1. Introduction
Since the level of social development has entered a new stage, the energy problem has also surfaced. Growing energy demand has led to a sharp decline in the stock of traditional primary energy sources, and environmental pressures have become increasingly prominent, and mankind has realized that the development of new energy sources is imminent. Among them, solar energy as a kind of access to simple, content and storage of huge, scalable and green clean energy, in the new energy power to provide guidance, fundamental role. At present, the development of mature solar cells include silicon-based solar cells, thin-film solar cells, composite solar cells and so on. There are also some new solar cells such as dye-sensitized solar cells (DSSCs), quantum dots solar cells and other solar cells with great potential in conversion efficiency, all of which provide a forward-looking, innovative contribution to the field of solar photovoltaic.
A common measure used to characterize the performance of solar cells is the photoelectric conversion efficiency (PCE), whose specific performance parameters include the short-circuit current (Jsc), open-circuit voltage (Voc), and fill factor (FF). Therefore, the photovoltaic performance of a solar cell can be represented by the J-V curve, and the intersection of the curve and the axis is the open-circuit voltage and short-circuit current.
Since the 21st century, researchers have discovered a new type of solar cell, which provides new ideas and new directions for the field of photovoltaics. This solar cell to perovskite material for light absorption layer, and electron transport layer (ETL) and hole transport layer (HTL) together to form a sandwich-like layer structure of its highest photoelectric conversion efficiency can reach 26%. It is worth mentioning that its photoelectric conversion efficiency from 3% to have been certified 26% of the research process of less than two decades, much smaller than the silicon-based solar cells with the conversion to improve the research of more than 80 years. Undeniably, the issues about the stability of the battery device, the mass production of the battery, the safety and cleanliness of the composition are still the core of today's perovskite solar cells to break the barrier. In this paper, we will summarize the material properties, device design and commercialization prospects of perovskite solar cells in detail from the device structure, material composition, crystal defects, and research costs, and seek to explore new paths to improve the photoelectric conversion efficiency by analyzing and discussing the new research results, and promote the release of perovskite solar cells from the upstream to the downstream of industrialization to broaden the scope of commercialization.
2. Perovskite Materials
Perovskite materials are organic-inorganic hybrid materials with the molecular formula ABX3. where A is usually an organic or inorganic cation (CH3NH3+, NH4+, Cs+, etc.), B is an inorganic metal cation (Sn4+, Pb2+, etc.), and X is a halogen anion (Cl-, Br-, and I-), and the cell structure is as shown in Fig1a. This structure is structured with the inorganic metal cations as the octahedral core, the halogen anions as the top corners of the octahedra, enclosing the cubic hexahedra, and the organic ions embedded as the body center. Because the cell structure is similar to perovskite, it is called perovskite material. Perovskite materials have excellent performance in ionizing radiation detection [1], solar cells, catalysts [2-4], quantum dot displays [5, 6] and other fields because of their excellent photoelectric properties (high light absorption, suitable bandwidth, high electron and hole mobility, and low complexity), which are shown in Figure 1. Usually, the C-V curve and energy-level diagram reflect PSCs’ work efficiency and whether the hole or electrons are easy to be delivery. Perovskite solar cells are solar cells in which perovskite material is the light absorbing material. Structurally perovskite solar cells can be categorized into mesoporous PSCs, planar heterojunction PSCs, and tandem PSCs. The mesoporous structure consists of mesoporous compounds such as TiO2 as the electron transport layer. Perovskite can be filled in the scaffold composed of mesoporous compounds, which can effectively improve the efficiency of electron transport, but this structure also limits its development in the field of flexibility. Planar heterojunction structures can be categorized into formal (n-i-p) and trans (p-i-n). The difference lies in the fact that the n-i-p structure has HTL on the top and ETL on the bottom, and the process is relatively mature; the p-i-n structure has ETL on the top and is suitable for flexible devices. However, with the deepening of the research, the HTL of n-i-p structure is a little less stable, and the preparation conditions are more severe; p-i-n structure can be prepared at room temperature, and the stability is better, and it has gradually become the mainstream PSC device structure. The stacked structure combines perovskite with silicon, copper indium gallium selenide (CIGS), etc. to form a multi-junction cell, which not only breaks through the limitations of single junction, but also realizes a new breakthrough in PCE.
As a semiconductor solar cell, perovskite solar cells use perovskite as an intermediate light absorption layer, utilizing a certain forbidden band width of perovskite to absorb photons. When the energy of the photons is greater than or equal to the band gap of the perovskite material, the electrons jump from the valence band to the conduction band, generating electron-hole pairs (excitons). Depending on the built-in electric field of the p-n junction and the long carrier diffusion distance of the perovskite material, the electron-hole pairs are separated in the perovskite material and then exported to the cathode and anode by entering the electron and hole transport layer respectively, and then form the electric current through the external circuit, realizing the photoelectric conversion.
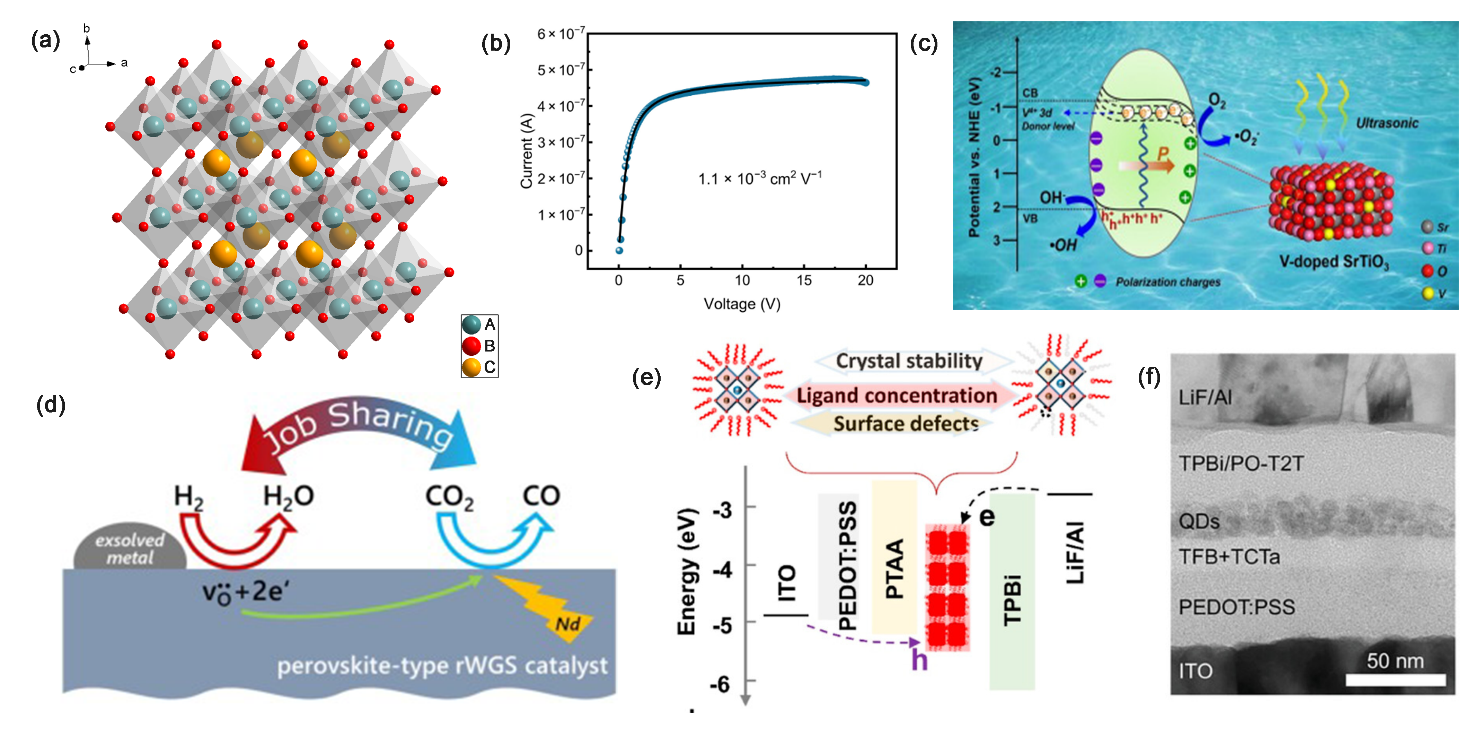
Figure 1: Perovskite materials are widely used in multiple applications. (a) Crystal structure of perovskite materials(b) Steady-state photoconductivity curve of MAPbI3 perovskite single-crystal devices under x-ray [1]. (c) Schematic diagram for the piezocatalytic mechanism of a kind of perovskite under ultrasonic vibration [3]. (d) Novel perovskite catalysts for CO2 utilization - Exsolution enhanced reverse water-gas shift activity [4]. (e) Structure and energy-level diagram of the CsPbI3 QD-based QLED [5]. (f) Cross-sectional TEM image for a kind of blue QLED device [6].
