1. Introduction
Electrochemical energy storage devices have been proposed as a feasible solution to meet the rising energy demand for a variety of applications, including renewable energy storage, portable devices, and electric vehicles, throughout the previous decades. With the development of the technology, people seek for the batteries with high energy capacity and safety. In the beginning, researchers developed Na-ion battery and Mg-ion battery which are approximately 150 Wh/kg to work as energy storage for the laptops, then in 2017, the vehicles company started to use Li-ion battery, which has the energy density around 250 Wh/kg, in the first generation electric vehicles [1]. 2020 is one significant year in the battery history because researchers developed Metal-sulphur battery which the energy density is 500 Wh/kg and Metal-air battery which the energy density is 1000 Wh/kg, and these kinds of batteries can be used for more advanced electric vehicles or airplane [1].
Electric vehicles have become the most popular vehicles trend and many counties or areas aims to develop more advanced electric vehicles. The battery is the core of the electric vehicles, which means the greater batteries can leads to the greater electric vehicles. China is one of the fastest developing countries for electric vehicles in the world. At the end of 2020, around 5 million electric vehicles were operating on China’s roads, but in 2006, China has only deployed 20,000 new energy vehicles nationwide [2]. Within 15 years, the number of electric vehicles had increased around 250 times and become one of the country with the most electric vehicles in the world. The Chinese government developed a range of policies tailored to their local conditions, including allowance, license plate incentives and more to boost sales and products.
However, the researchers are also countered by several issues like supply deficits and improvement of energy capacity and electrochemical stability. In this passage, the problems of energy capacity and electrochemical stability and some possible solutions are demonstrated.
2. Problems of the battery
2.1. Capacity
The most prevalent anode material for the battery is graphite, which is a a form of the carbon. Graphite only expand 10% its volume which shows great stable structure, but it has very poor energy density, which is only 372 mAh/g [3]. Other materials like lithium-based or silicon-based, they vary a lot in their volume but they shows excellent energy density.
2.2. Safety
There are three ways which will lead the battery explosion. First, the collision will deform the battery pack's structural integrity, which could lead to a separator inside the battery rupturing and causing an internal short circuit. Additionally, the electrolyte, which burns very easily, could leak due to the collision, starting a fire that could later explode. Second, overcharging and over discharge will cause the battery explode. Overcharging means that the battery still continue charge when it is reaching a standard cut-off voltage, so it will cause temperature increase and gas generation and lead battery explode. Over discharge refers to the decomposition of solid electrolyte interface due to excessive delithiation, which will cause gas and heat generation and expand the volume of the batteries. Third, long-term heating in a high-temperature environment will cause thermal abuse. This situation has three stages and is shown in figure 1. At first, the internal temperature of the batteries will rise under heat environment. Then the heat and gas generate in the battery and the temperature of the batteries rises sharply. The reactions between the batteries are accelerated and structure inside the batteries will be collapsed subsequently. At last, the batteries will be explored [4].
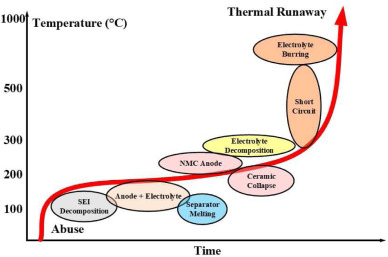
Figure 1. Schematic of the process of battery explosion under high temperature. Reproduced from reference [3].
3. Solutions
3.1. Structure
Researchers find different structures of battery packs to prevent battery cell from explosion. Air cooling system is widely used for dissipating heat and is one of the cheapest solution to improve safety of the battery. To be specific, air cooling system creates airflow through the fan operation. The flow uniformity of the cooling channel can be guaranteed through the Z-type of the inlet and outlet channel (left side of figure 2) and is extensively used in battery packs. This kind of geometry shape can increase the efficiency of cooling approximately 93% [5]. Currently, Kai Chen & Weixiong Wu [6] demonstrate that compared to a traditional Z-type flow circuit, the symmetrical air-cooled cooling system with the inlet and outlet in the middle of the static chamber (right side of figure 2) exhibited a greater cooling efficiency.
Figure 2. Schematic of the Z-type system (left) and symmetrical system (right). Reproduced from reference [4].
