1. Introduction
Since the advent of the Industrial Revolution, traditional fossil fuels, such as natural gas, coal, oil and so on, which have been important in the development of industry[1]. However, their environmental impact, including the formation of the ozone hole and global warming, has been significant. In the 21st century, the focus is on energy sources that can renew, such as water-power and wind-power, and there is also a growing need for large-scale energy storage. However, these sources can't provide uninterrupted electricity [2, 3]. Lithium-ion batteries are widely utilized due to their high energy density, long lifespan, low self-discharge rate, and lack of memory effect. These characteristics have enabled their extensive application in electric vehicles and mobile phones. Furthermore, they can maintain performance even with partial charges, which makes them durable and efficient. A lithium-ion battery is composed of four main components: the cathode, anode, electrolyte and separator. Battery’s operation depends on the lithium ions' intercalation and de-intercalation between the positive electrode and negative electrode (As shown in Figure 1). However, with the development of technology, the intensification of performance requirements has brought to light a few issues relating to the utilization of these applications. These include the necessity of higher energy density, the reduction in cycle life upon repeated utilization, and the emergence of thermal runaway incidents during periods of overcharge or over-discharge. It is of the utmost importance to address these challenges to facilitate the continued advancement of lithium-ion battery technology.
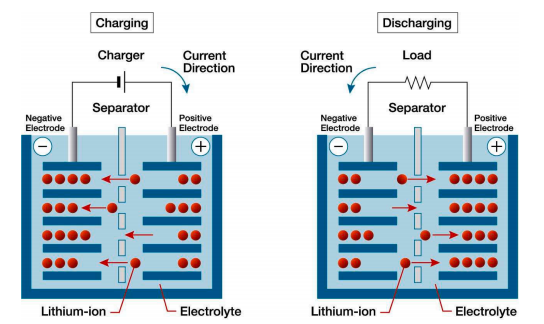
Figure 1: Figure with short caption (caption centred).
To address the needs of lithium battery development, researchers have sought to optimize the lithium-ion batteries’ efficiency by changing electrode materials’ type. It is successful that advances in anode materials have contributed to the progress of lithium battery. Young-Ok Kim and Su-Moon Park employed nuclear magnetic resonance (NMR) and electrochemical impedance spectroscopy (EIS) to investigate and clarify the mechanism of lithium-ion intercalation in graphite anodes [4]. Yuki Yamada and colleagues employed alternating current impedance spectroscopy to study the transport dynamics of lithium ions at the graphite anode-electrolyte interface [5]. Yixian Wang and collaborators [6] synthesized large interlayer spacing, ultra-thin amorphous carbon nanosheets using a tuneable molten salt method, while Wei Guo et al. prepared amorphous carbon nanosheets with a strong nitrogen doping through thermal decomposition, which enhanced lithium-ion storage capacity and fast transport [7]. Additionally, researchers have explored the use of silicon-based, tin-based and more recently, graphene-based materials in lithium-ion battery anodes, all achieving partial progress.
The article provides an overview of recent worldwide research on anode materials used in lithium-ion batteries. And it mainly focuses on the future and status of various anode materials and highlights the influence of each material on performance of batteries when they used as an anode. The objective of this review is to critically analyse the current state of research on anode materials used in lithium-ion batteries, with the aim of identifying the key challenges and proposing potential avenues for future investigation.
2. Research status of Lithium-ion battery anode materials
2.1. Graphite Anode Materials
Since the invention of lithium-ion batteries, carbon-based materials, particularly graphite, soft carbon and hard carbon, have been the preferred options for anodes. This is because carbon compounds have a high electrical conductivity, their low raw material cost and their versatility in structural design, encompassing 0D to 3D structures. Furthermore, carbon materials exhibit excellent mechanical properties and thermal conductivity. In recent decades, advances in battery technology have resulted in graphite becoming the predominant anode material, with hard carbon being gradually superseded. Compared to other materials made of carbon, such as carbon nanomaterials and graphene, graphite exhibits a lower lithium intercalation potential and offers a higher theoretical capacity, along with superior performance during the initial lithium intercalation. The material's outstanding physical properties stem from its layered crystal structure, where van der Waals forces and π-π interactions hold the layers together. During charging, lithium ions can intercalate between these layers due to the weak van der Waals forces, leading to the formation of lithium-rich compounds such as LiC6.