The most typical perovskite material is CH3NH3PbI3, with a direct forbidden bandwidth of 1.55 eV, which matches visible light, making it a good light absorbing material in the visible region; it produces excitons with a binding energy of only about 0.030 eV, implying that these excitons can easily decompose into free carriers without the excitons being easily complexed; it produces electrons and holes that have, due to the small effective mass, a larger mobility, the compounding time of electrons and holes is in the order of hundreds of nanoseconds, and thus the carriers have longer diffusion distances [7]. However, CH3NH3PbI3 is more affected by the environment, degrades under humid conditions, and is less stable. In addition, lead-containing compounds are more toxic and can have a negative impact on the environment Scientists are still exploring paths to improve PCE, and there is still no shortage of further exploration of perovskite materials in terms of stability, safety, and commercialization [8]. In this part, the direction of evolution of perovskite materials in recent years will be described in terms of stability, non-toxicity/low toxicity, and low cost.
2.1. Stability
The solution to the stability problem lies in the stabilization of the material at the test level under light, oxygen, damp or other chemical environments as well as the stabilization of the material at the preparation level by making up for surface defects through surface modification and other techniques. perovskite, which is occupied by iodide ions at the X-site, occupies a considerable portion of the whole system. The band gap of iodine-based perovskite is highly matched with the visible region, which can effectively realize photon absorption; in addition, the carrier diffusion length of iodine-based perovskite is longer than that of pure chlorine-based and pure bromine-based, which results in a higher efficiency in the separation and transport of electrons and holes. Although it occupies a great advantage only in terms of PCE size, its sensitivity to humidity, light, and heat, on the contrary, makes the lifetime of PSC devices made of it greatly reduced. Early on, Long et al. found that grain boundaries accelerate electron-hole complexation in CH3NH3PbI3, but replacing iodine with chlorine at grain boundaries reduces electron-hole complexation. By pushing the highest occupied molecular orbital (HOMO) density away from the boundary, chlorines restore the NA coupling of the PSCs. boundary, chlorines restore the NA coupling close to the value observed in pristine MAPbI3 [9].
Another solution is to modify or passivate the interface, which is currently the most common approach in the research field. They chemically attach one or more groups with specific functions to the perovskite surface. By reducing the density of defect states, non-radiative complexation is reduced or suppressed, and the carrier transport distance is elongated as much as possible to avoid loss of device efficiency. Some examples are shown in Figure 2. Muhammad et al who utilized fully encapsulated trapping tactic (FETT) introduced two chalcogen-molecules bridged via tetra-pyridine ligand for passivation of perovskite surface. These two substances were bridged with two lewis bases, chalcogen-thiophene (n-Bu4S) and selenophene (n-Bu4Se), respectively, using tetra-pyridine as a bridge, and utilizing the negative charge of the lewis bases to compensate for the Pb2+/iodine vacancies defects on the perovskite surface. perovskite surface. They found that the sulfur elements in n-Bu4Se, as opposed to n-Bu4S, effectively promoted grain growth during the annealing process, inhibited charge complexation, and were more favorable to compensate for the defects in the perovskite films. In addition, this method makes leads to PSC devices with a PCE as high as 25.37% and maintains an initial efficiency of 94% at 65℃ and 1300 h of solar irradiation[10]. Insights were provided on improving the stability of PSC devices in this field. LIN et al who doped a piperidinium salt solid ion[BMP]+[BF4]− into Cs0.17FA0.83Pb(IxBr1-x)3 , which is able to effectively inhibit Pb oxidation and calixarene deep trapping state, in other words, effectively compensated for perovskite localized defects and reduced photothermal degradation (peak efficiency of the encapsulated cell reached 95% after 1200h at 85℃ under full-spectrum simulated sunlight in ambient atmosphere) [11]. Reducing the crystal size by utilizing the crystallization control of perovskite precursors also facilitates the realization of low-trap state and highly crystal-depleted perovskite thin films. ZHENG et al developed a surface reconstruction method for the removal of defect-rich layer. They developed a wet polishing protocol utilizing anisole and nanometric abrasive particles to enhance the homogeneity of the perovskite film, which effectively solved the efficiency loss caused by the expansion of the device area, and the stability also showed good performance [12]. ZHU et al. used water-soluble lead acetate trihydrate (PbAc2·3H2O) and formamidine acetate (FAAc) as the raw materials dissolved in HI aqueous solution to synthesize FAPbI3 precursor (ASPM), which creatively utilized low-cost, low-purity raw materials for the preparation of high-purity precursors by hydrosynthesis and was able to achieve large-scale production of perovskite microcrystals with purity up to 99.996% at kilogram scale [13]. This is a major breakthrough in the commercialization of PSC devices.
