1. Introduction
Poly-fluoroalkyl substances (PFAS) are chemicals largely saturated by strong C-F bonds. As initially defined by Bucks et.al., PFASs are aliphatic substances that contain -CnF2n+1 moiety, where n is at least 1 [1]. Later reviews of PFASs continued to expand this definition, including that with functional groups on both ends, aromatic moieties, or cycloaliphatic substances in their studies. Due to the hydrophobic and oleophobic perfluorocarbon moieties, PFASs have extensive uses, especially as surfactants or surface protectors [2]. The recent overview of PFASs done by the Royal Society of Chemistry pinpointed 1400 individual PFASs for more than 200 uses[2]. Thus, the huge industrial manufacturing of PFASs has led to widespread contamination by PFASs, where PFASs have been released as industrial disposals since the 1940s [3]. As a result of the strong C-F covalent bonds, PFASs are not degradable under environmental conditions and thus accumulate in an extensive area of the world: the blood of polar bears in the Arctic, aquatic food webs for human seafood consumption, and human blood [3]. Human exposure to PFASs may cause severe health hazards. While significant correlations between elevated PFAS exposure and suppressed immune response are shown, the incidence of dyslipidemia is the strongest evidence for the correlated outcome of PFAS exposure [3]. As a result of various safety concerns, Europe and the USA gradually banned long-chain perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS), two of the predominant PFAS, in consumer products and also set drinking water standards for their concentrations [4]. These regulations on long-chain PFAS led to rising uses of short-chain PFAS, with less than six carbons in its backbone, as alternatives. However, the hazardous effects of short-chain PFAS are unclear now but may exceed that of long-chain PFAS, as short-chain compounds can more easily react with biomolecules due to less steric hindrance [3]. For example, “GenX” originally used as a PFOA alternative was found to possess higher toxicity than PFOA under considerations of toxicokinetic differences [5]. Thus, the removal of PFAS, especially short-chain ones, from drinking water is essential, as more short-chain PFAS are replacing their longer homologs. Three different removal methods of short-chain PFAS will be reviewed in this paper: Activated Carbon (AC), Ion Exchange Resins (IER), and Molecular Organic Frameworks (MOFs). This paper aims to summarize recent progress in short-chain PFAS removal and facilitate the future discussion of scholars.
2. Three removal methods of long-chain and short-chain PFAS
Common removal methods of PFAS are AC and IER, while the introduction of MOFs into this field of study has shown promising results. Figure 1 shows the structures and names of commonly detected aliphatic PFAS with -CnF2n+1 moiety. The three removal methods all involve different parts of those PFAS: their saturated C-F chain, unique functional groups, or sizes.
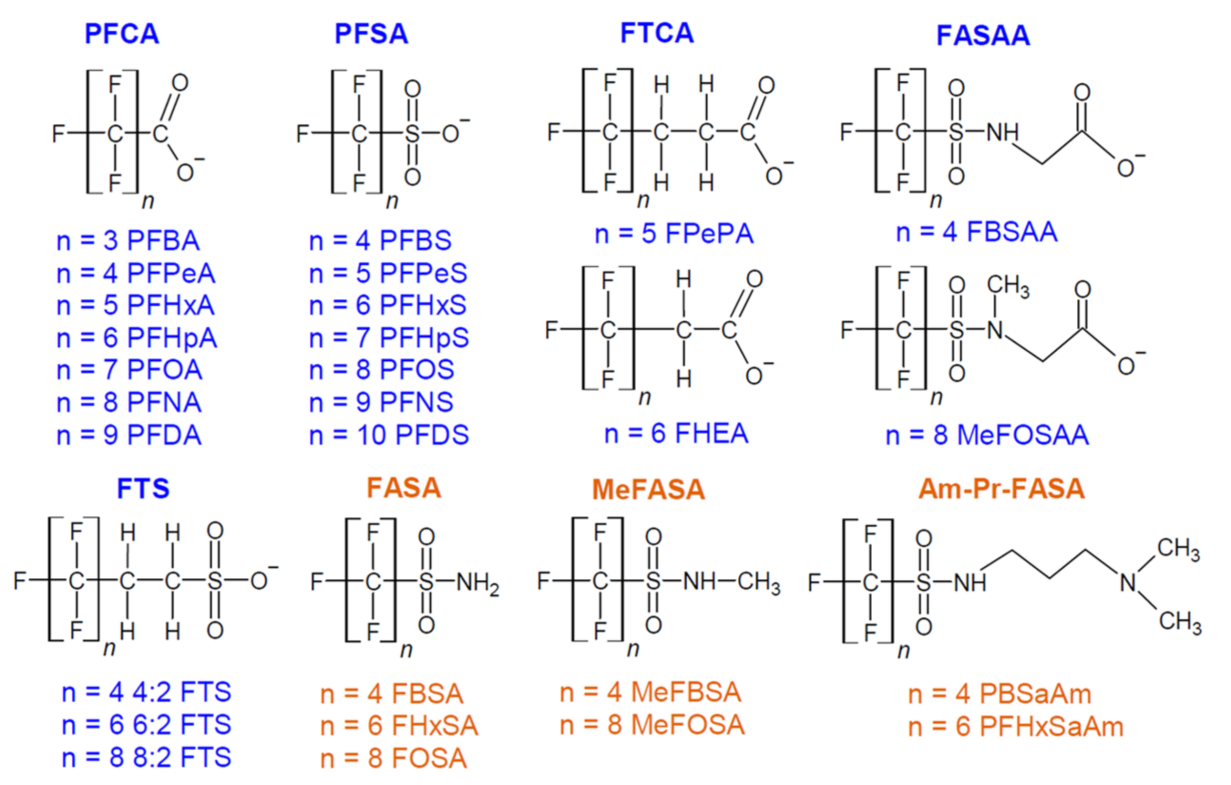
Figure 1: Structures of commonly detected long-chain and short-chain PFAS [6]. Some structures are deprotonated under neutral pH conditions. n notes the length of the individual C-F chains.
2.1. Activated Carbon