The stability of the graphite electrode-electrolyte interface is a crucial factor in the performance of lithium-ion batteries. The surface of the anode is coated with a solid electrolyte interphase (SEI). During initial charging, enabling the passage of lithium ions while impeding electron flow. However, the use of solvents containing lithium ions has been observed to result in the deterioration of the SEI, which in turn leads to a reduction in battery lifespan and efficiency. Additionally, the expansion of graphite during the charging process can potentially impact the structural integrity and safety of the battery.
2.2. Amorphous carbon materials
Amorphous carbon materials possess larger interlayer spacing and specific surface area compared to traditional graphite, offering more active sites for lithium-ion intercalation/deintercalation, thereby enhancing lithium-ion storage capacity. The disordered structure, with numerous micropores and nanopores, further improves lithium-ion intercalation in anode materials. Amorphous carbon can assume a variety of forms, including 0D nanocrystals, 1D nanotubes, 2D nanosheets and 3D porous structures[8]. These forms effectively shorten ion diffusion paths and enhance transmission capability.
By adjusting the carbonization temperature, it is possible to synthesize ultrathin amorphous carbon nanosheets with larger surface areas and higher thermal stability, which demonstrate exceptional reversible capacity and cycling performance[8]. Furthermore, amorphous carbon is also often combined with materials like graphite and silicon to form composites, serving as a conductive network and mitigating volume expansion with cycles of charge and discharge, thereby enhancing rate performance and stability.
In addition, amorphous carbon can be coated on solid electrolytes to enhance interfacial stability between lithium metal anodes and solid electrolytes, inhibiting lithium dendrite formation and extending battery life. Due to its unique structure and properties, amorphous carbon is a highly promising candidate for use as an anode material in lithium-ion batteries.
2.3. Transition Metal Oxide Materials
Research on anode materials made of transition metal oxides can be traced back to the 1990s. When used as anode electrodes, transition metal oxides show different electrochemical properties compared to graphite electrodes, as discovered by researchers[9]:
(1) Nanoscale transition metal oxides demonstrate an electrochemical capacity of approximately 700 mAh/g, which is nearly double the graphite electrodes' theoretical capacity (372 mAh/g). Additionally, metal oxide electrodes have shown an impressive 100% capacity retention over 100 cycles in experimental conditions, a feature not found in graphite electrodes.
(2) The transition metal oxide anode's reaction mechanism is unique, involving Li2O formation and decomposition (As shown in Eq.(1)), along with redox reactions, unlike traditional lithium insertion/extraction or alloying processes.
\( {M_{x}}{O_{y}}+z{Li_{2}}O→2zLiM{O_{n}} \) (1)
* M represents the transition metal (such as Co, Ni, Mn, etc.)
* \( x \) , \( y \) , \( z \) , and \( n \) are stoichiometric coefficients in the reaction, which depend on the chemical composition of the transition metal oxide.
Over the past 30 years, researchers have optimized transition metal oxide electrodes. Fe₃O₄ present certain challenges, namely low conductivity and volume expansion, but abundance, low cost, and high theoretical capacity (924 mAh/g) are also characteristics of this. Niobium-based oxides have gained attention for their higher working potential, stable lithium-ion transport channels, and ability to prevent lithium dendrites. Recently, nickel niobate has been seen as a prospective high-rate anode material, thanks to its unique crystal structure (octahedral structure) that allows efficient ion transport without structural modifications.