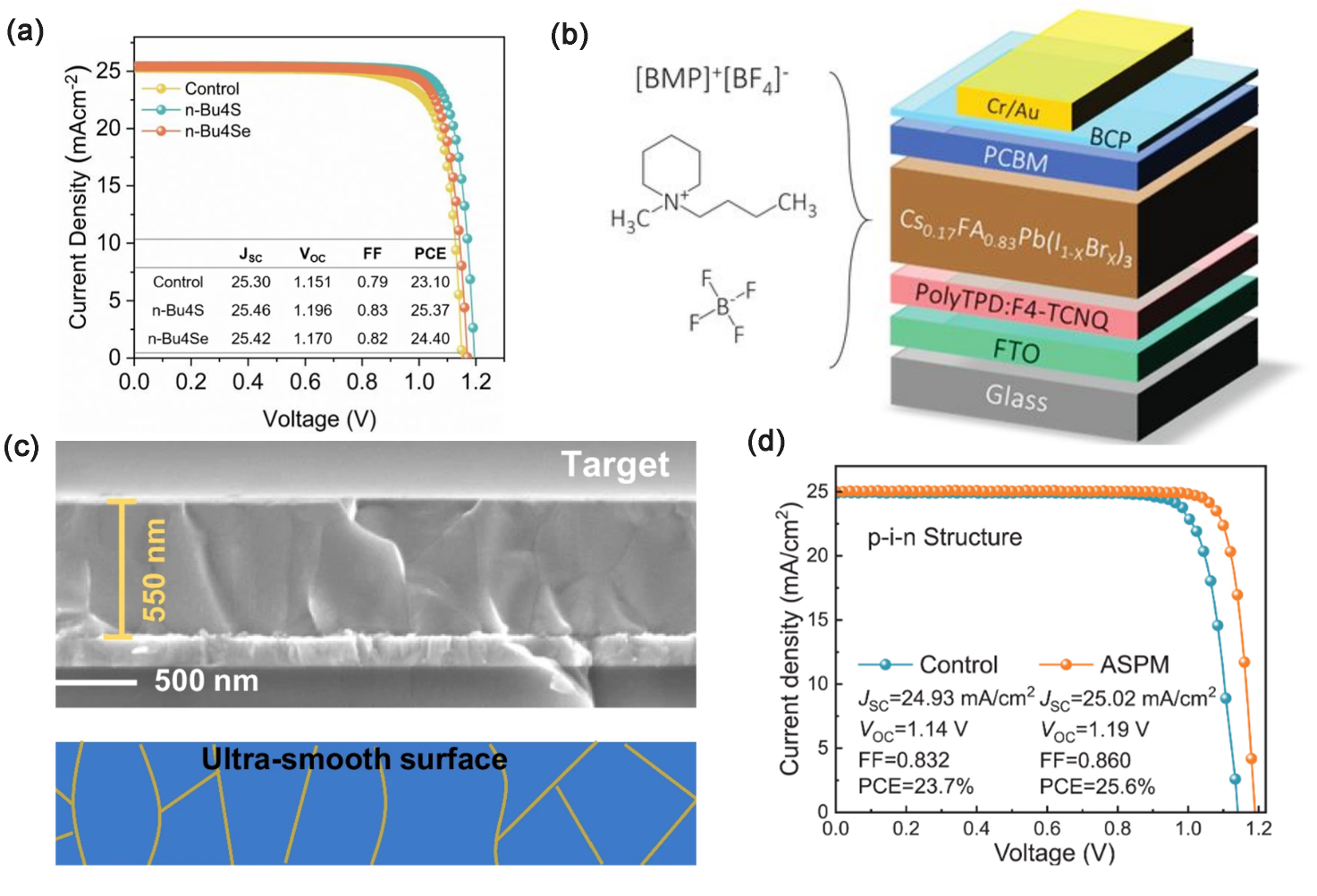
Figure 2: The surface modification of perovskite materials can effectively improve the photovoltaic characteristics of PCS devices. (a) J-V curves and related oarameters of best-performing PSCs (control, n-Bu4S, and n-Bu4Se-modified) [10]. (b) Schematic of the p-i-n perovskite solar cell and the chemical structure, [BMP]+[BF4]− and Cs0.17FA0.83Pb(IxBr1-x)3 are fixed to be the light-absorbed layer [11]. (c) Cross-sectional SEM images of perovskite films after nano-polishing treatment [12]. (d) J-V curves of control and ASPM precursor-based PSCs [13].
2.2. Non-toxicity/low toxicity
The non-toxic/low-toxicity treatment mainly focuses on the reduction or even removal of toxic elements during preparation and use, which can be explored from the perspectives of non-toxic/low-toxicity processing of PSC devices and non-toxic/low-toxicity materials. In this part, we will mainly focus on the non-toxic/low-toxicity of perovskite materials, i.e., the reduction or even removal of toxic components in perovskite, mainly Pb. Jain et al have developed a perovskite solar cell with the structure of FTO/c-TiO2/Meso- TiO2/CH3NH3Bi2I9/Spiro-OMeTAD/P3HT/C, which is made of Pb-free perovskite material and using methyl acetate as solvent, thus making the whole process non-toxic. At the same time, it is worth mentioning that they use better stability, hydrophobicity of the carbon paste, but also to avoid the penetration of metal electrodes into the battery; the choice of dense TiO2 and mesoporous TiO2 as the hole transport layer not only prevents perovskite holes and electrons and the bottom of the electrode contact, reducing the electron composite, to ensure that the electrons to the conductive direction of unidirectional transmission to ensure that the efficiency of electron transfer[14]. A few years ago there were also many researchers to replace the Pb element in the perovskite material with Sn, which can solve the possibility of Pb2+ poisoning from the root, but Sn2+ in the tin-based perovskite is easily oxidized to Sn4+, and the material stability is poor [15]. Adding SnF2 to it can effectively reduce the hysteresis effect of PSC films [16]. There are also some researches to grasp the growth direction by controlling the crystal going of perovskite materials, for example, WANG et al. investigated a 2D-quasi-2D-3D hierarchy of crystal structure with parallel orientation, where the substrate is now grown with 3D structure for charge transfer and then grow 2D structure for protection of the film [17]. Some studies tried to dope some co-additives in Sn-based PSCs, and there were also studies that crosslinked methylene-bis-acrylamide (MBAA) and acrylic acid (AA) to make a lead adsorption ion gel directly into MAPbI3, and effective adsorption can be achieved when lead leaks [18].
3. Device Structure and Preparation Technology
3.1. Device Processing
With the deepening of perovskite material research, PSC devices have more attractive new prospects in terms of high efficiency, flexibility, and low raw material cost. This section combines structural modification, encapsulation technology and PSC device product applications to explore diversified ideas for PSC device processing and explains further based on Figure 3.
3.1.1. Perovskite-based Tandem Solar Cells
Perovskite-based tandem solar cells are not pure perovskite solar cells in the traditional sense, but a kind of cell stacked by utilizing different semiconductor solar cells with increasing or decreasing bandgap step by step, so the value of PCE will be higher than other perovskite solar cells. Perovskite bandgap is adjustable, so it is easier to form tandem solar cells. silicon based solar cells are often stacked with PSCs to form perovskite/silicon tandem solar cells (PSTSC) [8, 19]. PSTSCs include three representative configurations: two-terminal (2T), in which the top cell is in direct contact with the bottom cell via a series circuit; four-terminal (4T), in which the top cell and the bottom cell are electrically isolated; and three-terminal (3T), in which the cell has an additional terminal that allows for the extraction of current from the perovskite and silica-based cells separately. It is worth mentioning that the power conversion efficiency of such solar cells has exceeded 31%, which is very promising and the theoretical limit can be higher [20]. There are also studies present PSTSCs with a gentle sinusoidal nanotexture connecting the advantages of structuring the silicon surface while preserving the material quality of the solution-processed perovskite and further implement a reflector with a dielectric buffer layer (RDBL) at the rear side of the silicon bottom cell, which enhances the optoelectronic properties of this PSTSC and gets a PCE of 29.8% [19]. In order to have more in-depth data on the outdoor operation and commercialization of the PSTSC, a study has derived the effect of current variation of PSTSC under outdoor operation through theoretical tests, such as the external quantum efficiency (EQE) of the tandem, the band gap of perovskite, the local solar spectrum, and the operating temperature range [21]. For commercialization, a study has generated a quasi-two-dimensional layered structure, CsPb2X5, on industrially textured silicon using a ternary co-evaporation solution (PbI2, PbCl2, and CsBr) method in conjunction with framework heat treatment (FHT), which achieves the full conversion of PbI2 on perovskite thin films, and after encapsulation, it is still able to achieve long time operation under solar conditions [22]. Recently, a self-assembled monolayer has been used to stabilize perovskite with a bandgap of 1.68 eV, and this monolayer was able to effectively achieve selective contacting of holes, and the 300 h PCE for operation under unfractionated conditions was still 95% of the previous one [23]. In addition, there are also studies to obtain an interesting result, the photovoltaic characteristics of the silicon bottom cells in the end-of-life PSTSC remain basically unchanged after recycling; the efficiency of the new cells made after recycling is also comparable to that of the previous ones [24]. This gives considerable hope for both environmental protection and PV energy cost reduction.