AC as a special type of carbonaceous material is a conventional method to adsorb or separate both long-chain and short-chain PFAS from water. Accounting for roughly 40% of PFAS removal studies, AC is a highly discussed method [7]. Overall, granular activated carbon (GAC), as a type of AC, has a high adsorption capacity reaching approximately 80% to 90% efficiency in removing a variety of PFAS [7]. The main mechanism of AC adsorption is the physical and hydrophobic interactions between non-polar carbonaceous materials and the hydrophobic C-F tail of PFAS [8]. Moreover, the anionic nature of most PFAS such as PFOA or PFOS also plays a role in interacting with basic sites of AC. A higher surface basicity may increase PFAS adsorption [8]. Recent studies have engineered AC to improve its basicity and hydrophobicity. While attaching carboxyl and hydroxyl groups through nanomaterials on AC significantly enhances the surface basicity and adsorption capacity, the addition of polymeric substances onto AC may increase the hydrophobicity, potentially exceeding the efficiency of AC made of natural materials [8]. Specifically, surface chemistry decides the adsorption capacity of PFAS, as basic and hydrophobic surfaces favor the process of multilayer adsorption mechanism [9].
Furthermore, AC made of different types of materials may present interesting features and benefits in PFAS removal. The creation of sludge-based activated carbon (SBAC) by Mohamed et al. is a novel generation of adsorbents [10]. The optimal SBAC developed from the manufactured sewage sludge that was dried, pyrolyzed, and activated may reach 90.6-100% removal efficiency, superior to that of GAC. SBAC also reduced the cost of producing AC for PFAS removal, having a price as low as 1.2 USD/kg when the commercial cost of AC was usually greater than 3 USD/kg. The additional benefit of SBAC not only brings down the price of PFAS removal but also reduces the polluting disposal of sewage waste, achieving a green circular economy [10]. Another interesting example of AC material is the biologically activated carbon (BAC). Bacteria can activate AC with a large surface area and microporous nature by forming biofilms [11]. While the microbial species are not changed by the maturity and depth of the carbon, PFAS removal can be affected by the species of the bacteria: Proteobacteria is crucial in removing PFAS by BAC. The performance of BAC reduced the PFAS content from 127.4 ng/L to 101.9 ng/L in raw water, which is more effective than the conventional process in drinking water treatment plants (DWTP). The additional benefits of using BAC lie within the long-term performance in years and the degradation potential of BAC [11].
Lastly, the PFAS removal efficiency of AC is also affected by the C-F chain length of PFAS. The predominant types of PFAS are PFOA and PFOS which both have a chain length of 8. Since higher chain length means higher hydrophobicity, long-chain PFAS are better adsorbed by AC than short-chain analogues. Using batch studies, Riegel et al. found that longer perfluoroalkyl carboxylic acids are adsorbed better than their short-chain analogues [12]. Also, the type of GAC materials influenced the result of long-chain and short-chain PFAS removal. Whereas the best adsorbent for PFOA was GAC made from lignite with an Iodine number of 650, it showed the lowest adsorption capacity for PFBA. For PFBA, GACs made from agglomerated bituminous coal performed the best. Eventually, since short-chain PFAS removal needs a much larger throughput of bed volume and regeneration to be effective, most short-chain PFAS removal is not recommended for AC. Considering operation and economic factors, GAC removal of PFAS is only effective for long-chain PFOA and short-chain PFHpA and PFHxA [12].