The performance of transition metal oxide electrodes is influenced by both the metal type and the electrode structure. To address poor conductivity and volume expansion during charge-discharge cycles, researchers suggest using nanostructured oxides. These nanostructures reduce ion diffusion pathways, improving transport rates and providing more active sites for lithium-ion insertion and extraction. Furthermore, nanostructured materials demonstrate enhanced resilience to volume fluctuations in comparison to their conventional counterparts, thereby improving the overall durability of the material in the process of cycling.
The latest research direction in anode materials is the development of transition metal oxide glasses, particularly for tin-based and vanadium-based glass materials[10]. The unique amorphous structure of glass materials offers superior structural stability and cycling life compared to traditional materials. The disordered structure can effectively mitigate the issue of anode volume expansion and enable higher conductivity and ion migration rates.
2.4. Other Materials
In addition to the above common lithium ion anode materials, researchers are also studying other new alternative materials.
2.4.1. Graphene material
Graphene's remarkable two-dimensional structure and exceptional electrical conductivity make it a potential choice as the anode material for lithium-ion batteries. Its theoretical capacity is 744 mAh/g, considerably higher than that of the traditional graphite anode. The researchers suggested improving the electrochemical performance of graphene anodes by utilizing porous, defective or doped graphene materials to boost lithium storage capacity and enhance overall performance. The use of graphene combined with silicon or oxide composite materials can effectively address the issue of electrode expansion during charge-discharge cycles, further optimizing performance.[11, 12].
2.4.2. Silicon-based Materials
Silicon's specific capacity can reach as high as 4200 mAh/g, more than ten times that of graphite, making it a key focus of research as a negative electrode material[11]. However, during the charge-discharge cycle, silicon can undergo volume expansion of more than 300%, resulting in structural damage and limiting its usefulness[13]. To solve this problem, the researchers synthesized silicon/graphene composites, which can buffer the silicon's volume change and reduce electrode pulverization, thereby improving cycle stability and capacity retention(As shown in Table.1). The conductivity and stability of silicon-based materials are further enhanced by the design of core-shell structure, hollow structure, and porous structure[11].
2.4.3. Tin-based Materials
Tin-based materials, with their high specific capacity and abundant availability, are theoretically excellent alternatives to graphite. The rapid capacity degradation of tin-based materials during charging and discharging leads to reduced battery life. To address this issue, Lei Chen et al. improved electrode performance by growing SnSSe on graphene and coating it with conductive carbon, which inhibited volume expansion [14]. Similarly, Hangjun Ying et al. adopted a dual fixation strategy of nitrogen-doped carbon and TiO2 to prevent the migration and Synthesis of SnOx nanoparticles and improve the material's stability and conductivity [15]. These strategies are designed to extend the life and improve tin-based materials in battery.
Table 1: Typical electrochemical characteristics of the anode of silicon/graphite composites.
