1. Introduction
1.1. Beta-blockers
Beta-blockers are a class of drugs primarily used in cardiovascular diseases and other conditions, the mechanism of which relies on blocking the receptor sites for hormones. Normally, the endogenous catecholamines epinephrine and norepinephrine possess the ability to activate beta-receptors by binding, causing various physiological reactions including hypertension, tachycardia and tremor [1]. The beta- receptors can be divided into three types according to their positions, namely, beta1, beta2 and beta3 receptors. Beta1-receptors are located mainly in the heart and kidneys. Beta2-receptors are located mainly in the lungs, gastrointestinal tract, liver, uterus and muscles. Beta3-receptors are located in fat cells, causing fat cells to break down and sometimes causing tremors [2]. Beta-blockers, as competitive antagonists, block beta-receptor sites, interfere with the binding of receptors with adrenaline and other stress hormones, thus weakening their effects. Some of them block activation of all types of beta-receptors and others are selective for one of the three known types, which categorizes beta-blockers into two groups according to binding affinity.
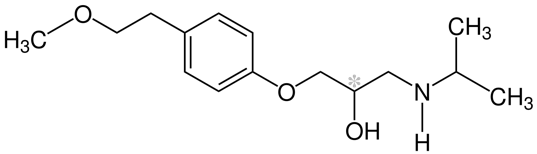
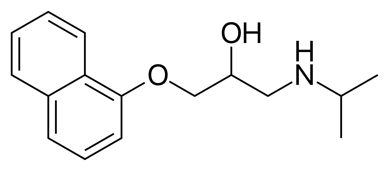
1.1.1. Structures of beta-blockers. The nomenclature for all beta-blockers contains a “LOL” suffix (Table 1). The general structure of beta-blockers contains an aromatic or heteroaromatic ring connected to a side alkyl chain incorporating amine and hydroxyl functional groups with at least one chiral center, possessing a high degree of enantioselectivity in binding to beta-adrenergic receptors. The amino group of beta-blockers is a main site for drug metabolism and inactivation, and the protonated amine group at physiologic pH (7.35-7.45) is required for beta-receptor binding [3].
Table.1 Structures and names of common beta-blockers | ||
name | Chemical structure | IUPAC names |
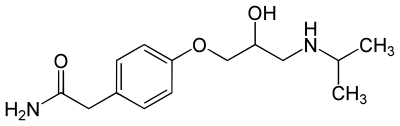
Atenolol/Tenormin |
| (RS)-2-{4-[2-Hydroxy-3-(propan-2-ylamino)propoxy]phenyl}acetamide |
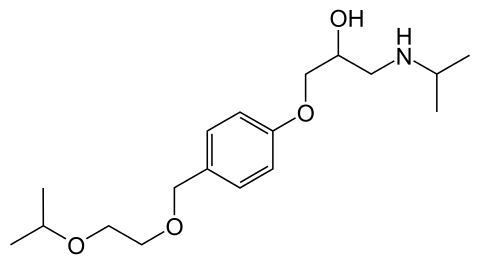
Bisoprolol/Concor |
| (RS)-14-[(2-isopropoxyethoxy)methyl]phenoxy 3-(isopropylamino)propan-2-ol |
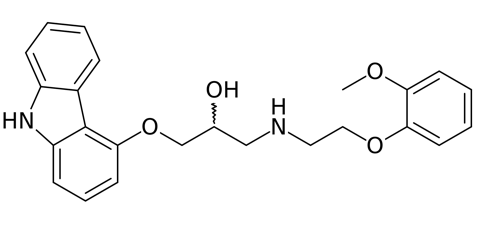
Carvedilol/Coreg |
| (±)-[3-(9H-carbazol-4-yloxy)-2-hydroxypropyl][2-(2-methoxyphenoxy)ethyl]amine |
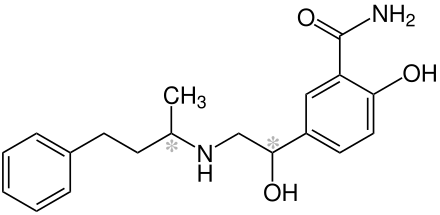
Labetalol/Trandate |
| (RS)-2-hydroxy-5-[1-hydroxy-2-(4-phenylbutan-2-ylamino)-ethyl]-benzamide |
Metoprolol /Betaloc or Lopresor |
| (RS)-1-(Isopropylamino)-3-[4-(2-methoxyethyl)phenoxy]propan-2-ol |
Propranolol /Inderal or Angilol |
| ((RS)-1-(1-methylethylamino)-3-(1-naphthyloxy)propan-2-ol |
1.1.2. Medical uses of beta-blockers. Through blockade of beta-receptors, beta-blockers are found and designed to reduce sympathetic nervous system activity, thus are widely used as cardiovascular drugs to decrease the number of deaths, strokes and heart attacks associated with hypertension and tachycardia [4]. Beta-blockers such as propranolol and atenolol have been prescribed for decades to provide anxiolytic treatment for anxiety disorder and social phobia, especially when the symptoms are related to the cardiovascular system [1]. Additionally, performance-enhancing effects of beta-blockers have been commonly reported for musicians, professional dancers and athletes for lowering heart rates and reducing tremors in events where accuracy and precision are vital. According to the Prohibited List by World Anti-Doping Agency (WADA), beta-blockers have been maintained as prohibited substances for athletes [5].
