saqib, S., & Bokhari, A. (2021). Review of biodiesel synthesis technologies, current trends, yield influencing factors and economical analysis of supercritical process. Journal of Cleaner Production, 309, 127388.
[3]. Pasha, M. K., Dai, L., Liu, D., Du, W., & Guo, M. (2021). Biodiesel production with enzymatic technology: progress and perspectives. Biofuels, Bioproducts and Biorefining, 15(5), 1526-1548.
[4]. Heo, H. Y., Heo, S., & Lee, J. H. (2019). Comparative techno-economic analysis of transesterification technologies for microalgal biodiesel production. Industrial & Engineering Chemistry Research, 58(40), 18772-18779.
[5]. Changmai, B., Vanlalveni, C., Ingle, A. P., Bhagat, R., & Rokhum, S. L. (2020). Widely used catalysts in biodiesel production: a review. RSC advances, 10(68), 41625-41679.
[6]. Lee, A. F., Bennett, J. A., Manayil, J. C., & Wilson, K. (2014). Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chemical Society Reviews, 43(22), 7887-7916.
[7]. Melchiorre, M., Amendola, R., Benessere, V., Cucciolito, M. E., Ruffo, F., & Esposito, R. (2020). Solvent-free transesterification of methyl levulinate and esterification of levulinic acid catalyzed by a homogeneous iron (III) dimer complex. Molecular Catalysis, 483, 110777.
[8]. Kibar, M. E., Hilal, L., Çapa, B. T., Bahçıvanlar, B., & Abdeljelil, B. B. (2023). Assessment of homogeneous and heterogeneous catalysts in transesterification reaction: a mini review. ChemBioEng Reviews, 10(4), 412-422.
[9]. Ma, X., Liu, F., Helian, Y., Li, C., Wu, Z., Li, H., ... & Zhou, S. (2021). Current application of MOFs based heterogeneous catalysts in catalyzing transesterification/esterification for biodiesel production: A review. Energy conversion and management, 229, 113760.
[10]. Ghosh, N., & Halder, G. (2022). Current progress and perspective of heterogeneous nanocatalytic transesterification towards biodiesel production from edible and inedible feedstock: A review. Energy Conversion and Management, 270, 116292.
[11]. Palitsakun, S., Koonkuer, K., Topool, B., Seubsai, A., & Sudsakorn, K. (2021). Transesterification of Jatropha oil to biodiesel using SrO catalysts modified with CaO from waste eggshell. Catalysis Communications, 149, 106233.