Electrode | Si: Graphite Ratio (by weight) | Mass loading (mg ·cm-2) | ICE | Reversible capacity (mAh/g) in the 1st cycle | Areal capacity (mAh/cm) | Cycle number | Capacity retention | Voltage range/1C |
Si: graphite[16] | 37.5 : 62.5 | 1 | 77% | 1001 at 0.1 A/g | — | 100 | 850 mAh/g, >80%, at 0.25A/g; 800 mAh/g at 5A/g | 1st cycle: 0.005- 0.9V after 1st cycle: 0.05-0.9V |
C/Si@MPC-G[17] | 9.72% Si | — | 90.9% | 665 at 0.5C | 3.6 | 50 | 95.6%, at 0.5C | 1st cycle: 0.01-1.5V After 1st cycle: 0.01-1.0V |
Si: Macropore- exploited graphite: Pitch: carbon (EGS)[18] | 17:73:10 | 1.7 | 92.9% | 914 at 0.1C | 3.4 | 50 | 789 mAh/g, 86%,at 0.5C | 0.005-1.0V |
Si: graphite (MGS)[19] | 6.3:93.7 | 6.9 | 93.0% | 527 at 0.1C | 3.5 | 50 | 516 mAh/g, >97% at 0.5C | 0.005-1.0V |
B-Si/CNT @ Graphite[20] | 90% graphite | 11.2 | — | — | 5.2 | 100 | 83.4%, at 1.12 mA/cm2 | 1.5V |
Si/graphite/carbon [21] | ~10% Si | — | 90.6% at 0.5C | 687.7 at 0.5C, 650 at 1C | — | 50 | 96.7%, at 1C | 0.001-1.5V, 1C=1000mAh/g |
Si/graphite/ pyrolytic Carbon (SiGC)[22] | 12.8% Si | 1.1 | >80% | 818 at 0.1 A/g | — | 300 | 610 mAh/g, 83.6%, at 0.5 A/g | 0.01-1.5V |
Porous Si/C-graphite[23] | Si/C : graphite 1:2 | — | 65% | 650 at 1/6C | 2.52 | 450 | 533 mAh/g, 82%,At 1/6C | 0.005-1.0V, 1C=2.4mAh/ cm2 |
Si/graphite(SEAG)[24] | 6.3% Si, 2.8% Ni | 7.1 | 93.8% | 525 at 0.1C | ≥3.4 | 50 | 99.3%, at 0.5C | 1st cycle: 0.005-1.5V after 1st cycle: 0.05-1.0V,1C=3.5 mAh/cm2 |
Si/C (Watermelon)[25] | 12.5:87.5 | 4.1 | 89.2% | 620 at 0.1C | 2.54 | 500 | 1.91 mAh/cm2, 75%,At 0.5C | 0.005-1.0V, 1C= 600mAh/g |
3. Challenges and bottlenecks of negative electrode materials
Significant progress has been achieved in the study of anode materials for lithium-ion batteries. However, the commonly used graphite electrodes are gradually failing to meet the demands of rapidly advancing technology. The selection of raw materials and the structural design of the anode directly influence the battery's capacity, cycle life, and rate performance. These limitations represent a significant obstacle to the further advance of lithium-ion batteries[3, 26, 27]The following section outlines the principal challenges and constraints associated with the advance of lithium-ion battery anode materials.
3.1. Volume Expansion
Volume expansion represents a significant challenge currently facing anode materials. Regardless of the material type, volume expansion occurs to varying degrees during the lithiation process. Given the limited space within the battery, excessive changes in the volume of electrode materials can lead to alterations in the battery structure, particle fracture, and increased contact resistance, ultimately resulting in capacity degradation[27]. As mentioned earlier, while high-capacity anode materials, such as silicon-based and tin-based materials, have higher theoretical capacities, they also have greater volume changes. To illustrate, silicon-based materials have been observed to undergo expansion of up to 300%.
3.2. Conductivity and Ion Transport Problems
The inherent disadvantage of high-capacity materials, such as silicon, is their low electrical conductivity. While charging and discharging, the ability of electrons to transfer within the anode is weak. This low conductivity directly reduces the efficiency of the battery in terms of charge and discharge, particularly under conditions of high rate. Poor electron conduction leads to increased internal resistance in the battery, hindering the rapid completion of electrode reactions. As a result, the battery's rate performance and power density are diminished[27].
In anode materials, not only electrons but also ions need to be transported. However, in high-capacity materials, the lithium-ion transport rate is relatively slow. Over extended cycle periods, the internal structure of the electrode undergoes alterations due to the processes of lithium de-intercalation and intercalation, which consequently impact the ion transport pathways[28]. The SEI generated on the negative electrode further increases the resistance to ion transport.