CIGS is also a good bottom cell, which has advantages in flexible devices, although the PCE is currently reported to be low relative to Si. A study demonstrated a monolithic perovskite/CIGS tandem solar cell with a certified PCE of 24.2%, and again, through simulations, confirmed the possibility of perovskite/CIGS tandem solar cells power conversion efficiency exceeding 30% [25].
3.1.2. Flexible PSC devices
The main advantages of flexible PSC (f-PSC) devices are flexibility, bendability, etc., making them key components for wearable electronic appliances, mobile power sources, and building integrated photovoltaics (BIPV) [26]. As opposed to other rigid PSC devices, f-PSCs often need to focus on their lifetime under mechanical conditions. The grain boundary is the weakest region in perovskite films, and under bending conditions, perovskite films are prone to stress and strain cracks, which not only create direct conditions for ion migration and reduce the power conversion efficiency, but may also be invaded by moisture, which can greatly reduce the lifetime [27]. It has been found that the introduction of an organic ammonium salt 4-(methoxy)benzylamine hydrobromide (MeOBABr) into the perovskite precursor solution forms a capping layer on the surface of the film. This soft buffer solution is effective in relieving residual stresses during bending of PSC devices and also passivates surface defects and reduces non-radiative compound losses [28]. Similarly another study found an initiator-free crosslinkable monomer (2,5-dioxopyrrolidin-1-yl) 5-(dithiolan-3-yl) pentanoate (FTA) passivating surface defects [29].
The development direction of PSC devices is also evident in the optimization of ETLs, such as stacking mesoporous TiO2 and dense TiO2 to make an ETL, using dense TiO2 to prevent the contact between the hole-transporting material and the external circuit, and mesoporous TiO2 to increase the contact area with the perovskite material and reduce the efficiency degradation caused by grain boundaries [14, 30]. There are also studies on doping graphene in TiO2 to improve conductivity and accelerate transport [31].
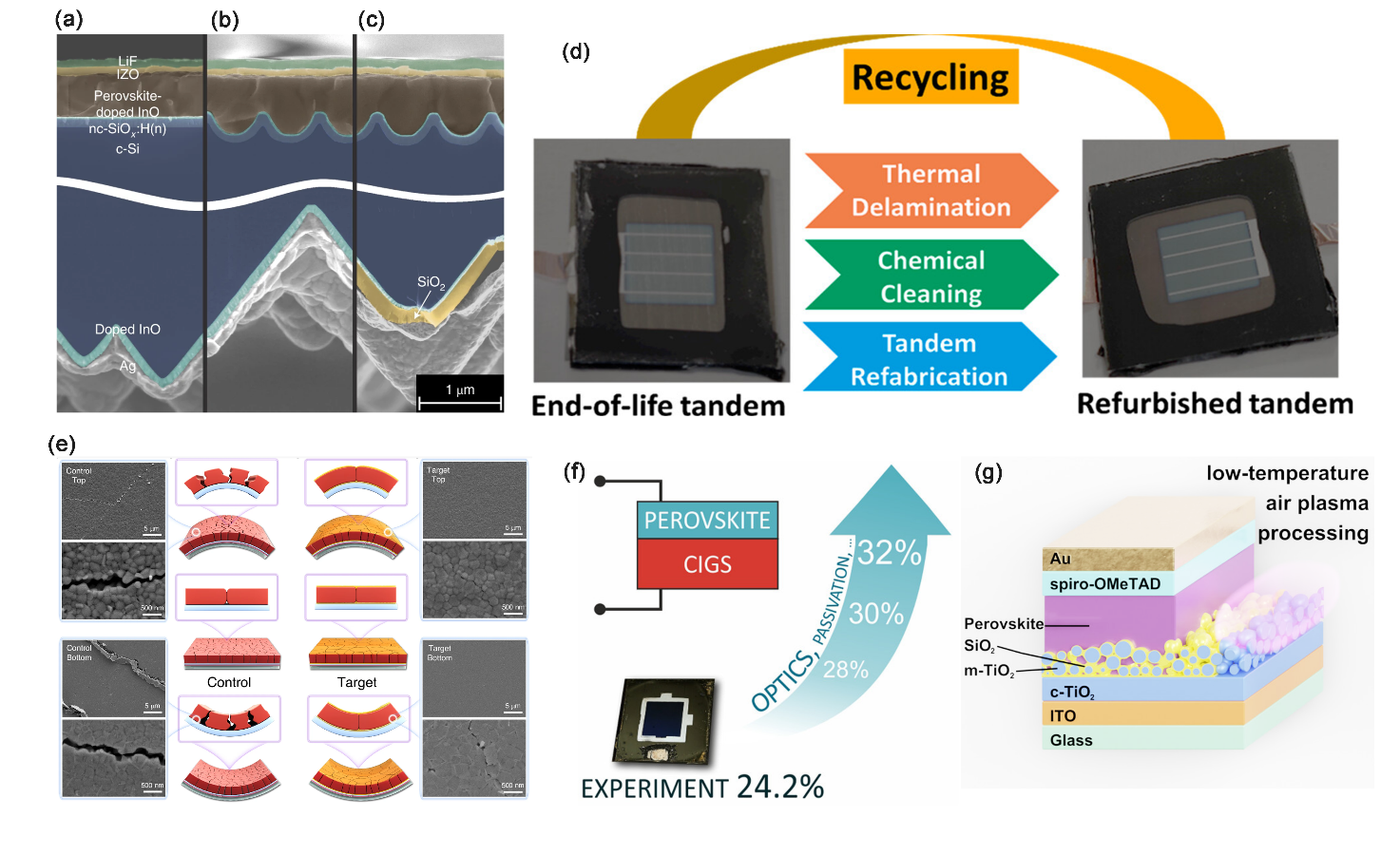
Figure 3: Device processing to realize diversified applications of perovskite solar cells. (a-c) SEM cross-section micrographs of the front and rear side of planar (a), nanotextured (b) and nanotextured + RDBL (c) PSTSCs [19]. (d)By recycling and remanufacturing, the optoelectronic properties of PSTSC still have a great performance [24]. (e)The theoretical value of perovskite/CIGS tandem solar cells’ PCE has exceeded 30% [25]. (f)After MeOBABr capping, the cracks made by bending cycles are much less than before [28]. (g) Differences in crystal structure affect the use of materials in PSC devices[30].
3.2. Preparation Technology
PSC devices can have great potential to revolutionize the solar industry thanks to their high efficiency and low production cost. However, how to prepare perovskite materials with few surface defects, high quality and high stability has also become a central issue in the fabrication and even commercialization of PSC devices. As a light-absorbing layer, perovskite materials affect the efficient charge transfer inside PSC devices. Therefore, the preparation method for perovskite materials has a significant impact on the efficiency of the light absorbing layer and determines the fundamental performance of the device. In this section, the path to high quality and efficiency of PSC devices will be elaborated on the basis of the preparation technology of perovskite materials.
