1. Introduction
The limited reserves of fossil fuels and the high environmental impact of their combustion have led to the search for alternative energy sources, including solar, wind, hydrogen, geothermal, and even nuclear energy [1]. Unfortunately, the intermittency and instability of these sources pose challenges to the stable operation of power systems. To meet the growing demand for energy storage, researchers have made significant progress in recent decades. Batteries and capacitors, the main energy storage devices, have demonstrated significant performance and potential for the future.
Energy is stored by creating an electric field inside capacitors during the charging process that attracts charges of opposite polarity. This includes supercapacitors (SCs), which can store and release large amounts of charge, have longer life cycles, higher power density, and faster charge/discharge rates than batteries and conventional capacitors, as shown in Fig. 1(a). The properties of SCs are influenced by three major components: electrodes, electrolytes, and separators. In particular, electrode materials affect energy storage and conversion efficiency, which can be regarded as the core of SCs [2]. There is a wide range of electrode materials to choose from, including carbon materials, metal oxides, conductive polymers, and MXenes, which are the most advanced two-dimensional (2D) materials with high specific surface area (SSA), tunable surface chemistry, and exceptional electrical conductivity.
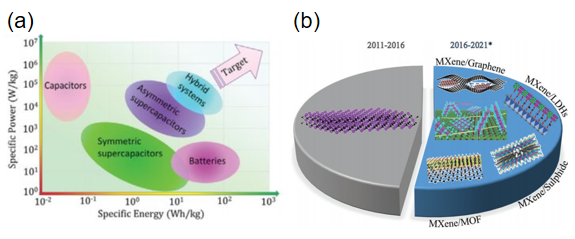
Figure 1: (a) Ragone Plot showing power density and energy density in capacitors and batteries [3]; (b) Research development from 2011 to 2021 for MXenes and MXene-based materials [4]
Discovered in 2011, MXenes are a class of two-dimensional transition metal carbides, nitrides, or carbonitrides with the general chemical structure My+1XyTx, where M represents an early transition metal, X indicates C, N, or CxNy, and Tx represents surface terminations [5]. Fig. 1(b) shows a ten-year trend in MXene materials, with a focus during the five years from 2011 to 2016 on materials with different M layers, such as unitary vanadium, titanium, molybdenum, and other metal compounds, or binary metal materials such as Mo2TiC2. While MXene (Ti3C2) can achieve electrical conductivity of 7,000 S·cm-1, a specific volume capacitance of 514 F·cm-3, and 99.75% capacitance retention after 2,000 cycles, restacking always hinders the transition of electrolyte ions. From 2016 to date, more researchers have focused on MXene composites, where synergies between materials in a heterogeneous structure can often drastically improve their electrical properties, allowing for enhanced energy storage. The particular structure of 2D/2D composites shortens or provides internal interconnects for fast electron transfer, thus mitigating the problem of restacking. Beyond the MXene/graphene, MXene/LDHs, MXene/sulfide, and MXene/MOF composites developed before 2021, there are also other emerging MXene composites. For example, MXene/Co3O4 shows a much higher specific capacitance of 1,081 F·g-1 at 0.5 A·g-1 when tested as an electrode in a three-electrode system with 6 M KOH electrolyte. In conclusion, the selection of materials exhibits great promising in SCs performance improvement. Different composite structures, device architectures design, and the strategy of performance improvement are worth exploring. Perhaps 2D/3D composites will emerge in the future, with unexpected structures and performance.
The growing demand for efficient energy storage devices has led to extensive research in the field of SCs. Among the various materials being investigated for SC applications, MXenes have emerged as promising candidates due to their unique properties. With the proliferation of research results in recent years, there is a need for an article that collects the preparation methods of these materials, reveals the mechanistic explanations, compares their physicochemical properties and electrochemical behavior, and summarizes their characterization methods and development trends. This review aims to critically analyze the recent developments in MXene-based SCs, highlighting their potential advantages, current limitations, and future prospects.
2. MXene-based Supercapacitors: Working Principles
MXene-based supercapacitors operate on the principles of electrical double-layer capacitance (EDLC) and pseudocapacitance [6]. The layered structure of MXenes provides a high surface area for charge storage, while their metallic conductivity ensures rapid charge transfer. An SC can be divided into three main parts: electrodes, electrolytes, and separators, each of which plays an important role in performance. When MXenes are used as electrodes, electron conductivity and energy storage capability are primarily determined by them. Additionally, the surface functional groups on MXenes contribute to pseudocapacitive charge storage through fast redox reactions. Conductivity can also be influenced by different types and concentrations of electrolytes.