High doses of Beta-blocker medications can result in many adverse effects including bradycardia, hypotension and arrhythmia [6]. In addition, researchers believe that the amine and amide functional groups of beta-blockers react with stomach acid to form derivatives with genotoxicity, carcinogenicity and mutagenicity, which can lead to severe liver injury [7]. Therefore, considering the adverse and toxic effects of beta-blockers along with the fact of being abused by athletes and professionals, it is of great significance to determine beta-blockers in human plasma and urine by sensitive, rapid and affordable approaches.
1.2. Determination of beta-blockers
Up to date, a variety of methods have been reported for beta-blocker determination [8] (Fig.1). Commonly, chromatographic methods are most widely used due to their easy access and sensitivity, and subsequent detectors are employed for quantification, such as UV detection, Mass Spectrometry detection and fluorescence detection. Though Liquid Chromatography (LC) and Gas Chromatography (GC) possess preferred high sensitivity and reliability, their time and labor-consuming procedure remains the major obstacle. In chromatography, sampling preparation is an essential step to improve sensitivity, which includes clean-up, extraction and sometimes derivatization for GC. Subsequent detections also require much preparation for quantification. Optical methods are based on the reaction of samples to electromagnetic radiation. Compared with chromatography, it is a relatively inexpensive and fast tool. However, optical methods are often characterized by quite narrow linear ranges of 2-3 folds. Their use is also limited in complex matrices such as serum and plasma [8].
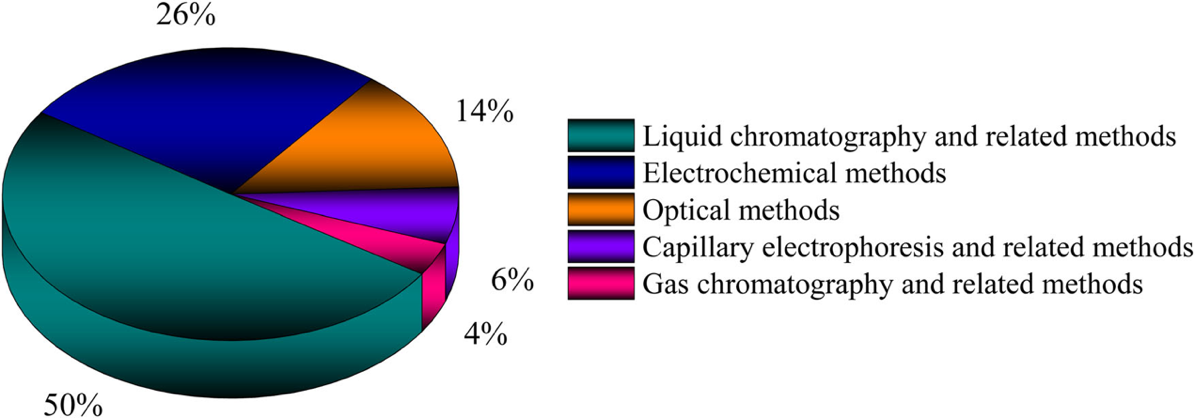 As shown in Fig.1, electrochemical methods make up 26% of the determination of beta-blockers, and their distinct advantage from others is the accessible procedure without separation or extraction process. The determination of targets usually requires separation and quantification, and electrochemical sensors combine these two steps readily through voltammetric methods where the current changes following changing potentials applied, and the peak current is in linearity with sample concentration in a specific range. By identifying beta-blockers from their specific oxidation/reduction potentials and by the enhanced selectivity and sensitivity of electrode modification, electrochemical sensors are excellent alternatives in determining beta-blockers in a rapid, sensitive and affordable way. This review focuses on improved sensitivity and selectivity due to electrode modifications of voltammetric sensors for beta-blockers.