3.2. Anode materials
In order to solve stable problem, the researchers have developed some advance materials. The first material is hollow graphite with a pore density of less than 45% [7]. This material has good thermodynamic stability and will enhanced electrical conductivity in the lithium-ion battery because the higher the hole density brings the more capacity and instability of Li-ion battery. The researchers set several experiments and conclude that pore density with 35% has the best performance during the Li-ion charging circle [7]. The second martial is surface coating of anode material. This material can be seen as anode material with artificial surface electrolyte interface. Surface electrolyte interface is the process to reduce the charge and discharge efficiency of the electrode materials, which can prevent battery from overcharging and over discharge, and prevent the co-embedding of the ions and side reactions. This covering layer can not only protect the graphite structure which is vulnerable to the deposited lithium in order to prolong the lifespan of the cell, but also enhance the battery’s Coulombic efficiencies [8].
Better capacity retention than normal material (graphite) was reported as a result of the dispersion of nanoparticles' reduced volume variation. After 15 cycles, a nanosized silicon anode material demonstrated a reversible capacity of 1700 mAh/g [9]. The initial reversible capacity of silicon/carbon composite was 1039 mAh/g, and a capacity of 794 mAh/g was maintained after 20 cycles, according to silicon/carbon composite anode in nano-size [10]. Nanotechnology will reduce the distance that lithium ions must travel to reach the electrolyte and will provide them a high specific surface area to fully contact in order to increase rate capability. Additionally, nanomaterials will increase cycle stability and reduce volume strain during lithiation and delithiation.
3.3. Li-air Battery
Li-air battery is one of the most prospect field which shows higher capacity and stability and also can replace gasoline as new way to store electric in order to power the vehicles. The theoretical density of Li-air battery is 13000 Wh/kg and practical density of Li-air battery is 1700 Wh/kg [11]. These two numbers are very close to those of gasoline. Li-air battery also has several benefits like lower cost and several electrolytes option (aqueous electrolyte, non-aqueous electrolyte, solid-state electrolyte, and hybrid electrolyte) [12]. Even though it has many benefits, the problems are also obvious and needed to be conquered.
3.3.1. Aqueous Electrolyte Li-air battery. Several chemical reactions will produce LiOH which is very soluble in water, so the product does not embed in the air electrode to block the porous and resist the cathode reactions. However, since Li will react with water and will lead to many strong side reactions during the charging circles, so anode and cathode of the battery in this condition will be damaged and cause poor stability. In this way, the researchers need to find solutions of reducing the activation barriers for both oxygen redox reaction and oxygen evolution reaction. Several researching groups have developed excellent solutions for enhancing stability of the battery and the most famous one came from PolyPlus Battery Company [12]. In this model (figure 3), lithium anode is protected by the film and organic electrolyte, which works like a wall to block any contact between water and the lithium metal. Also, researchers add catalysts in the positive electrode that reduce the activation barriers for both oxygen redox reaction and oxygen evolution reaction. Another famous model introduced by Toyota. This model use reservoir mechanism to make maximum energy density reach around 2300 Wh/L and he specific capacity of this system is 3020 mAh/g [13].
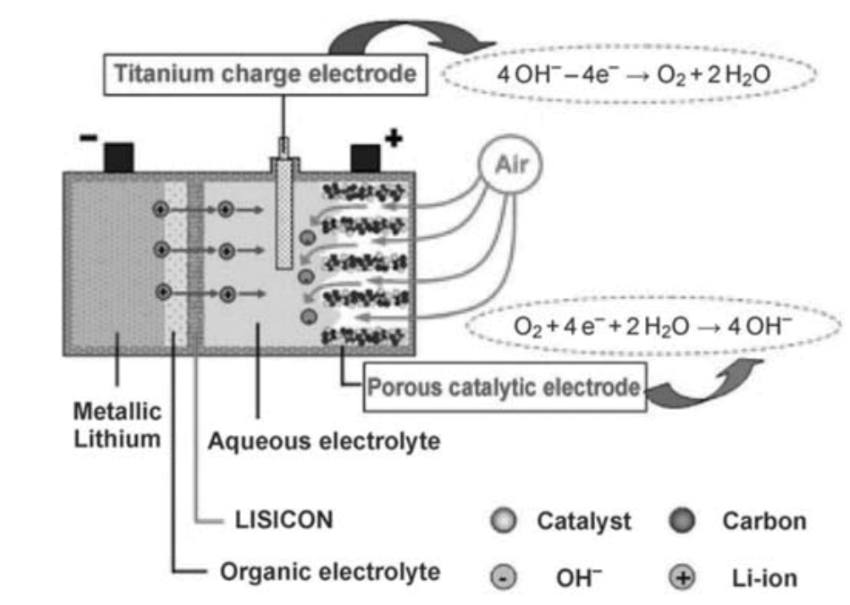
Figure 3. Schematic of the battery in an aqueous electrolyte condition from PolyPlus Company. Reproduced from reference [12].