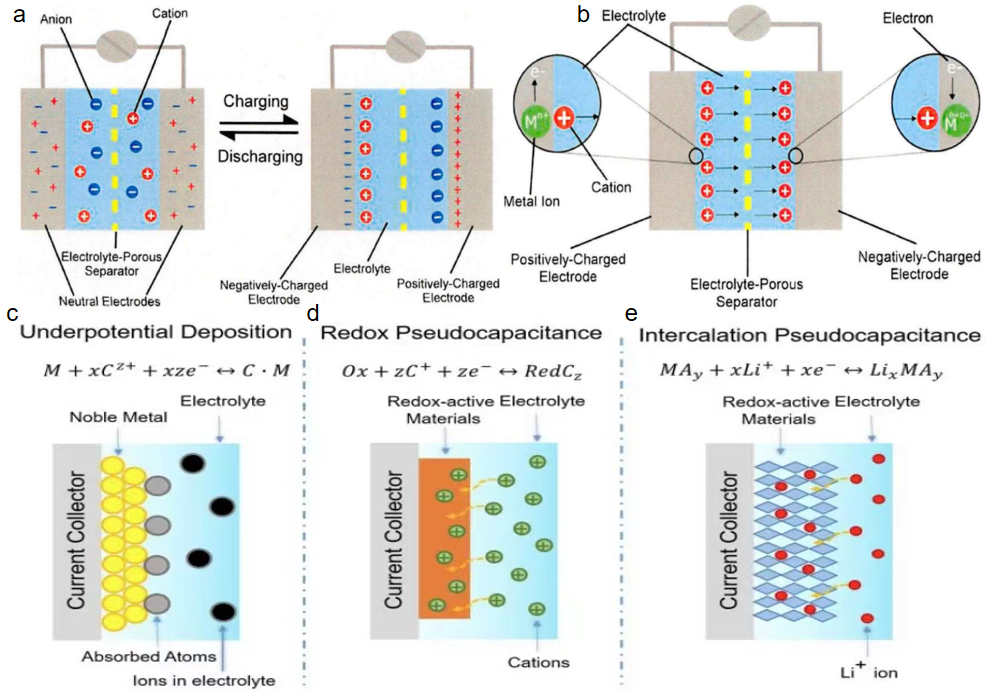
Figure 2: Two working principles: (a) EDLC and (b) pseudocapacitors for SCs; and three types of pseudocapacitance: (c) underpotential deposition, (d) redox pseudocapacitance, and (e) intercalation pseudocapacitance [7]
Figure 2 shows the different working principles and mechanisms of EDLCs and pseudocapacitors, even though they have a similar structure. Energy storage and release in EDLCs depend on the adsorption and ionization processes of charges near the interface. During the charging process, cations are attracted to the surface of the positive electrodes, while anions are adsorbed onto the negative electrodes, forming a bilayer and creating a potential difference. This potential difference generates an electric field inside the capacitor, where the charge, and therefore the electrical energy, is stored. When the external voltage is removed, the charge spontaneously returns to the neutral state, releasing the stored electrical energy.
Pseudocapacitor electrodes can undergo valence changes and rapidly reversible redox reactions that create potential differences near the interface. As a result, an electric field within the material holds the charges and enables the conversion of chemical energy into electrical energy. There are three types of pseudocapacitance: underpotential deposition, redox pseudocapacitance, and intercalation pseudocapacitance. Underpotential deposition refers to the adsorption of ions (H+) on catalytic noble metals (such as Pt, Rh, Ru, Ir) at positive reversible redox potentials and the electrodeposition of metal cations below the equilibrium potential. This process is highly reversible but has a narrow voltage window (0.3V-0.6V), resulting in low energy density. Redox pseudocapacitance is characterized by fast and reversible redox reactions on the surface of active materials, such as RuO2, MnO2, or certain conductive polymers, which have a high volume-specific capacitance of more than 5000 F·cm3. Finally, intercalation pseudocapacitance—also the operating principle for MXene materials—occurs when the electron transfer during the insertion or embedding of ions between the layers of the redox-active material stores electrical energy.