2.2. Ion Exchange Resins
IERs have shown to be an effective method to remove both long-chain and short-chain PFAS, generally having a high removal capacity, affordable cost, and ease of in-situ regeneration [8]. The anionic nature of PFAS can be attracted by anion exchange resins (AERs) which are usually positively charged. While the hydrophobic C-F tail of PFAS can be adsorbed by the neutral and hydrophobic part of the insoluble polymer backbone of AERs, the anionic head can be attracted by the positively charged surface sites of AERs. AERs are a relatively efficient material for attracting short-chain PFAS since the main interaction is anion exchange [8].
Although the mechanism of PFAS removal involves hydrophobic effects, van der Waals forces, hydrogen bonding, electrostatic interactions, and \( π-π \) bonding, electrostatic and hydrophobic interactions are the main mechanisms for IERs removing PFAS [13]. For the verification of the electrostatic interaction mechanism, changes in surface charges of IERs directly affected the uptake of anionic PFAS. The anion exchange process was further verified by plotting PFAS uptake against the release of chloride ions, showing a slope of 1. For the hydrophobic effects, the entropy-driven movements of water-repelling C-F chains of PFAS tended to move towards the hydrophobic surfaces of IERs. In some instances, the hydrophobic interaction overcame the electrostatic repulsions when anionic PFAS was adsorbed onto anionic surfaces. In addition, when the ionic concentration of water increases, the hydrophobic interactions become more significant [13].
Lenka et al. explored the adsorption of short-chain and ultrashort-chain PFAS by three different AERs and one AC [14]. Among the investigated adsorbents, activated carbon, strong base AER: A900, weak base AER: V1, and weak base AER: V3, AER: A900 performed the best adsorption of short-chain and ultrashort-chain PFAS such as PFBS, PFBA, and PFPrA. Several factors of adsorption were identified: adsorbate concentrations and characteristics, adsorbent properties, and the water matrix. At a high concentration (2 to 8 mg/L) of short-chain and ultrashort-chain PFAS, A900 presented a significantly higher capacity removing 94.42% of PFPrA, 91.18% of PFBA, and 59.66% of PFBS. At a pH lower than the pH at the point of zero charge of A900, its quaternary ammonium groups became positively charged and interacted with negatively charged PFAS through anion exchange. Then, the hydrophobic styrene/divinylbenzene backbone performed hydrophobic interactions with the C-F chains of PFAS. When the concentration of PFAS is low, the ionic interactions and the hydrophobic interactions become equally important in A900’s adsorptive removal of PFAS [14].
2.3. Metal-organic Frameworks
Metal organic frameworks (MOFs) are crystalline structures composed of metal ion nodes and organic linkers. Through coordination bonding, the nodes and linkers can self-assemble into 3D lattices [7]. Having high surface area, porosity, selectivity, and stability, it is widely used for gas storage, adsorption, separation, electrochemistry, etc. [15]. For reference, the pore volume and surface area of MOFs can achieve 3.9 cm3/g and 7130 m2/g, correspondingly. Hence, introducing MOFs into PFAS removal has become a recent target for researchers. However, MOFs need hydrolytic stability for effective adsorption of PFAS in water. For example, the Zeolitic imidazolate framework (ZIF) and chromium (III) terephthalate metal-organic framework (MIL-101) are water-stabilized and suitable for PFAS removal in water [7]. The interactions between MOFs and PFAS can be classified into ionic interactions, hydrophobic interactions, acid-base interactions, and fluorophilic interactions. By synthesizing a modified MOF, fluorinated zirconium-based MOF, UiO-66-(F4), researchers observed the strong interaction between the fluoride molecules [8].