As shown in Fig.1, electrochemical methods make up 26% of the determination of beta-blockers, and their distinct advantage from others is the accessible procedure without separation or extraction process. The determination of targets usually requires separation and quantification, and electrochemical sensors combine these two steps readily through voltammetric methods where the current changes following changing potentials applied, and the peak current is in linearity with sample concentration in a specific range. By identifying beta-blockers from their specific oxidation/reduction potentials and by the enhanced selectivity and sensitivity of electrode modification, electrochemical sensors are excellent alternatives in determining beta-blockers in a rapid, sensitive and affordable way. This review focuses on improved sensitivity and selectivity due to electrode modifications of voltammetric sensors for beta-blockers.
Fig.1 Reported analytical tools for determination of beta-blockers [8]
2. Electrochemical sensors for beta-blockers
In recent decades, electrochemical sensors have been widely used because of their simplicity and fast responses. Electrodes serve as transducers, translating the chemical signals into electronic signals to the output. The system usually consists of three electrodes. The working electrode is under investigation by the analytes. The complementary reactions take place at the counter electrode to maintain charge balance. A reference electrode with high stability is used to measure the potential of the working electrode. As far as the detected signal is concerned, the determination of beta-blockers can usually be based on potentiometry and voltammetry.
2.1. Potentiometric sensors
Potentiometric sensors measure the voltage between the working electrode and reference electrode, which is logarithmically correlated to the beta-blocker concentration according to the Nernst equation where ERed stands for standard half-cell reduction potential, R for the universal gas constant, T for temperature in Kelvins, z for the number of electrons transferred, F for Faraday constant and a for chemical activity (equation 1). However, the logarithmical relation has made it difficult for potentiometric methods to achieve high sensitivity, broad linear range and low detection limits.
\( {E_{Red}}=E_{Red}^{⊖}-\frac{RT}{zF}ln\frac{{a_{Red}}}{{a_{Ox}}}\ \ \ ( AUTONUM ) \)
2.2. Voltammetric sensors
To achieve a higher sensitivity and lower detection, the enhancement of signals stands out to be an excellent tool, leaving voltammetric sensors more favorable in determination of beta-blockers. Voltammetry is the study of current (usually current density) as a function of applied potential. The current signals can be enhanced by modification which enlarges the electrode surface area and increases the conductivity.
The most distinguishing characteristic of voltammetric methods is the unique oxidation/reduction potentials determined by both the target and electrode, with a defined peak at a specific potential and the height of the peak in linearity with beta-blocker concentration. Measuring methods for voltammetric sensors include Cyclic Voltammetry (CV), Linear Sweep Voltammetry (LSV), Differential Pause Voltammetry (DPV) and Squarewave Voltammetry (SWV), among which DPV is the most commonly applied for beta-blockers.
All beta-blockers can be irreversibly oxidized at the hydroxyl group, which turns into a carbonyl group by losing two electrons and two protons [2]. Considering different functional groups of beta-blockers, the cathodic reactions could happen in the carbonyl groups, or the heterocyclic aromatic rings. For the absence of reducible parts, nitrification and nitrosation are usually derivatization [7]. In addition, before cathodic analysis the system requires a degassing process to remove the dissolved oxygen. Therefore, the anodic analysis of beta-blockers has been studied in most research.
3. Increased sensitivity of voltammetric sensors by modifying nanomaterials
Sensitivity refers to how a sensor’s signal responds to changes in analyte concentration and often is related to the limit of detection (LOD), which is the lowest concentration of the species of interest that can be measured beyond the noise using a detecting method [9]. In voltammetric methods, the peak height (current value) is linearly correlated with beta-blocker concentration. With the current signals enhanced by modification due to enlarged surface area and higher conductivity, the sensitivity also increases. Nanomaterials are the most commonly used modifiers for their high surface-area-to-volume ratios and high electron transfer efficiency. Some of the negatively charged materials interacting with protonated beta-blockers result in adsorption on the electrode surface, which also amplifies current signals.