3.3.2. Non-aqueous Electrolyte Li-air battery. DMSO (dimethylsulfoxide) based electrolytes are common used in lithium-air battery, but they shows poor electrochemically stability when they contact with the lithium metal and will lead to many side reactions [14]. Lithium dendrites (figure 4) can potentially raise safety concerns because they form on Li metal even though a solid-electrolyte interphase (SEI) often forms on it in many non-aqueous electrolytes, allowing some experiments to be carried out without using solid electrolyte separator [14]. This is because the dendrites can penetrate the separator and reach the oxygen electrode and cause a short circuit. The battery also exhibits low Coulombic efficiency as a result of the capacity loss associated with the production of a new SEI and dendrites on the growing lithium surface area, even though this lithium metal structure can form SEI to separate each section and reduce side effects [14].
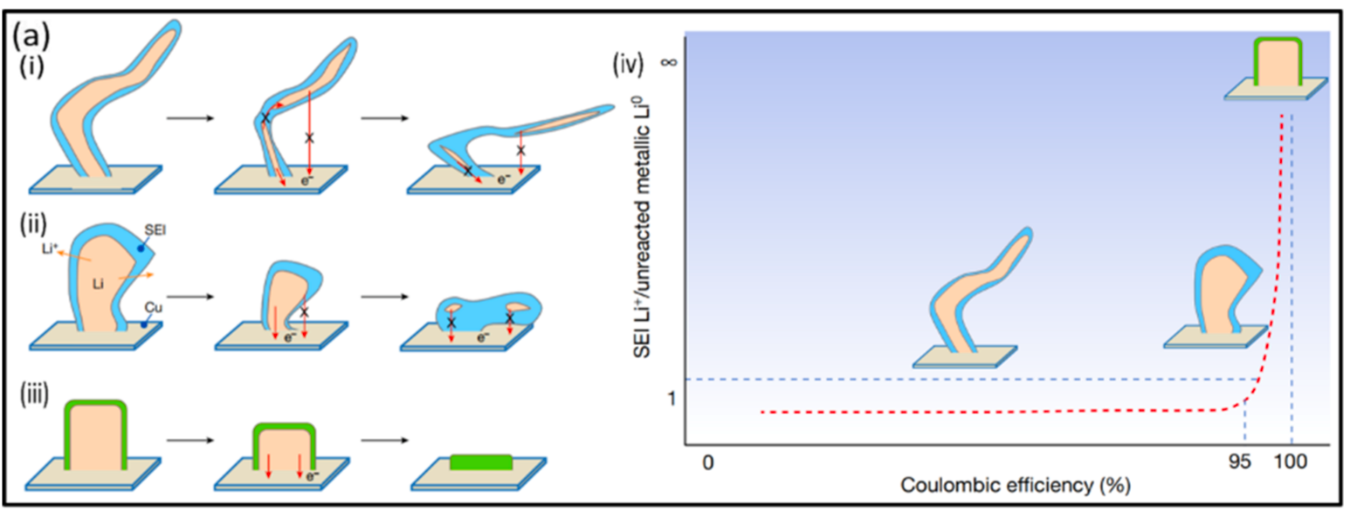
Figure 4. The formation of lithium dendrites and the relationship between coulomb efficiency and dendrites. Reproduced from reference [14].
3.3.3. Organic Electrolytes Li-air battery. The cycling performance was significantly poor. During discharge process, many organic products like lithium carbonate (Li2CO3) and alkyl carbonate (RO–(C=O)–OLi) are formed instead of lithium peroxide (Li2O2) which is an ideal chemical has great energy density [12].
3.3.4. Problems of Cathode. The cathode of this kind of battery also face some issues. Although oxygen in the air is free and accessible, the air contains CO2, N2, and H2O which will interact with cathode and lead short circuit. Li2CO3 and Li2O2 are produced during charging circle and hardly removed or solvated, so these chemicals will block the pore and decrease the charing efficiency and capacity of the battery [15]. In figure 5, red rings represent Li2O2 and black matters represent porous cathode. Red rings are stuck in the black matter and block yellow balls which represent Oxygen react with Li-ion, which will undermine the charing efficiency.