3. Recent Developments in MXene-based Supercapacitors
3.1. Synthesis and Processing of MXenes for Supercapacitor Applications
Generally, MXene preparation methods are divided into two categories: etched and non-etched, with each providing different surface chemistry, shape, and electrochemical properties. Therefore, depending on the specific application, a rational choice of preparation method can effectively meet performance requirements. This section summarizes the development of MXene synthesis over the past 10 years and analyzes the advantages and limitations of different synthesis methods. Additionally, the impact of novel delamination techniques and tuning of function groups on the structure and properties of MXenes will be discussed.
Progress in MXene synthesis from 2011 to 2023 is shown in Fig. 3. MXene was initially prepared by removing the intermediate A phase from the MAX phase, where M represents transition metal elements, A contains trivalent elements such as boron, aluminum, and gallium, and X stands for carbide or nitride. Due to the strong covalent bond between M and X, which is difficult to break, the weaker M-A bond can be etched using hydrofluoric acid (HF) because of the high reactivity of fluoride ions with aluminum. The concentration of HF, etching time, and temperature affect the structure and properties of MXene. Etching with a high HF concentration makes the MXene film more layered, resulting in an accordion-like structure. Mashtalir et al. [8] concluded that increasing the temperature and prolonging the etching time are favorable for etching, but excessive increases in temperature and time can lead to internal structural defects and oxidation of MXene.
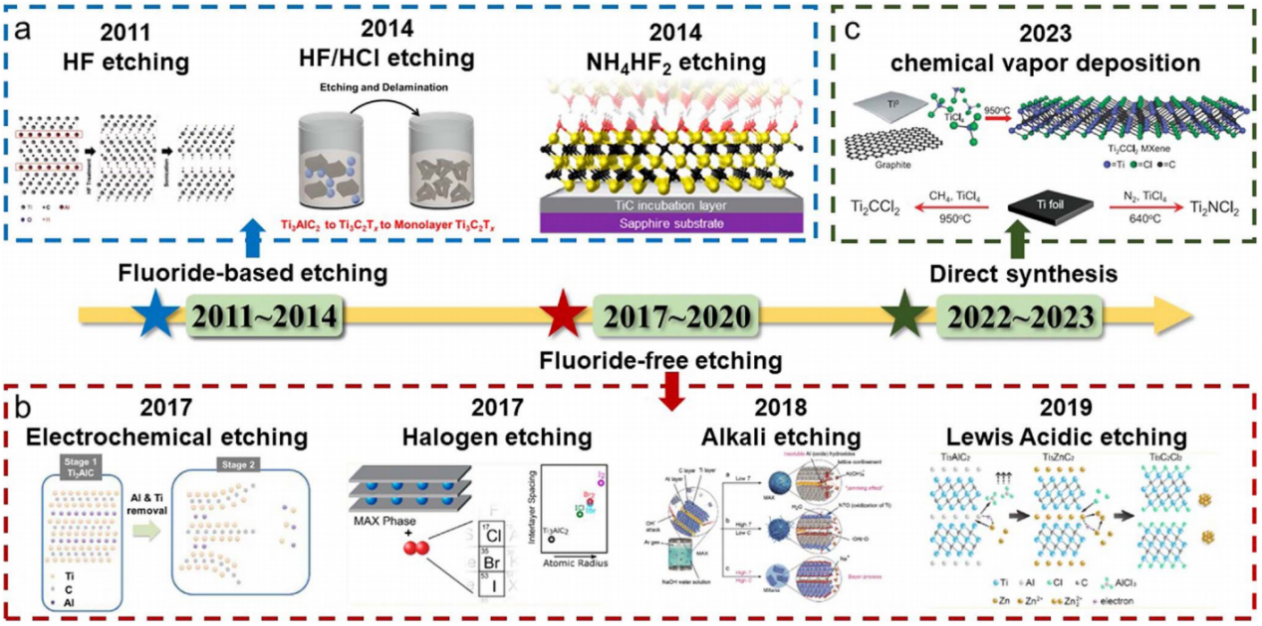
Figure 3: Developments in MXene synthesis from 2011 to 2023 with (a) fluoride-based etching [9], (b) fluoride-free etching [10], and (c) direct synthesis [11]
Although this method can produce MXene directly, HF, being a highly corrosive acid, poses significant safety hazards. Fluoride salt etching greatly reduces this risk. Instead of using HF directly, this approach involves adding fluorine salts and acids such as NH₄HF₂, HCl/LiF, HCl/NaF, HCl/KF, or HCl/FeF₃ to the reaction. This principle is also applied in radio frequency etching, where multiple centrifugations of the etched material by ultrasound allow safer and easier access to monolithic layers of MXene. While this method requires longer reaction times than the HF method, cationic insertion offers advantages such as larger sheets, fewer defects, higher electrical conductivity, and homogeneous layer spacing in the open accordion structure. Notably, different cation insertions result in varying layer spaces and properties.