3.3. Initial Coulomb efficiency problem
As outlined in Section 3.2, after the first cycle of charging and discharging, SEI film naturally forms on the outside of the anode material. Due to the significant amount of lithium ions consumed in this process, the initial coulombic efficiency is often below 80%. Such inefficiency not only leads to the depletion of valuable active lithium ions but, more significantly, imposes a restriction on the battery’s overall capacity output. The permanent depletion of lithium ions in forming the SEI layer hampers the battery’s performance from the outset, limiting its maximum potential energy storage. This not only impacts the battery’s lifespan and efficiency but also poses a significant challenge in the development of higher-performing lithium-ion batteries, especially in use where both energy density and lifecycle are critical, such as E-car and renewable energy storage systems.
3.4. Lithium Dendrite Formation and Security Threat
The lithium dendrites’ formation is also one of the challenges to be solved. The growth of lithium dendrites will not only reduce the efficiency of the battery, but also pose great threats to the safety of the battery. These threats mainly include the following.
The growth of lithium dendrites may lead to membrane damage, short circuits in batteries, uncontrolled heat generation due to internal temperature rise, and uncontrolled internal chemical reactions[26]. Along with the generation of a large amount of heat, a lot of gas is also released, increasing the threat of battery explosion or fire. The formation of lithium dendrites can lead to the deactivation of active lithium ions, resulting in a reduction in battery capacity.
4. Conclusion and Future Direction
The content above summarizes the current research status, issues and challenges faced by negative electrode materials for lithium-ion batteries. By comparing and analyzing the effects of different materials on the performance of negative electrodes, further thinking and data support are provided for the improvement and development of negative electrode materials. Notwithstanding the considerable advances that have been made in the field of battery negative electrode materials, several challenges remain, including issues such as low original coulombic efficiency and volume expansion. In addition to the issues inherent to the negative electrode material itself, current research has mostly focused on the electrode's performance under conventional conditions. There is a lack of understanding regarding the efficiency performance of the electrode in extreme environments.
In the future research, the following aspects will be the first focus of investigation. Firstly, increase research on composite materials. The electrochemical properties exhibited by different materials under different conditions are different. By combining different materials, their respective advantages can be fully utilized. Secondly, optimize the electrode structure design. In the future, further research will explore how nanotechnology can be used to modify electrode structures. By utilizing nanotechnology, the electrodes will gain a larger specific surface area and additional active sites for lithium-ion adsorption. Nanostructures can prevent the growth of electrode volume during charging and draining procedures, as well as decrease the diffusion path of lithium ions. Lithium-ion batteries are expected to remain the cornerstone of energy storage systems in the future, with research on negative electrode materials driving the development of next-generation advanced batteries. Global sustainable development goals will be achieved by the widespread use of high-performance pools in industries such as E-cars, manufacturing, and sustainable energy storage in the future.
References
[1]. T. Ahmad and D. Zhang, J. (2020). A critical review of comparative global historical energy consumption and future demand: The story told so far, Energy Reports, 6.
[2]. M. H. Hossain, M. A. Chowdhury, N. Hossain, M. A. Islam, and M. H. Mobarak, J. (2023). Advances of lithium-ion batteries anode materials-A review, Chemical Engineering Journal Advances, 16.
[3]. P. U. Nzereogu, A. D. Omah, F. I. Ezema, E. I. Iwuoha, and A. C. Nwanya, J. (2022). Anode materials for lithium-ion batteries: A review, Applied Surface Science Advances, 9.
[4]. Y. O. Kim and S. M. Park, J. (2001). Intercalation mechanism of lithium ions into graphite layers studied by nuclear magnetic resonance and impedance experiments, Journal of the Electrochemical Society,148.
[5]. Y. Yamada, Y. Iriyama, T. Abe, and Z. Ogumi, J. (2009). Kinetics of Lithium Ion Transfer at the Interface between Graphite and Liquid Electrolytes: Effects of Solvent and Surface Film, Langmuir, 25.
[6]. Y. X. Wang et al., J. (2018). A Tunable Molten-Salt Route for Scalable Synthesis of Ultrathin Amorphous Carbon Nanosheets as High-Performance Anode Materials for Lithium-Ion Batteries, Acs Applied Materials & Interfaces, 10.
