1. Introduction
The process of self-assembly occurs when the components of a system (such as molecules) congregate and arrange into a regular form without the involvement of external forces. Self-assembled structures have lower entropy than isolated monomers because they are arranged toward the lowest energy of configuration at equilibrium. The self-assembly process typically transforms the system from a disordered to an ordered state, which can operate on different scales [1]. For example, the molecules first gather into nano-sized supramolecular clusters (such as the self-assembly of surfactant molecules into microcells). The forces between these supramolecular units further urge them to make regular arrangement in space (for example, the arrangement of microcells into a body centered cubic lattice), so that the system has a hierarchical structure as shown in Figure 1. This is widely known as the ‘bottom-up’ approach in the manufacture of nanoparticles.
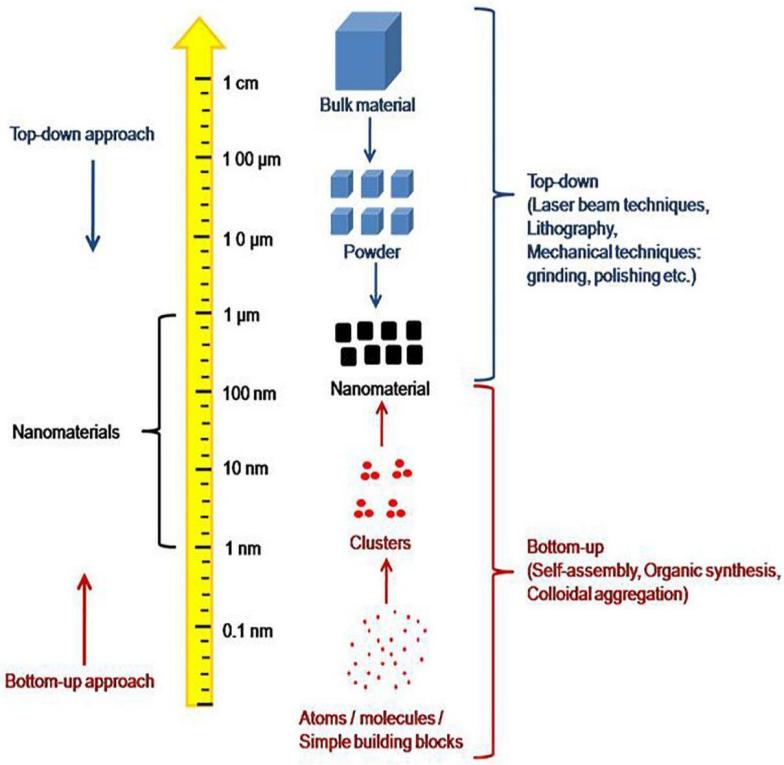
Figure 1: A display of the ‘bottom-up’ approach of self-assembly. The ‘top-down’ approach is the opposite of the ‘bottom-up’ which fabricate nanomaterial by breaking down bigger chunks of materials [2].
Self-assembly is common in nature: the cells of organisms are self-assembled by various biological molecules, and the crystallization of molecules is also a kind of self-assembly phenomenon. Due to its affordability, degradability, and adaptability, this approach is increasingly employed to create nanomaterials with unique optical, electrical, magnetic, sensing, and catalytic properties. In reality, researchers have been interested in the self-assembly technique used to assemble microspheres using monodisperse polymer microspheres since the early 1950s. For the first time, researchers at the University of Michigan were able to create mono-disperse polymer microspheres in polystyrene latex [3]. The ordered organization or close packing process of the microspheres under the influence of a driving force is referred to as self-assembly of microspheres. Since then, the technique of self-assembly has been applied to many new uses. For example, recent advances in drug delivery, for example, have opened up further pathways for developing innovative drug delivery systems (DDSs), and self-assembled colloids have demonstrated their immense potential to be employed as simple and efficient materials for this purpose [2]. This review ought to present an overview of the basic concepts of supramolecular self-assembly process and how these highly materials can be modified to be used in the contemporary generations of colloid structures such as hydrogels, micelles, and polymersomes whose developments are already shaped by the process of self-assembly.