3.2.1. Solution Method
The solution method is the most common and lowest cost method to prepare perovskite thin films, which is suitable for large-area preparation. The perovskite precursor is first dissolved in a solvent and coated on the substrate, and the perovskite film is obtained through solvent evaporation and post-processing. This method is simple, but the quality of the film is difficult to control and is prone to interfacial defects, resulting in lower photovoltaic conversion efficiency. Solution methods include the one-step spin-coating method and the two-step method. The one-step spin-coating method, which involves spin-coating a solution of perovskite precursor (e.g., γ-butyrolactone/DMSO solution of CH3NH3Pb(I1 - xBrx)3[32]) onto a substrate and subsequently inducing crystallization by an anti-solvent (e.g., chlorobenzene or ether), is suitable for large-area commercial production. The two-step method, in which a PbI₂ layer is deposited and then immersed in MAI solution to react to produce perovskite, is highly controllable and suitable for laboratory preparation [33]. Depending on the needs, researchers choose the process based on different characteristics.
3.2.2. Post Annealing Process
In the preparation of the required perovskite light absorbing layer, often need to control the growth of perovskite crystals to control the stability of the grain boundary, minimize surface defects, to avoid non-essential loss of PCE, post annealing process is the preparation of PSC devices to control the crystal growth of the typical process, through the control of the annealing temperature and duration of the quality of the perovskite layer is often produced under different conditions have a significant difference. In scientific research, the experimental environment (e.g., solvent, atmosphere) is also an influencing factor on the reaction results. A study inverted a glass dish on a perovskite film to simulate petri-plate assisted vapor annealing assisted vapor annealing (PVA), and it was found that, compared to no vapor annealing (w/o VA), the efficiency of the PSC devices treated with PVA increased by 20.03%, and the stability and electronic properties of the films were also improved [34]. With the continuous research, CHEN et al. used a 405 nm violet laser as a return laser, and through real-time measurements of the annealing data after laser refraction, it was confirmed that the continuous wave beam of mW class could provide kinetic energy for the crystals in the perovskite microwire, which provided a new idea for thermal annealing [35]. Of course, in order to minimize the complexity of the process, there are also studies to improve the preparation process by using the binary solvent precursor ink, and found a way to eliminate the need for annealing process, which is still expected to be a new direction of development, although with a slight but irrelevant reduction in performance [36].
4. Conclusion
In conclusion, perovskite solar cells (PSCs) have become a research hotspot in the field of photovoltaics in recent years due to the advantages of high power conversion efficiency, low cost, and tunable bandgap. Its light absorbing layer perovskite material has excellent photovoltaic properties, but poor stability, lead toxicity and mass production challenges constrain commercialization. Future commercialization is mainly focused on the following two aspects: improve stability, the development of moisture, heat-resistant materials, the introduction of the addition of stabilizers or chemical modification of the perovskite material; the development of non-toxic perovskite, although the current efficiency of lead-free materials is low, the stability of the lack of, but through the adjustment of the composition and structure of the material, the future is expected to develop a non-toxic alternative material with performance close to or exceeding the lead-based perovskite, so as to achieve environmentally friendly photovoltaic technology. With the maturity of the industry chain and the reduction of production costs, perovskite batteries will gradually enter the market and be widely used. In any case, these advances will push perovskite solar cells from the laboratory to the commercialization of the road, to achieve high power conversion efficiency and economic double harvest, and further promote the development of clean energy.
References
[1]. Yang, X. Y., Song, Y. L., Wang, L. X., Sun, Y., Jin, B. W., Wang, J., Liu, H., Yang, Y. J., Lin, Q. Q., Fang, Y. J. and Dong, Q. F. (2024). In-line tempering eliminates the domain boundary in perovskite single crystals for high-energy resolution ionizing radiation detectors. Science Advances, 10, 10.
[2]. Shang, C. Y., Xiao, X. and Xu, Q. (2023). Coordination chemistry in modulating electronic structures of perovskite-type oxide nanocrystals for oxygen evolution catalysis. Coordination Chemistry Reviews, 485, 13.
[3]. Lindenthal, L., Popovic, J., Rameshan, R., Huber, J., Schrenk, F., Ruh, T., Nenning, A., Löffler, S., Opitz, A. K. and Rameshan, C. (2021). Novel perovskite catalysts for CO2 utilization - Exsolution enhanced reverse water-gas shift activity. Applied Catalysis B-Environmental, 292, 12.
[4]. Zhou, Q., Li, N. J., Chen, D. Y., Xu, Q. F., Li, H., He, J. H. and Lu, J. M. (2022). Efficient removal of Bisphenol A in water via piezocatalytic degradation by equivalent-vanadium-doped SrTiO3 nanofibers. Chemical Engineering Science, 247, 11.
[5]. Wang, Y. F., Li, Y., Su, Y. Q., Chen, X. R., Wang, R., Xu, J. Y., Zhou, G. Q., Chen, Z. S., Xiang, H. Y., Zhidkov, I. S., Kurmaev, E. and Zeng, H. B. (2024). Fine Purification Engineering Enables Efficient Perovskite QLEDs with Efficiency Exceeding 23%. Acs Applied Materials & Interfaces, 16, 28853-28860.
[6]. Nong, Y. Y., Yao, J. S., Li, J. Q., Xu, L. M., Yang, Z., Li, C. and Song, J. Z. (2024). Boosting External Quantum Efficiency of Blue Perovskite QLEDs Exceeding 23% by Trifluoroacetate Passivation and Mixed Hole Transportation Design. Advanced Materials, 36, 8.
[7]. Grätzel, M. (2014). The light and shade of perovskite solar cells. Nature Materials, 13, 838-842.
[8]. Zhou, J., Fu, S. Q., Zhou, S., Huang, L. S., Wang, C., Guan, H. L., Pu, D. X., Cui, H. S., Wang, C., Wang, T., Meng, W. W., Fang, G. J. and Ke, W. J. (2024). Mixed tin-lead perovskites with balanced crystallization and oxidation barrier for all-perovskite tandem solar cells. Nature Communications, 15, 10.
[9]. Long, R., Liu, J. and Prezhdo, O. V. (2016). Unravelling the Effects of Grain Boundary and Chemical Doping on Electron-Hole Recombination in CH3NH3PbI3 Perovskite by Time-Domain Atomistic Simulation. Journal of the American Chemical Society, 138, 3884-3890.
[10]. Azam, M., Ma, Y., Zhang, B. X., Shao, X. F., Wan, Z. Q., Zeng, H. B., Yin, H. M., Luo, J. S. and Jia, C. Y. (2025). Tailoring pyridine bridged chalcogen-concave molecules for defects passivation enables efficient and stable perovskite solar cells. Nature Communications, 16, 11.
[11]. Lin, Y. H., Sakai, N., Da, P., Wu, J. Y., Sansom, H. C., Ramadan, A. J., Mahesh, S., Liu, J. L., Oliver, R. D. J., Lim, J., Aspitarte, L., Sharma, K., Madhu, P. K., Morales-Vilches, A. B., Nayak, P. K., Bai, S., Gao, F., Grovenor, C. R. M., Johnston, M. B., Labram, J. G., Durrant, J. R., Ball, J. M., Wenger, B., Stannowski, B. and Snaith, H. J. (2020). A piperidinium salt stabilizes efficient metal-halide perovskite solar cells. Science, 369, 96-102.
[12]. Fang, Z., Deng, B. R., Jin, Y. B., Yang, L., Chen, L. S., Zhong, Y. W., Feng, H. P., Yin, Y., Liu, K. K., Li, Y. J., Zhang, J. Y., Huang, J. R., Zeng, Q. H., Wang, H., Yang, X., Yang, J. X., Tian, C. B., Xie, L. Q., Wei, Z. H. and Xu, X. P. (2024). Surface reconstruction of wide-bandgap perovskites enables efficient perovskite/silicon tandem solar cells. Nature Communications, 15, 11.