3.1. Inorganic nanomaterials
Inorganic materials cover a wide range of substances, such as metals, metal oxides and metal salts, their sizes and morphologies controlled by synthetic routes. Inorganic nanomaterials amplify the current signals by providing a large surface area and fast electron transfer, and most of them serve as catalysts in the electrochemical oxidation of beta-blockers. Some inorganic species on the electrode can be oxidized, such as gold and copper. In the work of Shadjou et al., Cu(III) produced from CuO nanoparticles was proved to oxidize atenolol, carvedilol and propranolol at lower potentials [10]. Research by Casella et.al also suggested the involvement of beta-blockers in the oxidation of polycrystalline gold electrodes, with the anodic peak current proportional to concentration of propranolol and atenolol respectively [8]. These nanoparticles’ oxidation processes are surface-controlled and are associated with the adsorption steps between analytes and surface catalytic sites. Apart from this, medical studies have indicated magnesium improved the efficacy of all classes of antihypertensive drugs. Khairy et. al determined nifedipine and atenolol simultaneously on a magnesium oxide nanoplatelet modified screen-printed electrode, with detection limits of 0.032 μM and 1.76 μM, respectively [11]. For metal salts and metal oxides with lower electron conductivity, some still present reliable features in detecting beta-blockers. Zeolite-modified electrodes have been attractive in the past few years because of their molecular sieving and ion exchange properties. The incorporation of protonated atenolol by zeolite through electrostatic attraction resulted in a preconcentration before voltammetric measurement, with a LOD of 0.1 μM [12].
3.2. Organic nanomaterials
Graphene is a two-dimensional and single-layered carbon material with excellent electron conducting properties, thermal and mechanical stability, and every carbon atom is responsible for the environmental changes in its surroundings [13]. Appearing a spongy type structure, graphene provides a large surface area (2630m2 ··g-1), enhancing the current signals of -beta-blockers [14]. A reported electrochemical sensor for acebutolol with graphene-modified glassy carbon electrode as the working electrode presented a very low LOD of 0.0003 μM by DPV [13]. However, mass production of high-quality and low-cost graphene remains a challenge today due to the limited choices of solvent and expensive equipment. Graphene oxide (GO) and reduced graphene oxide (rGO) are derivatives of graphene. GO has the same structure as graphene but the single layer of carbon is covalently functionalized with oxygen-containing functional groups, and rGO is produced from GO with fewer oxygen functionalities. Though usually with more defects, residual oxygen and heteroatoms than graphene, rGO is an attractive alternative to graphene for its graphene-like properties and easy preparation. In the work of Purushothama and Nayaka, the rGO-modified electrochemical sensor for propranolol had a LOD of 0.002μM by adsorptive SWV [15].
Carbon nanotubes (CNTs) are cylindrically shaped allotropes of carbon possessing excellent mechanical strength, a large ratio of surface area to mass and high electrical conductivity. Multi-walled CNTs consist of multiple layers of concentric nanotubes of graphene inside other nanotubes. In the form of small bundles, CNTs act as a conductive channel for metal nanoparticles, the electrode and beta-blockers [16]. Additionally, CNTs are usually functionalized with carbonyl groups to maintain their stability, which contains a negative moiety. As mentioned earlier, the amine group of beta-blockers get protonated in physiological pH, which interacts well with carbonyl groups, resulting in a pre-concentration on the surface and enhancing the signals.
3.3. Quantum Dots
Recently, carbon Quantum Dots (QDs) have gained much attention in electrochemical sensing for their quantum size, biocompatibility, enzyme-like activity, high active area, and abundance of hydrophilic groups [17]. QDs have emerged as an attractive semiconductive material with extremely small sizes (0-10nm), tunable electronic and optical properties, their suitable band gap being the most characteristic feature for luminescent effects. Additionally, the band gap makes it possible for rapid formation of electron-hole pairs leading to high electron transfer efficiency. Generally, QDs are classified into two types, inorganic and organic. Inorganic QDs compose of an inner core, outer shell, stabilizing layer, ligands and the supporting linkers, usually ZnS, PbS and CdS etc. Organic QDs with better biocompatibility and lower toxicity, such as carbon quantum dots (CQDs) and graphene quantum dots (GQDs), can be synthesized in readily accessible and affordable approaches [18]. The synthetic routes differ between these two types of organic QDs and functional groups on their edges, with most CQDs being amorphous quasi-spherical nanoparticles, and most GQDs being more crystalline and resembling graphene nano-sheets. Following different precursors and routes, hydrophilic groups on the edges of organic QDs vary from hydroxyl, carboxyl or amino ones, which immobilize beta-blockers through hydrogen bonds and electrostatic forces, enhancing the connection between surface and beta-blockers. Due to its excellent biocompatibility with edgy functionalities, GQDs were also reported to adsorb the enzyme laccase on glassy carbon electrode for the determination of a neurotransmitter epinephrine. By forming covalent bonds, glutaraldehyde is used as a crosslinker to maintain a stable laccase layer on GQDs [19], where laccase is a green multi-copper oxidase and was proved to catalyze the electrochemical oxidation of atenolol [20]. Furthermore, the π-π network of GQDs also provides possibilities for the electrostatic adsorption of beta-blockers. Although there is only little research on QDs-modified electrochemical sensors for beta-blockers up to date, the future application will be promising. Especially for organic QDs, not only do they have higher electron transfer efficiency than GOs [21], a large surface area, but also possess excellent biocompatibility and low toxicity, and are synthesized from affordable and green raw materials.