2. Self-assembly in the manufacturing colloids
A colloid is a non-crystalline homogeneous material consisting up of macromolecules or nanoparticles of one component dispersed in another. [4]. Colloids include gels, sols, and emulsions; its particles do not settle and cannot be sorted out like those in a suspension by standard filtering or centrifugation. Although some define that colloids must have particles suspended exclusively in liquid, in this article, colloids include substances with particles suspended in air such as aerosols or other gels [5]. As previously mentioned in the introduction, three main categories of self-assembled colloids will be discussed.
2.1. Hydrogels
Hydrogels are a collection of hydrophilic cross-linked polymers which are insoluble in water. They have the ability to hold significant amounts of liquid in their structure while also having rigid shapes [6]. The two main types of hydrogels are those with covalent cross-linking bonds which are generally irreversible and those with non-covalent physical cross-linking bonds which allows them to be easily reversed and therefore used more commonly for numerous applications. By their versatility of form modification, the prior is often known as ‘permanent’ hydrogel and the latter ‘reversible’ hydrogels [7]. The structure of a common hydrogel can be demonstrated by Figure 2 below with chemical or physical crosslink between mesh of different polymers. Common examples of hydrogels in life include gelatin and collagen.
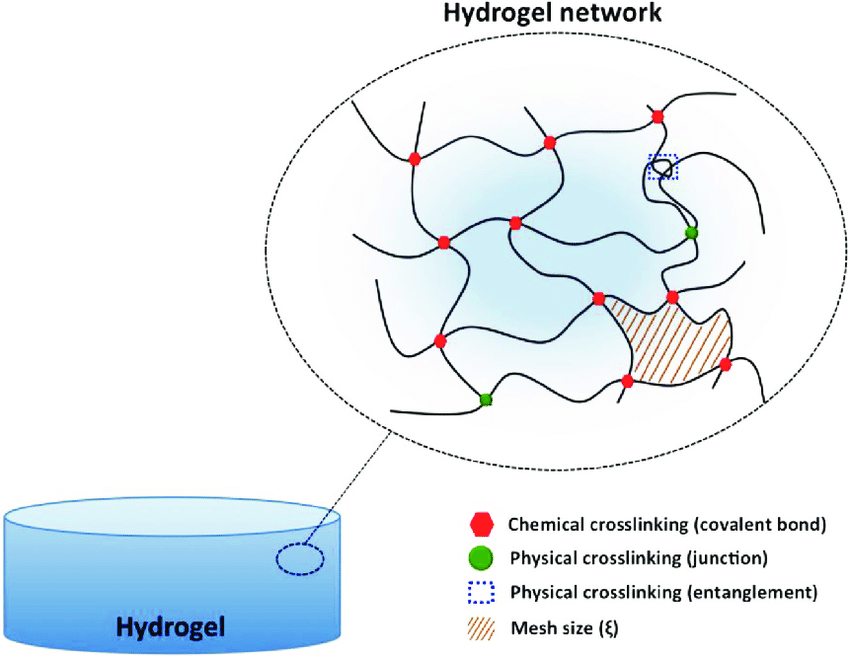
Figure 2 The diagram of possible crosslinking in a hydrogel, this article only covers hydrogels with one form of linkage [8].
The method of self-assembly is used in a wide variety of hydrogels both physical and chemical, and one type of self-assembled aerogel from each category (physical/chemical) will be discussed.
As previously mentioned in the introduction, one of the most well-known novel applications of self-assembly is the drug-delivery system. As biomaterials developed to fulfil more intricate roles in the last few decades, many polymers are tailor-made to treat certain diseases [9]. One of such polymer groups is poly(amino esters) (PBAEs). PBAEs have received a lot of attention due to their representation of a polymer collection for numerous biological uses, including delivery of proteins, antimicrobials, anticancer medicines, and tissue healing [10]. There are three general pathways to manufacture gels from PBAEs: creating chemical bonds at the cross-link sites of PBAEs, via the sol-gel transition of block polymers like PAEUPEG-PAEU or PBAE-PCLPEG-PCL-PBAE [11], and by using monomers of PBAEs at different reaction sites [12]. Bulk hydrogels made from PBAEs have very flexible shapes and can be readily injected. They can also be adequate carriers for both hydrophilic and hydrophobic drugs. One of the diseases whose treatment is significantly simplified by this technology is diabetes. Type 1 diabetics must continuously inject themselves with insulin in order to maintain blood sugar control. Researchers created hydrogels which can be injected beneath the skin and release insulin in the body. Insulin will gradually flow from the interior of the hydrogel into the bloodstream as it spontaneously diffuses from an environment with greater insulin concentration to one with lower insulin concentration, thus completing the delivery process [13]. Hyunh’s [14] successful synthesis of hydrogel based on PβAE achieved fantastic results: a single injection of hydrogel was able to maintain a healthy glucose concentration in the body of a diabetic patient for about a week, which achieves the same purpose as about 20 shots of insulin in the same span of time [15].