Even without direct HF involvement, the potential safety threat posed by HF cannot be completely eliminated. Fortunately, researchers have discovered fluorine-free etching methods, including electrochemical etching, molten salt etching, and alkali etching [1]. Although electrochemical etching can deliver high performance, it is not widely used due to its low yield and purity. Additionally, high-yield, hydrophilic, fluorine-free MXene can be obtained by reacting with sodium hydroxide or potassium hydroxide to form Al(OH)₄⁻ in a highly concentrated hot alkali solution. While the above methods can etch carbides, it is not applicable to nitrides. However, the recently developed molten salt method can etch both compounds. By using different molten salts, products with varying surface functional groups can be obtained. Unlike etching, the latest direct synthesis method utilizes chemical vapor deposition (CVD) to synthesize MXene from metal plates, graphite, and organics at high temperatures. This method can even synthesize products that cannot be etched. This bottom-up synthesis approach also allows for control of micromorphology to obtain the desired electrode material.
Due to the self-stacking behavior, MXene obtained through etching produces a multilayered accordion structure. Thus, the exfoliation of monolayers from MXene has become a critical issue, as monolayer flakes maintain a large specific surface area (SSA) and hydrophilicity. Therefore, further increasing the SSA to create more surface active sites has become the main objective of subsequent studies. Conventional delamination methods for multilayered materials generally achieve exfoliation by inserting organic macromolecules or inorganic ions and relying on ultrasound to further weaken interlayer interactions. Typically, the yields are low by ultrasonic method, but higher yields of MXene can be obtained by introducing hydrothermal or microwave reactions to assist in exfoliation. Recently, cyclic freeze-thaw methods have been discovered that can improve purity and prevent oxidation. Monolayer nanosheets are exfoliated by repeatedly freezing and thawing an aqueous solution of MXene, using the volume change of interlayer water during the solid-liquid conversion to weaken the interaction. The advantage of this method consists in require no insertion agents.
There are a wide variety of functional groups on the surface of the monolayer nanosheets after exfoliation, and their types and quantities have a significant impact on optical, electrical, magnetic, and piezoelectric properties. Consequently, many researchers focus on controlling these functional groups. Currently, several terminal groups have been identified, including halogen terminal groups, sulfur terminal groups, nitrogen-containing terminal groups, and vacancy terminal groups. MXene obtained through fluorine-containing etching typically has -F as the primary group. The chemical activity can be enhanced by replacing the resulting -OH with alkali, which also positively affects the redox activity of Ti atoms. Additionally, the electrostatic effect of -SO4 can promote ion penetration in the electrolyte, making sulfuric acid or sulfur-containing fluoride salts potential substitutes for hydrochloric acid. In terms of capacitance, -SO4 achieves 1316 mF·cm-2 at 3 mA·cm-2, compared to 542.7 mF·cm-2 for -F and 794.0 mF·cm-2 for -Cl [12]. Moreover, different salts can introduce pure groups into molten salts, allowing for easy modification of their species through substitution and elimination reactions. Vacancy group MXenes can also be obtained by reducing the elimination of halogen terminal groups. The latest redox-reactive group (-PO2) has shown higher carrier density, conductivity, and redox-active sites compared to -O, resulting in significantly increased charge storage capacity.
3.2. MXene-Based Electrode Materials and Their Performance
Electrodes are a crucial component of supercapacitors, responsible for achieving fast charge storage and stable output voltage. The structure of MXene is closely related to its performance, as specific surface area and surface chemistry are decisive factors in determining bulk specific capacitance, conductivity, and redox activity. These factors influence the type and number of reactive sites, which can be modified by tuning pore size, layer spacing, heteroatom doping, surface functional groups, and vacancies. Additionally, the size and thickness of the sheet layers also affect the electrochemical properties.