Nanomaterials are unstable independently due to their large surface energy. Thus, CNTs and polymers generally serve as supporting matrices for them in assurance of dispersibility and structural stability lacked by nanomaterials alone. In reported studies, nanocomposites combining metal nanoparticles or QDs with MWCNTs are favored as modifier of electrodes [22]. In the work of Arab et al., CdS@ZnS QDs were decorated on MWCNTs, and modification of CdS@ZnS/MWCNTs resulted in a nearly 3-times increase of the peak current for propranolol with a fairly low LOD of 14nM. The linear relation between peak current and scan rate suggested the adsorption-controlled process, in consistency with the electrostatic interaction between the negative CdS and protonated propranolol [23]. Polymers supporting nanomaterials also play a role in screening beta-blockers, as they are modified as selective layers. For conductive polymers where sp2 electrons are contiguous in the backbone, signals will further be enhanced.
4. Chemically selective polymeric layers
Though it is vital for electrochemical sensors to have high sensitivity, selectivity is more important than sensitivity and ensures accuracy, especially when biological samples contain many metabolites. While being insulators, many polymers interact with beta-blockers through hydrogen bonds, electrostatic forces, π-electrons and hydrophobic effect, etc. These interactions serve as screening gates for beta-blockers.
4.1. Preconcentration of beta-blockers
Nafion is a sulfonated tetrafluoroethylene-based fluoropolymer belonging to the first class of synthetic polymers with ionic properties. While the negatively charged sulfonate groups selectively preconcentrate beta-blocker cations, the hydrophobic backbone also provides selectivity through hydrophobic interactions [24]. The work by Nigovic et. al suggested a remarkable amplification of peak currents and a decrease in the overpotential for propranolol and bisoprolol on a Nafion-coated glassy carbon electrode. This Nafion-modified electrode successfully determined propranolol and bisoprolol in the presence of common interfering substances in biological samples, co-administrated drugs and other types of beta-blockers.
For polymers with conjugated π-electron structure, beta-blockers are preferentially adsorbed on the electrode through interactions between benzenic rings. Surface modification of polydopamine is realized through an oxidative polymerization reaction of dopamine, the thickness of which can be controlled by the number of cyclic voltammetry. Interestingly, the quinone/hydroquinone moieties of polydopamine can be oxidized/reduced at the surface. With atenolol added to the solution, they are adsorbed on the electrode surface through π-π interaction. Thus, the anodic peak current of polydopamine oxidation decreased, which is proportional to the concentration of atenolol. This well overcomes the problem that beta-blockers are oxidized at high potentials by measuring the oxidation of polydopamine. Additionally, the negative correlation between peak current and concentration of atenolol made it possible to lower the LOD, which turned out to be 2.7 × 10-8 M [25]. When the conjugated sp2 hybridized carbon bonded together to form the backbone of the polymer, the extended π-electron network shows excellent conductivity. Conductive polymers are broadly used in surface modification as they combine the advantages of organic polymers and semiconductors with good biocompatibility and high conductivity. In presence of 100-fold concentration of interferents in biological fluids, conductive polymers of melamine (1,3,5-triazine-2,4,6-triamine) had been reported to determine propranolol, which was also pre-concentrated on the surface through π-π interaction, with a very low LOD of 9nM [26].
As described above, beta-blockers are adsorbed on the electrode surface through interactions with the polymeric layers, which not only improved the selectivity but also enhanced the peak current signals. After modification with conductive polymer, the sensitivity will be further improved.