[7]. W. Guo, X. Li, J. T. Xu, H. K. Liu, J. M. Ma, and S. X. Dou, J. (2016). Growth of Highly Nitrogen-Doped Amorphous Carbon for Lithium-ion Battery Anode, Electrochimica Acta, 188.
[8]. J. Nai, X. Zhao, H. Yuan, X. Tao, and L. Guo, J. (2021). Amorphous carbon-based materials as platform for advanced high-performance anodes in lithium secondary batteries, Nano Res, 14.
[9]. P. Poizot, S. Laruelle, S. Grugeon, L. Dupont, and J. M. Tarascon, J. (2000). Nano-sized transition-metaloxides as negative-electrode materials for lithium-ion batteries, Nature, 407.
[10]. C. Shang, X. L. Li, R. Wei, X. Q. Liu, S. Q. Xu, and J. J. Zhang, J. (2023). Research progress of metal oxide glass anode materials for lithium-ion batteries: A Review, Journal of Non-Crystalline Solids, 618.
[11]. P. Sehrawat, A. Shabir, Abid, C. M. Julien, and S. S. Islam, J. (2021). Recent trends in silicon/graphene nanocomposite anodes for lithium-ion batteries, Journal of Power Sources, 501.
[12]. B. Mand Khan, W. Chun Oh, P. Nuengmatch, and K. Ullah, J. (2023). Role of graphene-based nanocomposites as anode material for Lithium-ion batteries, Materials Science and Engineering: B, 287.
[13]. H. J. Alathlawi and K. F. Hassan, J. (2024). Review-Recent Advancements in Graphene-Based Electrodes for Lithium-Ion Batteries, Ecs Journal of Solid State Science and Technology, 13.
[14]. L. Chen et al., J. (2020). Integrated Structure of Tin-Based Anodes Enhancing High Power Density and Long Cycle Life for Lithium Ion Batteries, Acs Applied Energy Materials, 3.
[15]. H. J. Ying, T. T. Yang, S. L. Zhang, R. N. Guo, J. L. Wang, and W. Q. Han, J. (2020). Dual Immobilization of SnOx Nanoparticles by N-Doped Carbon and TiO2 for High-Performance Lithium-Ion Battery Anodes, Acs Applied Materials & Interfaces, 12.
[16]. M. Cabello, E. Gucciardi, A. Herrán, D. Carriazo, A. Villaverde, and T. Rojo, J. (2020). Towards a high-power Si@ graphite anode for lithium ion batteries through a wet ball milling process," Molecules, vol. 25, no. 11, p. 2494, 2020.
[17]. Y. Son et al., J. (2020). Calendering‐compatible macroporous architecture for silicon–graphite composite toward high‐energy lithium‐ion batteries, Advanced Materials, 32.
[18]. J. Ma et al., J. (2020). Strategic pore architecture for accommodating volume change from high Si content in lithium‐ion battery anodes, Advanced Energy Materials, 10.
[19]. J. Ma et al., J. (2019). Towards maximized volumetric capacity via pore-coordinated design for large-volume-change lithium-ion battery anodes, Nature Communications, 10.
[20]. P. Li, J.-Y. Hwang, and Y.-K. Sun, J. (2019). Nano/microstructured silicon–graphite composite anode for high-energy-density Li-ion battery, ACS nano, 13.
[21]. C. Xiao, P. He, J. Ren, M. Yue, Y. Huang, and X. He, J. (2018). Walnut-structure Si–G/C materials with high coulombic efficiency for long-life lithium ion batteries, RSC advances, 8.
[22]. D. Sui et al., J. (2018). A high-performance ternary Si composite anode material with crystal graphite core and amorphous carbon shell, Journal of Power Sources, 384.
[23]. X. Li et al., J. (2017). Design of porous Si/C–graphite electrodes with long cycle stability and controlled swelling, Energy & Environmental Science, 10.