[13]. Zhu, P. D., Wang, D., Zhang, Y., Liang, Z., Li, J. B., Zeng, J., Zhang, J. Y., Xu, Y. T., Wu, S. Y., Liu, Z. X., Zhou, X. Y., Hu, B. H., He, F., Zhang, L., Pan, X., Wang, X. Z., Park, N. G. and Xu, B. M. (2024). Aqueous synthesis of perovskite precursors for highly efficient perovskite solar cells. Science, 383, 524-531.
[14]. Jain, S. M., Edvinsson, T. and Durrant, J. R. (2019). Green fabrication of stable lead-free bismuth based perovskite solar cells using a non-toxic solvent. Communications Chemistry, 2, 7.
[15]. Diau, E. W.-G., Jokar, E. and Rameez, M. (2019). Strategies To Improve Performance and Stability for Tin-Based Perovskite Solar Cells. ACS Energy Letters, 4, 1930-1937.
[16]. Kumar, M. H., Dharani, S., Leong, W. L., Boix, P. P., Prabhakar, R. R., Baikie, T., Shi, C., Ding, H., Ramesh, R., Asta, M., Graetzel, M., Mhaisalkar, S. G. and Mathews, N. (2014). Lead-Free Halide Perovskite Solar Cells with High Photocurrents Realized Through Vacancy Modulation. Advanced Materials, 26, 7122-7127.
[17]. Wang, F., Jiang, X. Y., Chen, H., Shang, Y. Q., Liu, H. F., Wei, J. L., Zhou, W. J., He, H. L., Liu, W. M. and Ning, Z. J. (2018). 2D-Quasi-2D-3D Hierarchy Structure for Tin Perovskite Solar Cells with Enhanced Efficiency and Stability. Joule, 2, 2732-2743.
[18]. ]Xiao, X., Wang, M. X., Chen, S. S., Zhang, Y. H., Gu, H. Y., Deng, Y. A., Yang, G., Fei, C. B., Chen, B., Lin, Y. Z., Dickey, M. D. and Huang, J. S. (2021). Lead-adsorbing ionogel-based encapsulation for impact-resistant, stable, and lead-safe perovskite modules. Science Advances, 7, 9.
[19]. Tockhorn, P., Sutter, J., Cruz, A., Wagner, P., Jäger, K., Yoo, D., Lang, F., Grischek, M., Li, B. R., Li, J. Z., Shargaieva, O., Unger, E., Al-Ashouri, A., Köhnen, E., Stolterfoht, M., Neher, D., Schlatmann, R., Rech, B., Stannowski, B., Albrecht, S. and Becker, C. (2022). Nano-optical designs for high-efficiency monolithic perovskite-silicon tandem solar cells. Nature Nanotechnology, 17, 1214-1221.
[20]. Shi, Y., Berry, J. J. and Zhang, F. (2024). Perovskite/Silicon Tandem Solar Cells: Insights and Outlooks. 9, 1305-1330.
[21]. Babics, M., Bristow, H., Pininti, A. R., Allen, T. G. and De Wolf, S. (2023). Temperature Coefficients of Perovskite/Silicon Tandem Solar Cells. ACS Energy Letters, 8, 3013-3015.
[22]. Luo, H. W., Zheng, X. T., Kong, W. C., Liu, Z., Li, H. J., Wen, J., Xia, R., Sun, H. F., Wu, P., Wang, Y. R., Mo, Y., Luo, X., Huang, Z. L., Hong, J. J., Chu, Z. J., Zhang, X. L., Yang, G. T., Chen, Y. F., Feng, Z. Q., Gao, J. F. and Tan, H. R. (2023). Inorganic Framework Composition Engineering for Scalable Fabrication of Perovskite/Silicon Tandem Solar Cells. ACS Energy Letters, 8, 4993-5002.
[23]. Al-Ashouri, A., Köhnen, E., Li, B., Magomedov, A., Hempel, H., Caprioglio, P., Márquez, J. A., Vilches, A. B. M., Kasparavicius, E., Smith, J. A., Phung, N., Menzel, D., Grischek, M., Kegelmann, L., Skroblin, D., Gollwitzer, C., Malinauskas, T., Jost, M., Matic, G., Rech, B., Schlatmann, R., Topic, M., Korte, L., Abate, A., Stannowski, B., Neher, D., Stolterfoht, M., Unold, T., Getautis, V. and Albrecht, S. (2020). Monolithic perovskite/silicon tandem solar cell with >29% efficiency by enhanced hole extraction. Science, 370, 1300-1309.
[24]. Yang, G., Wang, M. R., Fei, C. B., Gu, H. Y., Yu, Z. J., Alasfour, A., Holman, Z. C. and Huang, J. S. (2023). Recycling Silicon Bottom Cells from End-of-Life Perovskite-Silicon Tandem Solar Cells. ACS Energy Letters, 6.
[25]. Jost, M., Köhnen, E., Al-Ashouri, A., Bertram, T., Tomsic, S., Magomedov, A., Kasparavicius, E., Kodalle, T., Lipovsek, B., Getautis, V., Schlatmann, R., Kaufmann, C. A., Albrecht, S. and Topic, M. (2022). Perovskite/CIGS Tandem Solar Cells: From Certified 24.2% toward 30% and Beyond. ACS Energy Letters, 7, 1298-1307.
[26]. Tavakoli, M. M., Tsui, K. H., Zhang, Q. P., He, J., Yao, Y., Li, D. D. and Fan, Z. Y. (2015). Highly Efficient Flexible Perovskite Solar Cells with Antireflection and Self-Cleaning Nanostructures. Acs Nano, 9, 10287-10295.
[27]. Hu, X. T., Meng, X. C., Yang, X., Huang, Z. Q., Xing, Z., Li, P. W., Tan, L. C., Su, M., Li, F. Y., Chen, Y. W. and Song, Y. L. (2021). Cementitious grain-boundary passivation for flexible perovskite solar cells with superior environmental stability and mechanical robustness. Science Bulletin, 66, 527-535.
[28]. Jin, J. J., Zhu, Z. K., Ming, Y. D., Zhou, Y., Shang, J. T., Wang, S. F., Cui, X. X., Guo, T. H., Zhang, D., Tang, G. Q., Lin, Q. Q., Li, J. H., Liu, X. W., Liu, S., Chen, Z. W., Hu, Z., Meng, H. and Tai, Q. D. (2025). Spontaneous bifacial capping of perovskite film for efficient and mechanically stable flexible solar cell. Nature Communications, 16, 11.
[29]. Zhang, W. F., Liu, J., Song, W., Shan, J. H., Guan, H. W., Zhou, J., Meng, Y. Y., Tong, X. Y., Zhu, J. T., Yang, M. J. and Ge, Z. Y. (2025). Chemical passivation and grain-boundary manipulation via in situ cross-linking strategy for scalable flexible perovskite solar cells. Science Advances, 11, 15.
[30]. Homola, T., Pospisil, J., Shekargoftar, M., Svoboda, T., Hvojnik, M., Gemeiner, P., Weiter, M. and Dzik, P. (2020). Perovskite Solar Cells with Low-Cost TiO2 Mesoporous Photoanodes Prepared by Rapid Low-Temperature (70 °C) Plasma Processing. Acs Applied Energy Materials, 3, 12009-12018.
[31]. Khan, M. T. and Khan, F. (2022). Enhancement in photovoltaic performance of perovskites solar cells through modifying the electron transport layer with reduced graphene oxide. Materials Letters, 323, 4.