4.2. Molecularly imprinted polymers
As beta-blockers are generally prescribed with other drugs simultaneously and their detection is carried out in complex biological samples, it is of great significance that the electrode shows very high selectivity. Molecularly imprinted polymers (MIPs) are artificial receptors for target molecules with antibody-antigen-like recognition. The synthesis involves the formation of a complex between template molecules and functional monomers in a polymerization solvent containing cross-linkers and polymerization initiators. The complex is usually formed through strong interactions between protonated beta-blockers and the negative carboxyl groups of functional monomers, and other weaker bonds such as π-electron interaction and sometimes hydrophobic effects. In recent years, MIPs have emerged as a perfect candidate for electrode modification. Firstly, their unique selectivity is determined both by the molecular structure of templates and interactions between templates and functional monomers. Secondly, MIPs show excellent stability and mechanical strength due to the cross-linking reactions. In voltammetric sensors, only predetermined analytes can rebind to cavities after template extraction, resulting in specific oxidation for analytical determination. In order to gain an intimate integration between the MIP and the electrode, usually electropolymerization is carried out in a solution containing essential reactants and a suitable solvent, and the thickness is controlled by polymerization time or cycle numbers [27]. Due to the nonconducting feature of common polymers, many conductive materials such as CNTs and metal nanoparticles are composited with them to achieve higher sensitivity.
With selectivity being the most vital feature of MIP-based electrochemical sensors, decisions of the other three types of reactants (functional monomers, cross-linkers and polymerization initiators) and the solvent remain a major problem. Because of the complicated process, it is beneficial to determine the appropriate calculation method of functional monomer and solvents when calculating the binding energy and stability energies in different solvents. In the work of Nezhadali and Mojarrab, pyrrole and water were selected as functional monomers and polymerization solvents based on the computational results. In the presence of 4-fold of all interfering species, including other beta-blockers and analogs substances, metoprolol was successfully examined with a LOD of 2.88nM [28].
Many pharmaceutical compounds prescribed today are enantiomers with different pharmacological and pharmacokinetic activities, and some differences can be very profound. S-enantiomers of beta-blockers are 50-500 times more potent than R-enantiomers because S-enantiomers present greater affinity on beta-receptors [29]. Therefore, it is of great necessity that analytical tools show a chiral determination of beta-blockers. Compared to other polymeric layers, MIPs have complementary cavities of template molecules with both geometrical and chemical features, which makes them sensitive to molecules with nuanced structures. In the work by Iacob et. al, the MIP-modified electrode with R(+)-atenolol as a template simultaneously determined four types of R(+)-antipodes of beta-blockers by chiral discrimination of MIP and different oxidation potentials. [30]
5. Conclusion and prospect
Beta-blockers are a type of cardiovascular drug generally prescribed in treating hypertension for its ability to reduce sympathetic nervous system activity. Professionals also use it to lower heart rates and avoid tremors in events requiring high accuracy. The medical use, adverse reactions and abuse of many beta-blockers have prompted people to develop analytical tools for them. Although many methods provide high sensitivity, such as chromatography and mass spectrometry, the complicated pre-treatment and high cost are obstacles to the fast and convenient determination of beta-blockers.
This review briefly introduces the electrochemical sensors for beta-blockers, which has the characteristics of short analysis time, high sensitivity, low cost and so on. Voltammetric sensors are the most common electrochemical sensors for beta-blockers, where a changing potential is applied to the system and current signals are recorded for further analysis. In order to improve the sensitivity, nano-materials including metal nanoparticles, metal oxides, carbon nano-materials and quantum dots were modified on electrodes. They mainly enhance the signals by providing large surface area and higher electron transfer, and some inorganic nanoparticles present catalytic activity such as gold and copper. The negatively charged modified layer is beneficial to the electrostatic force between nanomaterials and protonated beta-blockers, which leads to signal enhancement pre-concentration. One possible way to lower the LOD is employing beta-blockers as an inhibitor to certain electrochemical reactions whose reduced signals are recorded.
Generally, in voltammetry, beta-blockers are identified by their anode potentials, but other substances might be interfered by overlapping peaks, and the oxidation potentials can be quite high. Therefore, electrodes are modified with materials that have preferences toward beta-blockers and can lower the potentials. Chemically selective polymers interact well with protonated beta-blockers because of their functional groups and π-electrons, which block many interfering substances. The conjugated π-electron network of conductive polymers can further amplify current signals. Common polymers such as Nafion and polydopamine are used as modifiers showing both selectivity and pre-concentration of beta-blockers. The MIP-based electrodes provide very high selectivity due to their key-lock recognition, and studies have proven the successful chiral determination of 4 beta-blockers when chiral analysis is important for pharmaceutical drugs. However, the chemical substances necessary for MIP synthesis are relatively expensive and hazardous to the human body. Considering the biocompatibility of some metals and organic QDs, it might be possible for electrodes to be modified with these materials where bio-compounds are incorporated, such as enzymes as they present excellent selectivity and are harmless. The chemical behavior between beta-blockers and bio-compounds is therefore recorded on the electrode.