In a systematic review of three specific MOFs, NU-1000, UiO-66, and ZIF-8, on both ionic and non-ionic PFAS adsorption, Li et al. considered the effects of porosity and node composition of MOFs [6]. Within the three MOFs, NU-1000 has shown the highest rate of removal for both anionic and non-ionic PFAS. While similar hydrophobic interactions existed in adsorbing both anionic and non-ionic PFAS, other factors contribute to NU-1000’s excellent adsorption capacity. For anionic PFAS, the deprotonated carboxylic or sulfonic group of PFAS might replace the terminally coordinated hydroxo ligands of Zr6 nodes in NU-1000 and achieve anion exchange. Second, the hydrophobic interactions existed: the pyrene-based ligands of NU-1000 formed hydrophobic pockets and interacted with the C-F chain of PFAS. Third, based on Pearson’s acid-base concept, small highly charged species, considered hard acids, should interact strongly with small highly electronegative species, considered hard bases. The sulfonates or carboxylates as hard bases interacted strongly with Zr6 as strong acids. For non-ionic PFAS, the hydrophobic interactions were similar to that of anionic PFAS. Nevertheless, the amine groups of non-ionic PFAS were fitted the definition of hard bases that interacted with Zr6-nodes. In addition, the μ3-bridging hydroxo groups of the Zr6-nodes might be deprotonated by amine groups of non-ionic PFAS. As a result, the strong electrostatic μ3-O2−−NH3+ interactions held NU-1000 and non-ionic PFAS together. Furthermore, the fast adsorption kinetics of NU-1000 and PFAS could be explained by the large pore sizes of NU-1000. As the pores (33 Å and 13 Å) were greatly larger than the kinetic diameter of PFOS (2.9-3.8 Å), NU-1000 took only one minute to reach the equilibrium state compared with commercial GAC which took more than 48 hours in this study. Eventually, regarding the C-F chain length, Li et al. found out that longer chain length led to higher adsorption, while the initial concentrations did not affect this chain-length dependency [6].
3. Conclusion
This paper reviewed the current process of adsorbing long-chain and short-chain PFAS by AC, IER, and MOF. The adsorption mechanism and modification of each removal method are presented. AC remains an economical method for removing long-chain PFAS, especially made from sewage sludge. IER shows a high removal capacity for both long-chain and short-chain PFAS. MOF emerges as a fast and effective method for removing long-chain PFAS and certain short-chain PFAS. This paper only summarized the features and process of each method, lacking a comparative analysis of these materials. Also, the removal of short-chain PFAS is relatively discussed, as most removal mechanisms of short-chain PFAS are not clear. Subsequent studies may focus on the comparative analysis of these materials and the potential direction for effectively removing short-chain PFAS.
References
[1]. Buck, R. C.; Franklin, J.; Berger, U.; Conder, J. M.; Cousins, I. T.; de Voogt, P.; Jensen, A. A.; Kannan, K.; Mabury, S. A.; van Leeuwen, S. P. Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integrated Environmental Assessment and Management 2011, 7 (4), 513–541. https://doi.org/10.1002/ieam.258.
[2]. Glüge, J.; Scheringer, M.; Cousins, I. T.; DeWitt, J. C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C. A.; Trier, X.; Wang, Z. An Overview of the Uses of Per- and Polyfluoroalkyl Substances (PFAS). Environmental Science: Processes & Impacts 2020, 22 (12). https://doi.org/10.1039/d0em00291g.
[3]. Sunderland, E. M.; Hu, X. C.; Dassuncao, C.; Tokranov, A. K.; Wagner, C. C.; Allen, J. G. A Review of the Pathways of Human Exposure to Poly- and Perfluoroalkyl Substances (PFASs) and Present Understanding of Health Effects. Journal of Exposure Science & Environmental Epidemiology 2019, 29 (2), 131–147. https://doi.org/10.1038/s41370-018-0094-1.
[4]. Wang, J.; Shen, C.; Zhang, J.; Lou, G.; Shan, S.; Zhao, Y.; Yu Bon Man; Li, Y. Per- and Polyfluoroalkyl Substances (PFASs) in Chinese Surface Water: Temporal Trends and Geographical Distribution. Science of The Total Environment 2024, 915, 170127–170127. https://doi.org/10.1016/j.scitotenv.2024.170127.
[5]. Wang, Z.; Cousins, I. T.; Scheringer, M.; Hungerbühler, K. Fluorinated Alternatives to Long-Chain Perfluoroalkyl Carboxylic Acids (PFCAs), Perfluoroalkane Sulfonic Acids (PFSAs) and Their Potential Precursors. Environment International 2013, 60, 242–248. https://doi.org/10.1016/j.envint.2013.08.021.