Adjustment of both pore and layer spacing can lead to changes in specific surface area, increasing the number of reactive sites while improving electron transport efficiency. The porous structure reduces restacking behavior and enhances the accessibility of electrolyte ions. Ren et al. used the partial oxidation of MXene by O₂ over a CuSO₄ catalyst to increase the specific surface area of the macroporous material from 19.6 to 93.6 m²·g⁻¹ [13]. On the other hand, layer spacing can be increased by the insertion of water molecules or ions, providing more ion transport channels and optimizing electrolyte ion transfer. Ghidiu et al. achieved a layer spacing of 5 to 28 Å by controlling the alkyl chain length of cations inserted into Ti₃C₂ and demonstrated that the cation radius determines the insertion effect [14].
Depending on heteroatom doping (e.g., N, O, S, P) and adjustments to the surface terminal groups (e.g., -F, -O, -Se, -NH₂) and vacancies, diverse reactive sites can be obtained. The -SO₄ group introduced by Guo et al. resulted in the expansion of the interlayer ion channel from 10.02 to 12.23 Å [15]. Meanwhile, providing more redox-active groups such as -O, -NH₄, and -PO₂ exposed additional surface-active sites. Additionally, the introduced defects and vacancies can act as extra redox-active sites, thereby increasing the material’s capacitance.
In addition, Zeraati et al. obtained MXene flakes with a large size using blade coating technology, which exhibited a conductivity of up to 20,000 s·cm⁻¹, significantly higher than other 2D materials [16]. This demonstrated that larger flake sizes lead to higher charge transfer efficiency. Furthermore, the distribution and quantity of surface-active sites determine the capacitance. Deeper active sites have higher activation energy compared to surface-active sites, which hinders ion movement; therefore, thinner flakes are preferable.
3.3. Hybrid MXene-based Materials for Enhanced Capacitance
MXenes can complement the properties of other 2D materials, and their composites can address defects while retaining the unique capabilities of both components. These advantages include an increase in the number of active sites, an enlargement of the specific surface area (SSA), and an improvement in mechanical properties. When other materials are integrated into MXene’s unique 2D structure, the active sites can be fully exposed, and agglomeration can be avoided, resulting in a stable structure and superior performance. As a substrate, MXenes could accelerate ion conversion and increase the active surface area due to their high conductivity. There are several known types of MXene composites, such as MXene/Metal Oxides, MXene/Metal Sulfides, MXene/Conductive Polymers, and MXene/Carbon-based materials [17].
Although transition metals as electrodes can increase capacitance, their low conductivity can be a limitation. Combining these metals with MXenes helps to address this shortcoming. When metal oxides are grown on MXene substrates using traditional polymerization methods, the enlarged specific surface area effectively increases the number of active sites and ion transport channels, while the excellent conductivity of MXenes further enhances the active surface area. Consequently, the synergistic of these composites can improve the electrochemical properties. Different synthesis methods and composites will yield varying effects, but the fundamental principle is to enhance ion transfer and increase the number of active sites by expanding the surface area. In contrast, metal sulfides offer good capacitance and conductivity, but volume changes can reduce cycle life; thus, MXenes are used to provide buffer space, extending the material’s lifespan.
Considering that adding MXene to each of the above two materials has potential to address their issues, the MXene/conductive polymer composite emerges as a strong combination. Conductive polymers are suitable for wearable devices due to their unique mechanical properties and biocompatibility, and the introduction of MXene can enhance energy density and capacitance retention. By increasing the layer spacing of MXene through the insertion of large molecules, multi-dimensional electron transport channels are created, significantly boosting conductivity. Additionally, incorporating carbon materials can effectively mitigate the problem of MXene self-accumulation. Depending on the characteristics of different carbon materials, such as graphene, carbon nanotubes, and reduced graphene oxide (rGO), the properties of the composite will exhibit varying degrees of improvement. Notably, MXene/rGO demonstrates higher volumetric capacitance compared to other carbon-based composites.
3.4. Device Architectures and Applications
To create MXene-based supercapacitors, the MXene-coated electrode or MXene composite electrode must be in contact with the electrolyte and separator and be enclosed in an aluminum housing. Recently, emerging methods such as 3D printing, inkjet printing, and pattern etching have been used to produce microcapacitors with custom geometries and uniform properties. It is crucial to note that the reaction conditions used to prepare MXene electrodes significantly impact product performance, necessitating strict control over reaction temperature, time, and etchant concentration. Additionally, factors such as heteroatom doping, porosity control, choice of intercalator type, and selection of functional groups all affect volumetric capacitance and conductivity. Similarly, the choice of electrolyte and separator is a key performance factor. Therefore, it is essential to produce the appropriate materials based on the specific requirements and to tailor their functions according to their different properties.