[32]. Jeon, N. J., Noh, J. H., Kim, Y. C., Yang, W. S., Ryu, S. and Seok, S. I. (2014). Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nature Materials, 13, 897- 903.
[33]. Im, J.-H., Jang, I.-H., Pellet, N., Grätzel, M. and Park, N.-G. (2014). Growth of CH3NH3PbI3 cuboids with controlled size for high-efficiency perovskite solar cells. Nature Nanotechnology, 9, 927-932.
[34]. Murugesan, V. S., Maitani, M., Segawa, H. and Miyasaka, T. (2025). Enhancement of power conversion efficiency in cesium-based mixed cation halide perovskite solar cells by controlled perovskite crystal out of glove box using post vapor annealing. Surfaces and Interfaces, 59, 105924
[35]. Chen, X., Wang, Z., Wu, R.-J., Cheng, H.-L. and Chui, H.-C. (2021). Laser-Induced Thermal Annealing of CH3NH3PbI3 Perovskite Microwires. Photonics, 8, 30.
[36]. Cassella, E. J., Spooner, E. L. K., Smith, J. A., Thornber, T., O'Kane, M. E., Oliver, R. D. J., Catley, T. E., Choudhary, S., Wood, C. J., Hammond, D. B., Snaith, H. J. and Lidzey, D. G. (2023). Binary Solvent System Used to Fabricate Fully Annealing-Free Perovskite Solar Cells. Advanced Energy Materials, 13, 14.
Cite this article
Li,Y. (2025). Research Progress on High-Efficiency Perovskite Solar Cells Based on Material Property. Applied and Computational Engineering,149,92-102.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of CONF-MSS 2025 Symposium: Automation and Smart Technologies in Petroleum Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Yang, X. Y., Song, Y. L., Wang, L. X., Sun, Y., Jin, B. W., Wang, J., Liu, H., Yang, Y. J., Lin, Q. Q., Fang, Y. J. and Dong, Q. F. (2024). In-line tempering eliminates the domain boundary in perovskite single crystals for high-energy resolution ionizing radiation detectors. Science Advances, 10, 10.
[2]. Shang, C. Y., Xiao, X. and Xu, Q. (2023). Coordination chemistry in modulating electronic structures of perovskite-type oxide nanocrystals for oxygen evolution catalysis. Coordination Chemistry Reviews, 485, 13.
[3]. Lindenthal, L., Popovic, J., Rameshan, R., Huber, J., Schrenk, F., Ruh, T., Nenning, A., Löffler, S., Opitz, A. K. and Rameshan, C. (2021). Novel perovskite catalysts for CO2 utilization - Exsolution enhanced reverse water-gas shift activity. Applied Catalysis B-Environmental, 292, 12.
[4]. Zhou, Q., Li, N. J., Chen, D. Y., Xu, Q. F., Li, H., He, J. H. and Lu, J. M. (2022). Efficient removal of Bisphenol A in water via piezocatalytic degradation by equivalent-vanadium-doped SrTiO3 nanofibers. Chemical Engineering Science, 247, 11.
[5]. Wang, Y. F., Li, Y., Su, Y. Q., Chen, X. R., Wang, R., Xu, J. Y., Zhou, G. Q., Chen, Z. S., Xiang, H. Y., Zhidkov, I. S., Kurmaev, E. and Zeng, H. B. (2024). Fine Purification Engineering Enables Efficient Perovskite QLEDs with Efficiency Exceeding 23%. Acs Applied Materials & Interfaces, 16, 28853-28860.
[6]. Nong, Y. Y., Yao, J. S., Li, J. Q., Xu, L. M., Yang, Z., Li, C. and Song, J. Z. (2024). Boosting External Quantum Efficiency of Blue Perovskite QLEDs Exceeding 23% by Trifluoroacetate Passivation and Mixed Hole Transportation Design. Advanced Materials, 36, 8.
[7]. Grätzel, M. (2014). The light and shade of perovskite solar cells. Nature Materials, 13, 838-842.
[8]. Zhou, J., Fu, S. Q., Zhou, S., Huang, L. S., Wang, C., Guan, H. L., Pu, D. X., Cui, H. S., Wang, C., Wang, T., Meng, W. W., Fang, G. J. and Ke, W. J. (2024). Mixed tin-lead perovskites with balanced crystallization and oxidation barrier for all-perovskite tandem solar cells. Nature Communications, 15, 10.
[9]. Long, R., Liu, J. and Prezhdo, O. V. (2016). Unravelling the Effects of Grain Boundary and Chemical Doping on Electron-Hole Recombination in CH3NH3PbI3 Perovskite by Time-Domain Atomistic Simulation. Journal of the American Chemical Society, 138, 3884-3890.
[10]. Azam, M., Ma, Y., Zhang, B. X., Shao, X. F., Wan, Z. Q., Zeng, H. B., Yin, H. M., Luo, J. S. and Jia, C. Y. (2025). Tailoring pyridine bridged chalcogen-concave molecules for defects passivation enables efficient and stable perovskite solar cells. Nature Communications, 16, 11.
[11]. Lin, Y. H., Sakai, N., Da, P., Wu, J. Y., Sansom, H. C., Ramadan, A. J., Mahesh, S., Liu, J. L., Oliver, R. D. J., Lim, J., Aspitarte, L., Sharma, K., Madhu, P. K., Morales-Vilches, A. B., Nayak, P. K., Bai, S., Gao, F., Grovenor, C. R. M., Johnston, M. B., Labram, J. G., Durrant, J. R., Ball, J. M., Wenger, B., Stannowski, B. and Snaith, H. J. (2020). A piperidinium salt stabilizes efficient metal-halide perovskite solar cells. Science, 369, 96-102.
[12]. Fang, Z., Deng, B. R., Jin, Y. B., Yang, L., Chen, L. S., Zhong, Y. W., Feng, H. P., Yin, Y., Liu, K. K., Li, Y. J., Zhang, J. Y., Huang, J. R., Zeng, Q. H., Wang, H., Yang, X., Yang, J. X., Tian, C. B., Xie, L. Q., Wei, Z. H. and Xu, X. P. (2024). Surface reconstruction of wide-bandgap perovskites enables efficient perovskite/silicon tandem solar cells. Nature Communications, 15, 11.
[13]. Zhu, P. D., Wang, D., Zhang, Y., Liang, Z., Li, J. B., Zeng, J., Zhang, J. Y., Xu, Y. T., Wu, S. Y., Liu, Z. X., Zhou, X. Y., Hu, B. H., He, F., Zhang, L., Pan, X., Wang, X. Z., Park, N. G. and Xu, B. M. (2024). Aqueous synthesis of perovskite precursors for highly efficient perovskite solar cells. Science, 383, 524-531.
[14]. Jain, S. M., Edvinsson, T. and Durrant, J. R. (2019). Green fabrication of stable lead-free bismuth based perovskite solar cells using a non-toxic solvent. Communications Chemistry, 2, 7.
[15]. Diau, E. W.-G., Jokar, E. and Rameez, M. (2019). Strategies To Improve Performance and Stability for Tin-Based Perovskite Solar Cells. ACS Energy Letters, 4, 1930-1937.
[16]. Kumar, M. H., Dharani, S., Leong, W. L., Boix, P. P., Prabhakar, R. R., Baikie, T., Shi, C., Ding, H., Ramesh, R., Asta, M., Graetzel, M., Mhaisalkar, S. G. and Mathews, N. (2014). Lead-Free Halide Perovskite Solar Cells with High Photocurrents Realized Through Vacancy Modulation. Advanced Materials, 26, 7122-7127.