[6]. Li, R.; Alomari, S.; Islamoglu, T.; Farha, O. K.; Fernando, S.; Thagard, S. M.; Holsen, T. M.; Wriedt, M. Systematic Study on the Removal of Per- and Polyfluoroalkyl Substances from Contaminated Groundwater Using Metal–Organic Frameworks. Environmental Science & Technology 2021, 55 (22), 15162–15171. https://doi.org/10.1021/acs.est.1c03974.
[7]. Amen, R.; Ibrahim, A.; Shafqat, W.; Hassan, E. B. A Critical Review on PFAS Removal from Water: Removal Mechanism and Future Challenges. Sustainability 2023, 15 (23), 16173. https://doi.org/10.3390/su152316173.
[8]. Vu, C. T.; Wu, T. Recent Progress in Adsorptive Removal of Per- and Poly-Fluoroalkyl Substances (PFAS) from Water/Wastewater. Critical Reviews in Environmental Science and Technology 2020, 52 (1), 1–40. https://doi.org/10.1080/10643389.2020.1816125.
[9]. Kim, G.; Mengesha, D. N.; Choi, Y. Adsorption Dynamics of PFAS on Activated Carbon: Interplay of Surface Chemistry and PFAS Structural Properties. Separation and Purification Technology 2024, 349, 127851–127851. https://doi.org/10.1016/j.seppur.2024.127851.
[10]. Mohamed, B. A.; Li, L. Y.; Hamid, H.; Jeronimo, M. Sludge-Based Activated Carbon and Its Application in the Removal of Perfluoroalkyl Substances: A Feasible Approach towards a Circular Economy. Chemosphere 2022, 294, 133707. https://doi.org/10.1016/j.chemosphere.2022.133707.
[11]. Zhong, T.; Lin, T.; Zhang, X.; Jiang, F.; Chen, H. Impact of Biological Activated Carbon Filtration and Backwashing on the Behaviour of PFASs in Drinking Water Treatment Plants. Journal of Hazardous Materials 2023, 446, 130641–130641. https://doi.org/10.1016/j.jhazmat.2022.130641.
[12]. Riegel, M.; Haist-Gulde, B.; Sacher, F. Sorptive Removal of Short-Chain Perfluoroalkyl Substances (PFAS) during Drinking Water Treatment Using Activated Carbon and Anion Exchanger. Environmental Sciences Europe 2023, 35 (1). https://doi.org/10.1186/s12302-023-00716-5.
[13]. Dixit, F.; Dutta, R.; Barbeau, B.; Berube, P.; Mohseni, M. PFAS Removal by Ion Exchange Resins: A Review. Chemosphere 2021, 272, 129777. https://doi.org/10.1016/j.chemosphere.2021.129777.
[14]. Lenka, S. P.; Kah, M.; Chen, J.; Andres, B.; Lokesh Padhye. Adsorption Mechanisms of Short-Chain and Ultrashort-Chain PFAS on Anion Exchange Resins and Activated Carbon. Environmental Science Water Research & Technology 2024, No. 5. https://doi.org/10.1039/d3ew00959a.
[15]. Saeed, T.; Ahmad, S.; Naeem, A.; Haleem, A.; Ahmad, B.; Sayed, M.; Nazish Huma Khan; Rida Ihsan. An Overview of Investigation of Metal and Covalent Organic Frameworks for Various Applications. Journal of Molecular Structure 2024, 1312, 138475–138475. https://doi.org/10.1016/j.molstruc.2024.138475.
Cite this article
Ji,Z. (2025). Recent Methods of Effectively Removing Long-chain and Short-chain Per-and Poly-fluoroalkyl Substances from Drinking Water. Applied and Computational Engineering,126,83-88.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Buck, R. C.; Franklin, J.; Berger, U.; Conder, J. M.; Cousins, I. T.; de Voogt, P.; Jensen, A. A.; Kannan, K.; Mabury, S. A.; van Leeuwen, S. P. Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integrated Environmental Assessment and Management 2011, 7 (4), 513–541. https://doi.org/10.1002/ieam.258.
[2]. Glüge, J.; Scheringer, M.; Cousins, I. T.; DeWitt, J. C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C. A.; Trier, X.; Wang, Z. An Overview of the Uses of Per- and Polyfluoroalkyl Substances (PFAS). Environmental Science: Processes & Impacts 2020, 22 (12). https://doi.org/10.1039/d0em00291g.
[3]. Sunderland, E. M.; Hu, X. C.; Dassuncao, C.; Tokranov, A. K.; Wagner, C. C.; Allen, J. G. A Review of the Pathways of Human Exposure to Poly- and Perfluoroalkyl Substances (PFASs) and Present Understanding of Health Effects. Journal of Exposure Science & Environmental Epidemiology 2019, 29 (2), 131–147. https://doi.org/10.1038/s41370-018-0094-1.