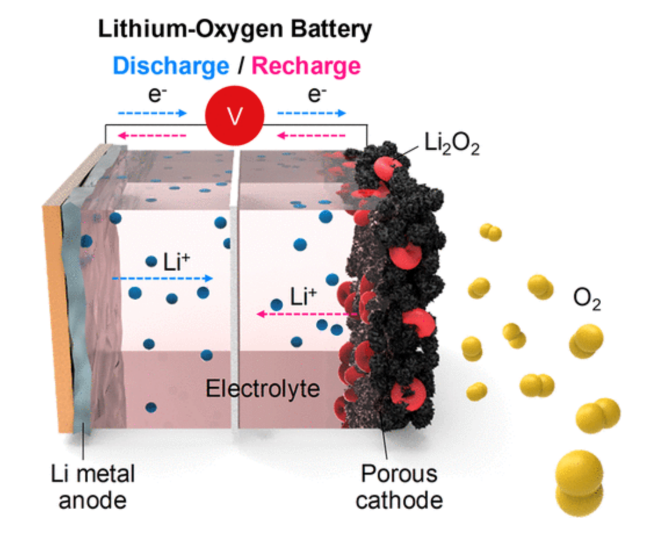
Figure 5. Schematic of Li-air battery in charging process. Reproduced from reference [15].
3.3.5. Membrane. Researchers [12] now find out that using proper membrane can solve the majority of issues of Li-air battery. Examples include covering the porous cathode's outer surface with an oxygen diffusion membrane to block H2 ,CO2 and water from the surrounding air and provide a high oxygen solvability through the membrane, as well as inhibiting the formation of unwanted discharge products like LiOH and Li2CO3 to prevent pore blockage and increase oxygen possibility into Li-air batteries. Additionally, the oxygen selective membrane at the lithium metal anode may limit the production of lithium dendrites and regulate the electrolyte reaction.
4. Discussion
In the recent decades, the development of the Li-based batteries grew rapidly. Many problems were found and solved during the period. Researchers mainly focus on how to overcome the obstacles of energy capacity and stability. surface coating technology and hollow graphite would be ideal ways to replace the ordinary graphite in the battery in order to increase the stability. Nano technology products like nano meter Silicon based materials and nano meter Silicon/Carbon composite materials can enhance the energy capacity and energy density of the Li-based battery. Researchers also developed several cell structures to help dissipate the heat to enhance the stability of the battery. Moreover, Li-air battery has become one of an intriguing field because it has great energy capacity and lower costs. Although Li-air battery has around four different electrolyte options (non-aqueous, aqueous, solid-state, and hybrid), many problems, like poor stability and lithium dendrites, are shown and hinder the further development of this battery. With the unremitting efforts of researchers, different kinds of membranes and electrolyte are introduced and have mitigated the issues of the Li-air battery.
References
[1]. K.Hayat, L.F.Vega, A.AlHajaj “What have we learned by multiscale models on improving the cathode storage capacity of Li-air batteries? Recent advances and remaining challenges,” Renewable and Sustainable Energy Reviews, February 2022
[2]. Hui He and Lingzhi Jin, “How China Put Nearly 5 Million New Energy Vehicles on The Road in One Decade”
[3]. Xiaomei Jiang, “The impact of electrode with carbon materials on safety performance of lithium-ion batteries: A review,” Carbon, Volume 191, May 2022, Pages 448-470
[4]. Xinghui Zhang, Zhao Li “A review on thermal management of lithium-ion batteries for electric vehicles,” Energy, Volume 238, Part A, 1 January 2022, 121652
[5]. Kai Chen, Weixiong Wu, "Cooling efficiency improvement of air-cooled battery thermal management system through designing the flow pattern,” Energy, 167 (2019), pp. 781-790
[6]. Taehoon Kim, Wentao Song, Dae-Yong Son, Luis K. Ono, and Yabing Qi, “Lithium-ion batteries: outlook on present, future, and hybridized technologies,” J. Mater. Chem. A, 2019
[7]. Chen Yang, Xiuying Zhang, Jingzhen, “Holey graphite: A promising anode material with ultrahigh storage for lithium-ion battery,” Electrochim. Acta, 346 (2020)
[8]. Gaoding Yang, Simeng Zhang, Suting Weng, “Anionic Effect on Enhancing the Stability of a Solid Electrolyte Interphase Film for Lithium Deposition on Graphite”, Nano Lett., 21 (12) (2021), pp. 5316-5323
[9]. Li, H.; Huang, X.; Chen, L.; Wu, Z.; Liang, Y, “A High capacity nano Si composite anode material for lithium rechargeable batteries. Electrochem,” Solid-State Lett. 1999, 2, 547–549.