[17]. Wang, F., Jiang, X. Y., Chen, H., Shang, Y. Q., Liu, H. F., Wei, J. L., Zhou, W. J., He, H. L., Liu, W. M. and Ning, Z. J. (2018). 2D-Quasi-2D-3D Hierarchy Structure for Tin Perovskite Solar Cells with Enhanced Efficiency and Stability. Joule, 2, 2732-2743.
[18]. ]Xiao, X., Wang, M. X., Chen, S. S., Zhang, Y. H., Gu, H. Y., Deng, Y. A., Yang, G., Fei, C. B., Chen, B., Lin, Y. Z., Dickey, M. D. and Huang, J. S. (2021). Lead-adsorbing ionogel-based encapsulation for impact-resistant, stable, and lead-safe perovskite modules. Science Advances, 7, 9.
[19]. Tockhorn, P., Sutter, J., Cruz, A., Wagner, P., Jäger, K., Yoo, D., Lang, F., Grischek, M., Li, B. R., Li, J. Z., Shargaieva, O., Unger, E., Al-Ashouri, A., Köhnen, E., Stolterfoht, M., Neher, D., Schlatmann, R., Rech, B., Stannowski, B., Albrecht, S. and Becker, C. (2022). Nano-optical designs for high-efficiency monolithic perovskite-silicon tandem solar cells. Nature Nanotechnology, 17, 1214-1221.
[20]. Shi, Y., Berry, J. J. and Zhang, F. (2024). Perovskite/Silicon Tandem Solar Cells: Insights and Outlooks. 9, 1305-1330.
[21]. Babics, M., Bristow, H., Pininti, A. R., Allen, T. G. and De Wolf, S. (2023). Temperature Coefficients of Perovskite/Silicon Tandem Solar Cells. ACS Energy Letters, 8, 3013-3015.
[22]. Luo, H. W., Zheng, X. T., Kong, W. C., Liu, Z., Li, H. J., Wen, J., Xia, R., Sun, H. F., Wu, P., Wang, Y. R., Mo, Y., Luo, X., Huang, Z. L., Hong, J. J., Chu, Z. J., Zhang, X. L., Yang, G. T., Chen, Y. F., Feng, Z. Q., Gao, J. F. and Tan, H. R. (2023). Inorganic Framework Composition Engineering for Scalable Fabrication of Perovskite/Silicon Tandem Solar Cells. ACS Energy Letters, 8, 4993-5002.
[23]. Al-Ashouri, A., Köhnen, E., Li, B., Magomedov, A., Hempel, H., Caprioglio, P., Márquez, J. A., Vilches, A. B. M., Kasparavicius, E., Smith, J. A., Phung, N., Menzel, D., Grischek, M., Kegelmann, L., Skroblin, D., Gollwitzer, C., Malinauskas, T., Jost, M., Matic, G., Rech, B., Schlatmann, R., Topic, M., Korte, L., Abate, A., Stannowski, B., Neher, D., Stolterfoht, M., Unold, T., Getautis, V. and Albrecht, S. (2020). Monolithic perovskite/silicon tandem solar cell with >29% efficiency by enhanced hole extraction. Science, 370, 1300-1309.
[24]. Yang, G., Wang, M. R., Fei, C. B., Gu, H. Y., Yu, Z. J., Alasfour, A., Holman, Z. C. and Huang, J. S. (2023). Recycling Silicon Bottom Cells from End-of-Life Perovskite-Silicon Tandem Solar Cells. ACS Energy Letters, 6.
[25]. Jost, M., Köhnen, E., Al-Ashouri, A., Bertram, T., Tomsic, S., Magomedov, A., Kasparavicius, E., Kodalle, T., Lipovsek, B., Getautis, V., Schlatmann, R., Kaufmann, C. A., Albrecht, S. and Topic, M. (2022). Perovskite/CIGS Tandem Solar Cells: From Certified 24.2% toward 30% and Beyond. ACS Energy Letters, 7, 1298-1307.
[26]. Tavakoli, M. M., Tsui, K. H., Zhang, Q. P., He, J., Yao, Y., Li, D. D. and Fan, Z. Y. (2015). Highly Efficient Flexible Perovskite Solar Cells with Antireflection and Self-Cleaning Nanostructures. Acs Nano, 9, 10287-10295.
[27]. Hu, X. T., Meng, X. C., Yang, X., Huang, Z. Q., Xing, Z., Li, P. W., Tan, L. C., Su, M., Li, F. Y., Chen, Y. W. and Song, Y. L. (2021). Cementitious grain-boundary passivation for flexible perovskite solar cells with superior environmental stability and mechanical robustness. Science Bulletin, 66, 527-535.
[28]. Jin, J. J., Zhu, Z. K., Ming, Y. D., Zhou, Y., Shang, J. T., Wang, S. F., Cui, X. X., Guo, T. H., Zhang, D., Tang, G. Q., Lin, Q. Q., Li, J. H., Liu, X. W., Liu, S., Chen, Z. W., Hu, Z., Meng, H. and Tai, Q. D. (2025). Spontaneous bifacial capping of perovskite film for efficient and mechanically stable flexible solar cell. Nature Communications, 16, 11.
[29]. Zhang, W. F., Liu, J., Song, W., Shan, J. H., Guan, H. W., Zhou, J., Meng, Y. Y., Tong, X. Y., Zhu, J. T., Yang, M. J. and Ge, Z. Y. (2025). Chemical passivation and grain-boundary manipulation via in situ cross-linking strategy for scalable flexible perovskite solar cells. Science Advances, 11, 15.
[30]. Homola, T., Pospisil, J., Shekargoftar, M., Svoboda, T., Hvojnik, M., Gemeiner, P., Weiter, M. and Dzik, P. (2020). Perovskite Solar Cells with Low-Cost TiO2 Mesoporous Photoanodes Prepared by Rapid Low-Temperature (70 °C) Plasma Processing. Acs Applied Energy Materials, 3, 12009-12018.
[31]. Khan, M. T. and Khan, F. (2022). Enhancement in photovoltaic performance of perovskites solar cells through modifying the electron transport layer with reduced graphene oxide. Materials Letters, 323, 4.
[32]. Jeon, N. J., Noh, J. H., Kim, Y. C., Yang, W. S., Ryu, S. and Seok, S. I. (2014). Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nature Materials, 13, 897- 903.
[33]. Im, J.-H., Jang, I.-H., Pellet, N., Grätzel, M. and Park, N.-G. (2014). Growth of CH3NH3PbI3 cuboids with controlled size for high-efficiency perovskite solar cells. Nature Nanotechnology, 9, 927-932.
[34]. Murugesan, V. S., Maitani, M., Segawa, H. and Miyasaka, T. (2025). Enhancement of power conversion efficiency in cesium-based mixed cation halide perovskite solar cells by controlled perovskite crystal out of glove box using post vapor annealing. Surfaces and Interfaces, 59, 105924
[35]. Chen, X., Wang, Z., Wu, R.-J., Cheng, H.-L. and Chui, H.-C. (2021). Laser-Induced Thermal Annealing of CH3NH3PbI3 Perovskite Microwires. Photonics, 8, 30.
[36]. Cassella, E. J., Spooner, E. L. K., Smith, J. A., Thornber, T., O'Kane, M. E., Oliver, R. D. J., Catley, T. E., Choudhary, S., Wood, C. J., Hammond, D. B., Snaith, H. J. and Lidzey, D. G. (2023). Binary Solvent System Used to Fabricate Fully Annealing-Free Perovskite Solar Cells. Advanced Energy Materials, 13, 14.