In addition, the choice of electrolytes is crucial for the electrochemical properties of MXene-based capacitors. Electrolytes are classified into aqueous and non-aqueous systems. Rapid reversible redox reactions in sulfuric acid environments yield extraordinary capacitance compared to neutral or alkaline environments. Unfortunately, in aqueous-phase systems, the narrow voltage window still limits the increase in energy density. In contrast, non-aqueous systems are unaffected by the decomposition of water molecules, thus providing a broader voltage window for capacitors to demonstrate their capacitive performance. Interestingly, the pseudocapacitance and redox pseudocapacitance properties are influenced by the collapse of the solvent molecular shell structure. When this structure collapses completely, solvent molecules do not insert into the interlayers, resulting in a constant interlayer spacing and allowing for more ions to be inserted. Conversely, if the structure does not collapse completely, ion insertion pulls molecules into the interlayer, leading to an expansion of the interlayer spacing.
MXene-based capacitors have promising prospects in transportation, power energy storage, and industrial fields due to their high energy density, rapid charging and discharging capabilities, and safety and reliability. They meet the demands for strong driving force and are used in new energy vehicles as well as in train starting and braking systems. Notably, they reduce the dependence of electric vehicles on batteries and extend their service life. Similarly, the use of supercapacitors in conjunction with batteries in internal combustion engines can extend battery life in the industrial sector. Additionally, the high power and rapid charging and discharging capabilities of MXene-based capacitors play a unique role in high-voltage substations, power grids, and wind power generation.
4. Conclusions and Outlook
Although MXene-based supercapacitors hold great potential in the energy storage and conversion technology market, high production costs, poor recycling and stability, and limited charging and discharging speeds remain challenges that need to be addressed. In the future, MXene-based supercapacitors can be improved in three key areas: material optimization, improvement of energy and power density, and exploration of new application areas. The comprehensive performance of MXene-based supercapacitors can be gradually enhanced by selecting appropriate preparation processes and materials.
Currently, Ti3C2-based 2D materials have been the primary focus of research in recent years. However, many other MXene materials are also worth exploring. Research should shift towards the preparation, characterization, and performance testing of these new materials. Additionally, although various etching methods exist, the topography of the product is constrained by reaction conditions. In contrast, product topography obtained through chemical vapor deposition (CVD) on a flat plate is easier to control. Therefore, exploring new non-etching synthesis methods is crucial to improving the material utilization of MXene-based supercapacitors.
Additionally, the choice between aqueous and non-aqueous electrolytes influences the behavior of MXene-based supercapacitors. Notably, sulfuric acid environments and certain organic solution electrolytes can significantly enhance performance. However, MXene-based supercapacitors also face challenges, including stability in both aqueous and non-aqueous electrolytes, volumetric capacitance, and large-scale synthesis. Addressing these challenges is crucial for the practical application of MXene-based supercapacitors in energy storage systems.
In conclusion, MXene-based supercapacitors represent a rapidly evolving field with immense potential for energy storage applications. Continued research efforts and interdisciplinary collaborations are essential to overcome current limitations and unlock the full capabilities of MXenes in supercapacitor technology.
References
[1]. Yi, S., Wang, L., Zhang, X., Li, C., Xu, Y., Wang, K., ... & Ma, Y. (2023). Recent advances in MXene-based nanocomposites for supercapacitors. Nanotechnology.
[2]. Bhat, A., Anwer, S., Bhat, K. S., Mohideen, M. I. H., Liao, K., & Qurashi, A. (2021). Prospects challenges and stability of 2D MXenes for clean energy conversion and storage applications. npj 2D Materials and Applications, 5(1), 61.
[3]. Zahir, N., Magri, P., Luo, W., Gaumet, J. J., & Pierrat, P. (2022). Recent advances on graphene quantum dots for electrochemical energy storage devices. Energy & Environmental Materials, 5(1), 201-214.
[4]. Nasrin, K., Sudharshan, V., Subramani, K., & Sathish, M. (2022). Insights into 2D/2D MXene heterostructures for improved synergy in structure toward next‐generation supercapacitors: a review. Advanced Functional Materials, 32(18), 2110267.