In summary, nanomaterials are modified for signal enhancement and polymers are modified for selectivity, though some of these two types can share functions such as catalysis, selectivity and pre-concentration. Nonetheless, immobilizing nanomaterials and polymers together will lead to desired analytical results. Future research might aim at the compatibility of these two electrode modifiers and their concurrent effect in detecting beta-blockers.
References
[1]. Dooley T. Mental Health in Family Medicine. 2015;11:89-99.
[2]. Sarvestani MRJ, Madrakian T, Afkhami A. J Electroanal Chem. 2021;899:115666.
[3]. Bekhradnia A, Ebrahimzadeh M. Med Chem. 2014.
[4]. do Vale GT, Ceron CS, Gonzaga NA, Simplicio JA, Padovan JC. Curr Hypertens Rev. 2019;15(1):22-31.
[5]. Heuberger JAAC, Cohen AF. Sports Medicine. 2019;49(4):525-39.
[6]. Reith DM, Dawson AH, Whyte IM, Buckley NA, Sayer GP. Journal of Toxicology: Clinical Toxicology. 1996;34(3):273-8.
[7]. Belal F, Al-Deeb OA, Al-Majed AA, Gad-Kariem EAR. Il Farmaco. 1999;54(10):700-4.
[8]. Yıldırım S, Erkmen C, Uslu B. Crit Rev Anal Chem. 2022;52(1):131-69.
[9]. Desimoni E, Brunetti B. Electroanalysis. 2013;25(7):1645-51.
[10]. Shadjou N, Hasanzadeh M, Saghatforoush L, Mehdizadeh R, Jouyban A. Electrochim Acta. 2011;58:336-47.
[11]. Khairy M, Khorshed AA, Rashwan FA, Salah GA, Abdel-Wadood HM, Banks CE. Sens. Actuators, B. 2017;252:1045-54.
[12]. Arvand M, Vaziri M, Vejdani M. Materials Science and Engineering: C. 2010;30(5):709- 14.
[13]. Bagoji AM, Nandibewoor ST. New J Chem. 2016;40(4):3763-72.
[14]. Er E, Çelikkan H, Erk N. Sens Actuators, B. 2017;238:779-87.
[15]. Purushothama HT, Nayaka YA. Anal Bioanal Electrochem. 2019;11:1575-89.
[16]. Kun Z, Yi H, Chengyun Z, Yue Y, Shuliang Z, Yuyang Z. Electrochim Acta. 2012;80:405- 12.
[17]. Hassanvand Z, Jalali F, Nazari M, Parnianchi F, Santoro C. ChemElectroChem. 2021;8(1):15-35.
[18]. Elugoke SE, Adekunle AS, Fayemi OE, Mamba BB, Sherif E-SM, Ebenso EE. Biosensors. 2020;10(11):162.
[19]. Baluta S, Lesiak A, Cabaj J. Electroanalysis. 2018;30(8):1781-90.
[20]. Bathinapatla A, Gorle G, Kanchi S, Puthalapattu RP, Ling YC. Bioelectrochemistry. 2022:108212.
[21]. Li M, Ni W, Kan B, Wan X, Zhang L, Zhang Q, et al. Physical Chemistry Chemical. Physics. 2013;15(43):18973-8.
[22]. Arumugasamy SK, Govindaraju S, Yun K. Appl Surf Sci. 2020;508:145294.
[23]. Arab N, Fotouhi L, Salis A. Microchem J. 2021;168:106453.
[24]. Nigović B, Marušić M, Jurić S. J Electroanal Chem. 2011;663(2):72-8.
[25]. Amiri M, Amali E, Nematollahzadeh A. Sens Actuators, B. 2015;216:551-7.
[26]. Raj M, Gupta P, Goyal RN. J Electrochem Soc. 2016;163(6):H388-H94.
[27]. Antuña Jiménez D, Díaz Díaz G, Blanco López MdC, Lobo Castañón MJ, Miranda. Ordieres AJ, Tuñón Blanco P. Molecularly Imprinted Sensors. 2012.
[28]. Nezhadali A, Mojarrab M. Anal Chim Acta. 2016;924:86-98.
[29]. Cizmarikova R. Ceska a Slovenska Farmacie: Casopis Ceske Farmaceuticke Spolecnosti a Slovenske Farmaceuticke Spolecnosti. 2002;51(3):121-8.