However, the self-delivery process of PβAE-based hydrogels does have potential drawbacks. As the hydrogels are extremely stimuli-responsive, minor changes in the environment might lead to dramatic changes in the hydrogels’ structures [16]. Also, the residual of certain organic solvents used during the synthesis process can also be of great harm to tissues [17].
The PBAE hydrogels and its fellow physical hydrogels have great advantages as vessels for drug delivery due to their flexibility and immediate responses to exterior stimuli. However, their lack of mechanical strength restricts their use in other contexts, such soft robotics (chemomechanical systems) [18]. This is where liquid-crystalline particles come into play: they can be integrated into chemical and physical hydrogels as a monomer or dopant during the polymerization process and have outstanding optical, electric, and magnetic characteristics. These gels are commonly utilized to create chemomechanical actuators such as mechano-optical detectors because they have reasonably superior mechanical strength. To make chemically or physically crosslinked hydrogels, liquid-crystalline monomers can be polymerized individually or in copolymerization with additional monomers. Mesogens can then be piled on the main polymer chains or attached to the primary chains as pendants. In dimethylformamide solution, copolymerization of AA with an LC monomer 11-(4'-cyanobiphenyloxy)undecyl acrylate (11CBA) resulted in LC hydrogels with high water retention. The phases of poly(11CBA-co-AA) after stretching in water vary on the monomer compositions and water concentration, displaying multiphase behavior. Later investigations demonstrated that variations in the molar percentage of 11CBA, water concentration, and temperature all had a significant impact on the functionality of LC monomer assemblies. When the molar fraction of 11CBA is greater than 0.26 and water content is rather low, because of the hydrophobic contact between the mesogenic side chains, the copolymers expanded to form hydrogels, leading to the establishment of a physical pelinking junction with the side chains formed bilayers oriented perpendicular to the main-chain. When water content exceeds 70%, the hydrogel will transform into an amorphous state [19]. Structures formed by these same monomers can shrink and expand freely if the three aforementioned parameters are adjusted to suitable levels, making them great candidates as soft actuators [18].
2.2. Micelles
A micelle is an amphiphilic molecule cluster that is dispersed in liquid as a colloidal suspension. They are produced when amphiphilic molecules self-assemble in an aqueous solution due to the molecule having polarity. The hydrophilic polar component of these structures consists the micelle's outer surface, while the hydrophobic, nonpolar section forms the core as shown in Figure 3. [20]. This makes micelles have the special property of containing lipophilic molecules in their hydrophobic core, which is the principle of cleansing with soap.
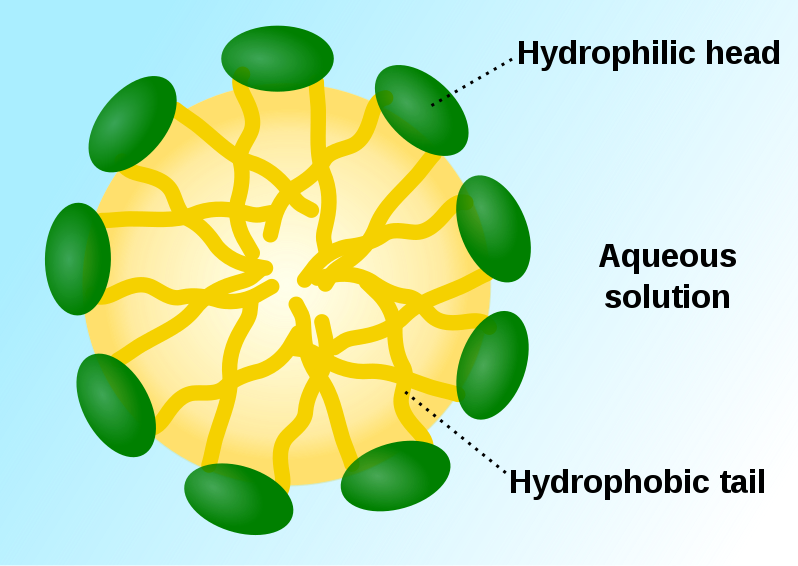
Figure 3. The ‘head’ and ‘tail’ of the phospholipid which forms the micelles [21]
One of the most intriguing new technologies involving self-assembled micelles are the micellar self-assembled fluorescent sensors. Fluorescence-based chemical species sensing has received a lot of attention due to the fluorescent signal's strong intensity, convenience of reading, abundance of suitable fluorophores, and the significant role that chemical species plays in a fluorophore's emission properties. Early applications of this technology such as the fluorophores were not very flexible and could only sense several types of ions unselectively. Later, FSR (fluorophore–spacer–receptor) was developed with the fluorophore sensor covalently linked to the receptor component by a spacer. FSR molecular devices behave in a simple ON-OFF mechanism for the ion that it tries to detect. Based on works involving FSR, novel micellar self-assembled fluorescent sensors were devised to detect cation, anions and pH windows.