[5]. Hart, J. L., Hantanasirisakul, K., Lang, A. C., Anasori, B., Pinto, D., Pivak, Y., ... & Taheri, M. L. (2019). Control of MXenes’ electronic properties through termination and intercalation. Nature communications, 10(1), 522.
[6]. Zang, X., Wang, J., Qin, Y., Wang, T., He, C., Shao, Q., ... & Cao, N. (2020). Enhancing capacitance performance of Ti 3 C 2 T x MXene as electrode materials of supercapacitor: from controlled preparation to composite structure construction. Nano-Micro Letters, 12, 1-24.
[7]. Shao, Y., El-Kady, M. F., Sun, J., Li, Y., Zhang, Q., Zhu, M., ... & Kaner, R. B. (2018). Design and mechanisms of asymmetric supercapacitors. Chemical reviews, 118(18), 9233-9280.
[8]. Mashtalir, O., Naguib, M., Mochalin, V. N., Dall'Agnese, Y., Heon, M., Barsoum, M. W., & Gogotsi, Y. (2023). Intercalation and delamination of layered carbides and carbonitrides. In MXenes (pp. 359-377). Jenny Stanford Publishing.
[9]. Halim, J., Lukatskaya, M. R., Cook, K. M., Lu, J., Smith, C. R., Näslund, L. Å., ... & Barsoum, M. W. (2014). Transparent conductive two-dimensional titanium carbide epitaxial thin films. Chemistry of Materials, 26(7), 2374-2381.
[10]. Sun, W., Shah, S. A., Chen, Y., Tan, Z., Gao, H., Habib, T., ... & Green, M. J. (2017). Electrochemical etching of Ti 2 AlC to Ti 2 CT x (MXene) in low-concentration hydrochloric acid solution. Journal of Materials Chemistry A, 5(41), 21663-21668.
[11]. Wang, D., Zhou, C., Filatov, A. S., Cho, W., Lagunas, F., Wang, M., ... & Talapin, D. V. (2023). Direct synthesis and chemical vapor deposition of 2D carbide and nitride MXenes. Science, 379(6638), 1242-1247.
[12]. Otgonbayar, Z., Yang, S., Kim, I. J., & Oh, W. C. (2023). Recent advances in two-dimensional MXene for supercapacitor applications: progress, challenges, and perspectives. Nanomaterials, 13(5), 919.
[13]. Ren, C. E., Zhao, M. Q., Makaryan, T., Halim, J., Boota, M., Kota, S., ... & Gogotsi, Y. (2016). Porous two‐dimensional transition metal carbide (MXene) flakes for high‐performance Li‐ion storage. ChemElectroChem, 3(5), 689-693.
[14]. Ghidiu, M., Kota, S., Halim, J., Sherwood, A. W., Nedfors, N., Rosen, J., ... & Barsoum, M. W. (2017). Alkylammonium cation intercalation into Ti3C2 (MXene): effects on properties and ion-exchange capacity estimation. Chemistry of Materials, 29(3), 1099-1106.
[15]. Guo, M., Geng, W. C., Liu, C., Gu, J., Zhang, Z., & Tang, Y. (2020). Ultrahigh areal capacitance of flexible MXene electrodes: electrostatic and steric effects of terminations. Chemistry of Materials, 32(19), 8257-8265.
[16]. Zeraati, A. S., Mirkhani, S. A., Sun, P., Naguib, M., Braun, P. V., & Sundararaj, U. (2021). Improved synthesis of Ti 3 C 2 T x MXenes resulting in exceptional electrical conductivity, high synthesis yield, and enhanced capacitance. Nanoscale, 13(6), 3572-3580.
[17]. Sun, M., Ye, W., Zhang, J., & Zheng, K. (2024). Structure, Properties, and Preparation of MXene and the Application of Its Composites in Supercapacitors. Inorganics, 12(4), 112.
Cite this article
Zhu,H. (2025). Recent Developments in MXene-Based Supercapacitors. Applied and Computational Engineering,124,45-53.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Yi, S., Wang, L., Zhang, X., Li, C., Xu, Y., Wang, K., ... & Ma, Y. (2023). Recent advances in MXene-based nanocomposites for supercapacitors. Nanotechnology.
[2]. Bhat, A., Anwer, S., Bhat, K. S., Mohideen, M. I. H., Liao, K., & Qurashi, A. (2021). Prospects challenges and stability of 2D MXenes for clean energy conversion and storage applications. npj 2D Materials and Applications, 5(1), 61.