[4]. Wang, J.; Shen, C.; Zhang, J.; Lou, G.; Shan, S.; Zhao, Y.; Yu Bon Man; Li, Y. Per- and Polyfluoroalkyl Substances (PFASs) in Chinese Surface Water: Temporal Trends and Geographical Distribution. Science of The Total Environment 2024, 915, 170127–170127. https://doi.org/10.1016/j.scitotenv.2024.170127.
[5]. Wang, Z.; Cousins, I. T.; Scheringer, M.; Hungerbühler, K. Fluorinated Alternatives to Long-Chain Perfluoroalkyl Carboxylic Acids (PFCAs), Perfluoroalkane Sulfonic Acids (PFSAs) and Their Potential Precursors. Environment International 2013, 60, 242–248. https://doi.org/10.1016/j.envint.2013.08.021.
[6]. Li, R.; Alomari, S.; Islamoglu, T.; Farha, O. K.; Fernando, S.; Thagard, S. M.; Holsen, T. M.; Wriedt, M. Systematic Study on the Removal of Per- and Polyfluoroalkyl Substances from Contaminated Groundwater Using Metal–Organic Frameworks. Environmental Science & Technology 2021, 55 (22), 15162–15171. https://doi.org/10.1021/acs.est.1c03974.
[7]. Amen, R.; Ibrahim, A.; Shafqat, W.; Hassan, E. B. A Critical Review on PFAS Removal from Water: Removal Mechanism and Future Challenges. Sustainability 2023, 15 (23), 16173. https://doi.org/10.3390/su152316173.
[8]. Vu, C. T.; Wu, T. Recent Progress in Adsorptive Removal of Per- and Poly-Fluoroalkyl Substances (PFAS) from Water/Wastewater. Critical Reviews in Environmental Science and Technology 2020, 52 (1), 1–40. https://doi.org/10.1080/10643389.2020.1816125.
[9]. Kim, G.; Mengesha, D. N.; Choi, Y. Adsorption Dynamics of PFAS on Activated Carbon: Interplay of Surface Chemistry and PFAS Structural Properties. Separation and Purification Technology 2024, 349, 127851–127851. https://doi.org/10.1016/j.seppur.2024.127851.
[10]. Mohamed, B. A.; Li, L. Y.; Hamid, H.; Jeronimo, M. Sludge-Based Activated Carbon and Its Application in the Removal of Perfluoroalkyl Substances: A Feasible Approach towards a Circular Economy. Chemosphere 2022, 294, 133707. https://doi.org/10.1016/j.chemosphere.2022.133707.
[11]. Zhong, T.; Lin, T.; Zhang, X.; Jiang, F.; Chen, H. Impact of Biological Activated Carbon Filtration and Backwashing on the Behaviour of PFASs in Drinking Water Treatment Plants. Journal of Hazardous Materials 2023, 446, 130641–130641. https://doi.org/10.1016/j.jhazmat.2022.130641.
[12]. Riegel, M.; Haist-Gulde, B.; Sacher, F. Sorptive Removal of Short-Chain Perfluoroalkyl Substances (PFAS) during Drinking Water Treatment Using Activated Carbon and Anion Exchanger. Environmental Sciences Europe 2023, 35 (1). https://doi.org/10.1186/s12302-023-00716-5.
[13]. Dixit, F.; Dutta, R.; Barbeau, B.; Berube, P.; Mohseni, M. PFAS Removal by Ion Exchange Resins: A Review. Chemosphere 2021, 272, 129777. https://doi.org/10.1016/j.chemosphere.2021.129777.
[14]. Lenka, S. P.; Kah, M.; Chen, J.; Andres, B.; Lokesh Padhye. Adsorption Mechanisms of Short-Chain and Ultrashort-Chain PFAS on Anion Exchange Resins and Activated Carbon. Environmental Science Water Research & Technology 2024, No. 5. https://doi.org/10.1039/d3ew00959a.
[15]. Saeed, T.; Ahmad, S.; Naeem, A.; Haleem, A.; Ahmad, B.; Sayed, M.; Nazish Huma Khan; Rida Ihsan. An Overview of Investigation of Metal and Covalent Organic Frameworks for Various Applications. Journal of Molecular Structure 2024, 1312, 138475–138475. https://doi.org/10.1016/j.molstruc.2024.138475.