[10]. Wang, C.S.; Wu, G.T.; Zhang, X.B.; Qi, Z.F.; Li, W.Z, “Lithium insertion in carbon‐silicon composite materials produced by mechanical milling,” J. Electrochem. Soc. 1998, 145, 2751–2758
[11]. K.Hayat, L.F.Vega, A.AlHajaj, “What have we learned by multiscale models on improving the cathode storage capacity of Li-air batteries? Recent advances and remaining challenges,” Volume 154, Renewable and Sustainable Energy Reviews, February 2022, 111849
[12]. Jake Christensen, Paul Albertus, Roel S. Sanchez-Carrera, “A Critical Review of Li/Air Batteries,” J. Electrochem. Soc. 2011, Volume 159, Issue 2, Pages R1-R30
[13]. K. Suto, S. Nakanishi, H. Iba, and K. Nishio, “An Aqueous Li-Air Battery Based on a Novel Reservoir Concept,” The 15th International Meeting on Lithium Batteries - IMLB 2010
[14]. Tao Liu, J. Padmanabhan.Vivek “Current Challenges and Routes Forward for Nonaqueous Lithium− Air Batteries,” Chem. Rev. 2020, 120, 14, 6558–6625
[15]. Won-Jin Kwak, Rosy, Daniel Sharon, Chun Xia, Hun Kim, “ Lithium–Oxygen Batteries and Related Systems: Potential, Status, and Future”, March 5, 2020, Chem. Rev. 2020, 120, 14, 6626–6683
Cite this article
Wu,Y. (2023). A review of current problems and plausible solutions of lithium-based battery. Applied and Computational Engineering,3,225-230.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Materials Chemistry and Environmental Engineering (CONF-MCEE 2023)
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. K.Hayat, L.F.Vega, A.AlHajaj “What have we learned by multiscale models on improving the cathode storage capacity of Li-air batteries? Recent advances and remaining challenges,” Renewable and Sustainable Energy Reviews, February 2022
[2]. Hui He and Lingzhi Jin, “How China Put Nearly 5 Million New Energy Vehicles on The Road in One Decade”
[3]. Xiaomei Jiang, “The impact of electrode with carbon materials on safety performance of lithium-ion batteries: A review,” Carbon, Volume 191, May 2022, Pages 448-470
[4]. Xinghui Zhang, Zhao Li “A review on thermal management of lithium-ion batteries for electric vehicles,” Energy, Volume 238, Part A, 1 January 2022, 121652
[5]. Kai Chen, Weixiong Wu, "Cooling efficiency improvement of air-cooled battery thermal management system through designing the flow pattern,” Energy, 167 (2019), pp. 781-790
[6]. Taehoon Kim, Wentao Song, Dae-Yong Son, Luis K. Ono, and Yabing Qi, “Lithium-ion batteries: outlook on present, future, and hybridized technologies,” J. Mater. Chem. A, 2019
[7]. Chen Yang, Xiuying Zhang, Jingzhen, “Holey graphite: A promising anode material with ultrahigh storage for lithium-ion battery,” Electrochim. Acta, 346 (2020)
[8]. Gaoding Yang, Simeng Zhang, Suting Weng, “Anionic Effect on Enhancing the Stability of a Solid Electrolyte Interphase Film for Lithium Deposition on Graphite”, Nano Lett., 21 (12) (2021), pp. 5316-5323
[9]. Li, H.; Huang, X.; Chen, L.; Wu, Z.; Liang, Y, “A High capacity nano Si composite anode material for lithium rechargeable batteries. Electrochem,” Solid-State Lett. 1999, 2, 547–549.
[10]. Wang, C.S.; Wu, G.T.; Zhang, X.B.; Qi, Z.F.; Li, W.Z, “Lithium insertion in carbon‐silicon composite materials produced by mechanical milling,” J. Electrochem. Soc. 1998, 145, 2751–2758
[11]. K.Hayat, L.F.Vega, A.AlHajaj, “What have we learned by multiscale models on improving the cathode storage capacity of Li-air batteries? Recent advances and remaining challenges,” Volume 154, Renewable and Sustainable Energy Reviews, February 2022, 111849
[12]. Jake Christensen, Paul Albertus, Roel S. Sanchez-Carrera, “A Critical Review of Li/Air Batteries,” J. Electrochem. Soc. 2011, Volume 159, Issue 2, Pages R1-R30
[13]. K. Suto, S. Nakanishi, H. Iba, and K. Nishio, “An Aqueous Li-Air Battery Based on a Novel Reservoir Concept,” The 15th International Meeting on Lithium Batteries - IMLB 2010
[14]. Tao Liu, J. Padmanabhan.Vivek “Current Challenges and Routes Forward for Nonaqueous Lithium− Air Batteries,” Chem. Rev. 2020, 120, 14, 6558–6625
[15]. Won-Jin Kwak, Rosy, Daniel Sharon, Chun Xia, Hun Kim, “ Lithium–Oxygen Batteries and Related Systems: Potential, Status, and Future”, March 5, 2020, Chem. Rev. 2020, 120, 14, 6626–6683