[24]. N. Kim, S. Chae, J. Ma, M. Ko, and J. Cho, J. (2017). Fast-charging high-energy lithium-ion batteries via implantation of amorphous silicon nanolayer in edge-plane activated graphite anodes, Nature communications, 8.
[25]. Q. Xu, J. Y. Li, J. K. Sun, Y. X. Yin, L. J. Wan, and Y. G. Guo, J. (2017). Watermelon-Inspired Si/C Microspheres with Hierarchical Buffer Structures for Densely Compacted Lithium-Ion Battery Anodes, Advanced Energy Materials, 7.
[26]. C. Y. Du et al., J. (2023). The Status of Representative Anode Materials for Lithium-Ion Batteries, Chemical Record, 23.
[27]. X. Zhao and V.-P. Lehto, J. (2020). Challenges and prospects of nanosized silicon anodes in lithium-ion batteries, Nanotechnology, 32.
[28]. Z. Cheng, H. Jiang, X. Zhang, F. Cheng, M. Wu, and H. Zhang, J. (2023). Fundamental Understanding and Facing Challenges in Structural Design of Porous Si‐Based Anodes for Lithium‐Ion Batteries, Advanced Functional Materials, 33.
Cite this article
Li,Z. (2025). Research Status of Lithium-Ion Battery Anode Materials. Applied and Computational Engineering,127,154-161.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. T. Ahmad and D. Zhang, J. (2020). A critical review of comparative global historical energy consumption and future demand: The story told so far, Energy Reports, 6.
[2]. M. H. Hossain, M. A. Chowdhury, N. Hossain, M. A. Islam, and M. H. Mobarak, J. (2023). Advances of lithium-ion batteries anode materials-A review, Chemical Engineering Journal Advances, 16.
[3]. P. U. Nzereogu, A. D. Omah, F. I. Ezema, E. I. Iwuoha, and A. C. Nwanya, J. (2022). Anode materials for lithium-ion batteries: A review, Applied Surface Science Advances, 9.
[4]. Y. O. Kim and S. M. Park, J. (2001). Intercalation mechanism of lithium ions into graphite layers studied by nuclear magnetic resonance and impedance experiments, Journal of the Electrochemical Society,148.
[5]. Y. Yamada, Y. Iriyama, T. Abe, and Z. Ogumi, J. (2009). Kinetics of Lithium Ion Transfer at the Interface between Graphite and Liquid Electrolytes: Effects of Solvent and Surface Film, Langmuir, 25.
[6]. Y. X. Wang et al., J. (2018). A Tunable Molten-Salt Route for Scalable Synthesis of Ultrathin Amorphous Carbon Nanosheets as High-Performance Anode Materials for Lithium-Ion Batteries, Acs Applied Materials & Interfaces, 10.
[7]. W. Guo, X. Li, J. T. Xu, H. K. Liu, J. M. Ma, and S. X. Dou, J. (2016). Growth of Highly Nitrogen-Doped Amorphous Carbon for Lithium-ion Battery Anode, Electrochimica Acta, 188.
[8]. J. Nai, X. Zhao, H. Yuan, X. Tao, and L. Guo, J. (2021). Amorphous carbon-based materials as platform for advanced high-performance anodes in lithium secondary batteries, Nano Res, 14.
[9]. P. Poizot, S. Laruelle, S. Grugeon, L. Dupont, and J. M. Tarascon, J. (2000). Nano-sized transition-metaloxides as negative-electrode materials for lithium-ion batteries, Nature, 407.
[10]. C. Shang, X. L. Li, R. Wei, X. Q. Liu, S. Q. Xu, and J. J. Zhang, J. (2023). Research progress of metal oxide glass anode materials for lithium-ion batteries: A Review, Journal of Non-Crystalline Solids, 618.
[11]. P. Sehrawat, A. Shabir, Abid, C. M. Julien, and S. S. Islam, J. (2021). Recent trends in silicon/graphene nanocomposite anodes for lithium-ion batteries, Journal of Power Sources, 501.