[30]. Iacob B-C, Bodoki E, Florea A, Bodoki AE, Oprean R. Anal Chem. 2015;87(5):2755-63.
Cite this article
Jiang,P. (2023). Electrochemical determination of beta-blockers: A review on voltammetric sensors with chemically modified electrode in terms of sensitivity and selectivity. Applied and Computational Engineering,7,47-55.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Materials Chemistry and Environmental Engineering (CONF-MCEE 2023), Part II
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Dooley T. Mental Health in Family Medicine. 2015;11:89-99.
[2]. Sarvestani MRJ, Madrakian T, Afkhami A. J Electroanal Chem. 2021;899:115666.
[3]. Bekhradnia A, Ebrahimzadeh M. Med Chem. 2014.
[4]. do Vale GT, Ceron CS, Gonzaga NA, Simplicio JA, Padovan JC. Curr Hypertens Rev. 2019;15(1):22-31.
[5]. Heuberger JAAC, Cohen AF. Sports Medicine. 2019;49(4):525-39.
[6]. Reith DM, Dawson AH, Whyte IM, Buckley NA, Sayer GP. Journal of Toxicology: Clinical Toxicology. 1996;34(3):273-8.
[7]. Belal F, Al-Deeb OA, Al-Majed AA, Gad-Kariem EAR. Il Farmaco. 1999;54(10):700-4.
[8]. Yıldırım S, Erkmen C, Uslu B. Crit Rev Anal Chem. 2022;52(1):131-69.
[9]. Desimoni E, Brunetti B. Electroanalysis. 2013;25(7):1645-51.
[10]. Shadjou N, Hasanzadeh M, Saghatforoush L, Mehdizadeh R, Jouyban A. Electrochim Acta. 2011;58:336-47.
[11]. Khairy M, Khorshed AA, Rashwan FA, Salah GA, Abdel-Wadood HM, Banks CE. Sens. Actuators, B. 2017;252:1045-54.
[12]. Arvand M, Vaziri M, Vejdani M. Materials Science and Engineering: C. 2010;30(5):709- 14.
[13]. Bagoji AM, Nandibewoor ST. New J Chem. 2016;40(4):3763-72.
[14]. Er E, Çelikkan H, Erk N. Sens Actuators, B. 2017;238:779-87.
[15]. Purushothama HT, Nayaka YA. Anal Bioanal Electrochem. 2019;11:1575-89.
[16]. Kun Z, Yi H, Chengyun Z, Yue Y, Shuliang Z, Yuyang Z. Electrochim Acta. 2012;80:405- 12.
[17]. Hassanvand Z, Jalali F, Nazari M, Parnianchi F, Santoro C. ChemElectroChem. 2021;8(1):15-35.
[18]. Elugoke SE, Adekunle AS, Fayemi OE, Mamba BB, Sherif E-SM, Ebenso EE. Biosensors. 2020;10(11):162.
[19]. Baluta S, Lesiak A, Cabaj J. Electroanalysis. 2018;30(8):1781-90.
[20]. Bathinapatla A, Gorle G, Kanchi S, Puthalapattu RP, Ling YC. Bioelectrochemistry. 2022:108212.
[21]. Li M, Ni W, Kan B, Wan X, Zhang L, Zhang Q, et al. Physical Chemistry Chemical. Physics. 2013;15(43):18973-8.
[22]. Arumugasamy SK, Govindaraju S, Yun K. Appl Surf Sci. 2020;508:145294.
[23]. Arab N, Fotouhi L, Salis A. Microchem J. 2021;168:106453.
[24]. Nigović B, Marušić M, Jurić S. J Electroanal Chem. 2011;663(2):72-8.
[25]. Amiri M, Amali E, Nematollahzadeh A. Sens Actuators, B. 2015;216:551-7.
[26]. Raj M, Gupta P, Goyal RN. J Electrochem Soc. 2016;163(6):H388-H94.
[27]. Antuña Jiménez D, Díaz Díaz G, Blanco López MdC, Lobo Castañón MJ, Miranda. Ordieres AJ, Tuñón Blanco P. Molecularly Imprinted Sensors. 2012.
[28]. Nezhadali A, Mojarrab M. Anal Chim Acta. 2016;924:86-98.
[29]. Cizmarikova R. Ceska a Slovenska Farmacie: Casopis Ceske Farmaceuticke Spolecnosti a Slovenske Farmaceuticke Spolecnosti. 2002;51(3):121-8.
[30]. Iacob B-C, Bodoki E, Florea A, Bodoki AE, Oprean R. Anal Chem. 2015;87(5):2755-63.