Micellar self-assembled sensors for metal cations integrate the ideas of FSR fluorescent sensor with intramicellar fluorescence quenching, potentially taking benefits of the enhancement of intramicellar contacts due to molecular component integrations in micelles. Each type of target metal cation to be detected in an aqueous solution requires a specific ligand for its detection as shown in Figure 4. To be preferentially integrated into micelles in a micellar solution, the ligand must be lipophilic or lipophilized (for example, with a long n-alkyl chain) and not able to prevent fluorescence in the pH range which it could detect the metal cation [22]. The micellar solution must comprise a lipophilic ligand and a hydrophobic fluorescent molecule. The ideal fluorophore linked to it in the FSR molecule could be the same if the receptor component of an FSR sensor is used as the ligand. When the amounts of both constituents and the surfactant are changed, a significant number of micelles that contain both the lipophilic fluorophore and the lipophilic ligand are discovered, and the fluorescence appears in the sensor. But it must be noted that in a certain pH range, ligands are very sensitive and the ON-OFF mechanism only reacts to the presence of one specific cation. However, outside of the desired range, fluorescence quenching occurs and hinders the possibility of cation detection. As early as 1999, scientists successfully detected Cu 2+ ions via this method.
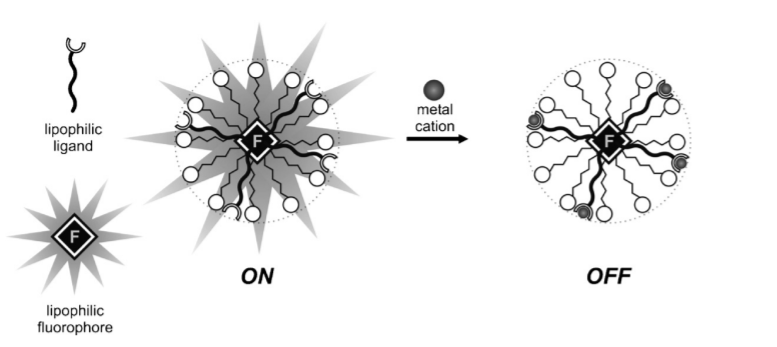
Figure 4. The ON-OFF mechanism of a metal ion FSR detector. [23]
Pallavicini and co. were able to successfully construct FSR ON-OFF sensors for Cu2+ cations using the non-ionic surfactant Titron X-100 as micellar containers, plain pyrene as fluorophores, and the dioxide-2,3,2 ligand to specifically detect Cu 2+ ions as in Figure 3. The dioxide-2,3,2 was lipophilized to create ion 1 by adding an n-dodecyl chain to the maleimidic carbon atom for use in the micellar detector. Later, these scientists were able to replace the dioxide-2,3,2 formed ligand to other lipophilized ligands while keeping the container and the fluorophores from the FSR sensors of copper to detect different cations, demonstrating the versatility and potential of this system [23]
An intriguing example of a luminous sensor of the chemosensing ensemble type that makes use of micellar inclusion and the resulting poor interaction of the molecular constituents with the solute was presented by Niikura and Anslyn in 2003 [24]. In methanol, the fluorescent 5-carboxy fluorescein molecule 1 and the molecular receptor 2 create a reliable sensor group that effectively signals the inositol triphosphate anion (with their chemical structure clearly shown in Figure 5). This is because favored charge pairing interactions make 2 and 5-carboxy fluorescein will interact and aid in the stability of the latter's yellow luminous open form. The addition of inositol triphosphate signals the preferred interaction between a and b, releasing c in a cyclized, colorless state, in the same solvent. Although the receptor does not work in a solution with plain water solvent as the fluorescent 5-carboxy fluorescein molecule was unstable in an open condition when 2 and inositol triphosphate anions met, when Triton X-100 micellar containers are added, low solvation and the local concentration rise improve the affinity of inositol triphosphate anion with 2 in Triton X-100 micellar containers. Additionally, the fluorescent 5-carboxy fluorescein molecules stay inside the micelles when inositol triphosphate anion replaces 5-carboxy fluorescein from 2 as the weakly polar environment there makes the enclosed system more stable. Thus, the presence of fluorescent 5-carboxy fluorescein molecules and 2 molecules in a Triton X-100 solution creates a successful indicator of inositol triphosphate anions.