[12]. B. Mand Khan, W. Chun Oh, P. Nuengmatch, and K. Ullah, J. (2023). Role of graphene-based nanocomposites as anode material for Lithium-ion batteries, Materials Science and Engineering: B, 287.
[13]. H. J. Alathlawi and K. F. Hassan, J. (2024). Review-Recent Advancements in Graphene-Based Electrodes for Lithium-Ion Batteries, Ecs Journal of Solid State Science and Technology, 13.
[14]. L. Chen et al., J. (2020). Integrated Structure of Tin-Based Anodes Enhancing High Power Density and Long Cycle Life for Lithium Ion Batteries, Acs Applied Energy Materials, 3.
[15]. H. J. Ying, T. T. Yang, S. L. Zhang, R. N. Guo, J. L. Wang, and W. Q. Han, J. (2020). Dual Immobilization of SnOx Nanoparticles by N-Doped Carbon and TiO2 for High-Performance Lithium-Ion Battery Anodes, Acs Applied Materials & Interfaces, 12.
[16]. M. Cabello, E. Gucciardi, A. Herrán, D. Carriazo, A. Villaverde, and T. Rojo, J. (2020). Towards a high-power Si@ graphite anode for lithium ion batteries through a wet ball milling process," Molecules, vol. 25, no. 11, p. 2494, 2020.
[17]. Y. Son et al., J. (2020). Calendering‐compatible macroporous architecture for silicon–graphite composite toward high‐energy lithium‐ion batteries, Advanced Materials, 32.
[18]. J. Ma et al., J. (2020). Strategic pore architecture for accommodating volume change from high Si content in lithium‐ion battery anodes, Advanced Energy Materials, 10.
[19]. J. Ma et al., J. (2019). Towards maximized volumetric capacity via pore-coordinated design for large-volume-change lithium-ion battery anodes, Nature Communications, 10.
[20]. P. Li, J.-Y. Hwang, and Y.-K. Sun, J. (2019). Nano/microstructured silicon–graphite composite anode for high-energy-density Li-ion battery, ACS nano, 13.
[21]. C. Xiao, P. He, J. Ren, M. Yue, Y. Huang, and X. He, J. (2018). Walnut-structure Si–G/C materials with high coulombic efficiency for long-life lithium ion batteries, RSC advances, 8.
[22]. D. Sui et al., J. (2018). A high-performance ternary Si composite anode material with crystal graphite core and amorphous carbon shell, Journal of Power Sources, 384.
[23]. X. Li et al., J. (2017). Design of porous Si/C–graphite electrodes with long cycle stability and controlled swelling, Energy & Environmental Science, 10.
[24]. N. Kim, S. Chae, J. Ma, M. Ko, and J. Cho, J. (2017). Fast-charging high-energy lithium-ion batteries via implantation of amorphous silicon nanolayer in edge-plane activated graphite anodes, Nature communications, 8.
[25]. Q. Xu, J. Y. Li, J. K. Sun, Y. X. Yin, L. J. Wan, and Y. G. Guo, J. (2017). Watermelon-Inspired Si/C Microspheres with Hierarchical Buffer Structures for Densely Compacted Lithium-Ion Battery Anodes, Advanced Energy Materials, 7.
[26]. C. Y. Du et al., J. (2023). The Status of Representative Anode Materials for Lithium-Ion Batteries, Chemical Record, 23.
[27]. X. Zhao and V.-P. Lehto, J. (2020). Challenges and prospects of nanosized silicon anodes in lithium-ion batteries, Nanotechnology, 32.
[28]. Z. Cheng, H. Jiang, X. Zhang, F. Cheng, M. Wu, and H. Zhang, J. (2023). Fundamental Understanding and Facing Challenges in Structural Design of Porous Si‐Based Anodes for Lithium‐Ion Batteries, Advanced Functional Materials, 33.