[3]. Zahir, N., Magri, P., Luo, W., Gaumet, J. J., & Pierrat, P. (2022). Recent advances on graphene quantum dots for electrochemical energy storage devices. Energy & Environmental Materials, 5(1), 201-214.
[4]. Nasrin, K., Sudharshan, V., Subramani, K., & Sathish, M. (2022). Insights into 2D/2D MXene heterostructures for improved synergy in structure toward next‐generation supercapacitors: a review. Advanced Functional Materials, 32(18), 2110267.
[5]. Hart, J. L., Hantanasirisakul, K., Lang, A. C., Anasori, B., Pinto, D., Pivak, Y., ... & Taheri, M. L. (2019). Control of MXenes’ electronic properties through termination and intercalation. Nature communications, 10(1), 522.
[6]. Zang, X., Wang, J., Qin, Y., Wang, T., He, C., Shao, Q., ... & Cao, N. (2020). Enhancing capacitance performance of Ti 3 C 2 T x MXene as electrode materials of supercapacitor: from controlled preparation to composite structure construction. Nano-Micro Letters, 12, 1-24.
[7]. Shao, Y., El-Kady, M. F., Sun, J., Li, Y., Zhang, Q., Zhu, M., ... & Kaner, R. B. (2018). Design and mechanisms of asymmetric supercapacitors. Chemical reviews, 118(18), 9233-9280.
[8]. Mashtalir, O., Naguib, M., Mochalin, V. N., Dall'Agnese, Y., Heon, M., Barsoum, M. W., & Gogotsi, Y. (2023). Intercalation and delamination of layered carbides and carbonitrides. In MXenes (pp. 359-377). Jenny Stanford Publishing.
[9]. Halim, J., Lukatskaya, M. R., Cook, K. M., Lu, J., Smith, C. R., Näslund, L. Å., ... & Barsoum, M. W. (2014). Transparent conductive two-dimensional titanium carbide epitaxial thin films. Chemistry of Materials, 26(7), 2374-2381.
[10]. Sun, W., Shah, S. A., Chen, Y., Tan, Z., Gao, H., Habib, T., ... & Green, M. J. (2017). Electrochemical etching of Ti 2 AlC to Ti 2 CT x (MXene) in low-concentration hydrochloric acid solution. Journal of Materials Chemistry A, 5(41), 21663-21668.
[11]. Wang, D., Zhou, C., Filatov, A. S., Cho, W., Lagunas, F., Wang, M., ... & Talapin, D. V. (2023). Direct synthesis and chemical vapor deposition of 2D carbide and nitride MXenes. Science, 379(6638), 1242-1247.
[12]. Otgonbayar, Z., Yang, S., Kim, I. J., & Oh, W. C. (2023). Recent advances in two-dimensional MXene for supercapacitor applications: progress, challenges, and perspectives. Nanomaterials, 13(5), 919.
[13]. Ren, C. E., Zhao, M. Q., Makaryan, T., Halim, J., Boota, M., Kota, S., ... & Gogotsi, Y. (2016). Porous two‐dimensional transition metal carbide (MXene) flakes for high‐performance Li‐ion storage. ChemElectroChem, 3(5), 689-693.
[14]. Ghidiu, M., Kota, S., Halim, J., Sherwood, A. W., Nedfors, N., Rosen, J., ... & Barsoum, M. W. (2017). Alkylammonium cation intercalation into Ti3C2 (MXene): effects on properties and ion-exchange capacity estimation. Chemistry of Materials, 29(3), 1099-1106.
[15]. Guo, M., Geng, W. C., Liu, C., Gu, J., Zhang, Z., & Tang, Y. (2020). Ultrahigh areal capacitance of flexible MXene electrodes: electrostatic and steric effects of terminations. Chemistry of Materials, 32(19), 8257-8265.
[16]. Zeraati, A. S., Mirkhani, S. A., Sun, P., Naguib, M., Braun, P. V., & Sundararaj, U. (2021). Improved synthesis of Ti 3 C 2 T x MXenes resulting in exceptional electrical conductivity, high synthesis yield, and enhanced capacitance. Nanoscale, 13(6), 3572-3580.
[17]. Sun, M., Ye, W., Zhang, J., & Zheng, K. (2024). Structure, Properties, and Preparation of MXene and the Application of Its Composites in Supercapacitors. Inorganics, 12(4), 112.