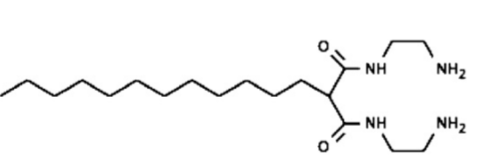 1
1
 2
2
Figure 5: The aforementioned atoms 1 and 2
Moreover, Pallavicini and colleagues created bright pH window sensors by fusing together different components in micelles. Based on the few systems published in the literature, they utilized the identical quenching groups as different lipophilic components and self-assemble them in Titron X-100 micelles. One tertiary amine, a lipophilized pyridine, and pyrene as the fluorophore were present in the combination. The OFF-ON-OFF reaction is produced by moving along the pH axis [23] When the pH crosses the pKa of the pyridinium group (lipophilized pyridine) or the ammonium group (tertiary amine), the hydrogen atom bonded to the pyridinium group will first fall off at its pKa , turning on the FSR, while the hydrogen atom will fall off the ammonium group after its pKa is reached. Effectively, the device is turned on within the pH range between these two pKa values.
2.3. Vesicles
A vesicle is a structure found within or outside of a cell that is made up of cytoplasm or liquid and is surrounded by a lipid bilayer. Natural activities such as exocytosis, endocytosis, and material flow inside the plasma membrane result in the formation of vesicles. They may also be created artificially created to perform more specific roles compared to natural ones.
2.3.1 Polymersomes
Polymersomes are artificially created vesicles whose membranes are created using amphiphilic synthetic block copolymers which are very similar to those in micelles [25]. However, vesicles contain an aqueous core bounded by a second layer of hydrophilic membrane whose hydrophilic heads are on opposite sides of each other, essentially the same structure as lipid based liposomes as in Figure 6. Amphiphilic block copolymers' capacity to self-assemble in picky solvents has been extensively researched in academics and used in a variety of industrial goods. This advancement is being pioneered by colloids constructed from self-assembled amphiphilic block polymers, which has countless applications, from biomedicine to nanosized enzymatic reactors.
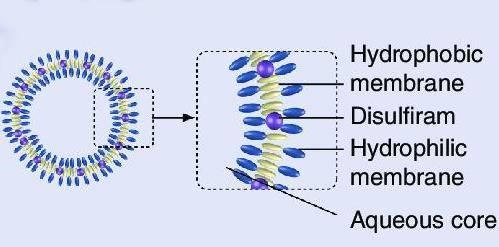
Figure 6 Structure of a polymersome [26]
In aqueous solution, block copolymer amphiphiles self-assemble to reduce energetically unfavorable hydrophobe-water interactions. Because amphiphilic surfactant head-groups are either hydrated or charged, they prefer to keep a particular separation from other surfactants, which is represented by the area, a. Tail interactions, which result from variations in energy and enthalpy between the solvent and the surroundings of hydrophobic tails, can be represented by the area v/lc, with v and lc being the length and volume of the tail. The ratio of these two areas is the packing parameter. This equation usually gives the geometry of the assembled structure. Generally, spherical micelles are formed when p ≤ 1/3, cylindrical micelles when 1/3 ≤ p ≤ 1/2, and polymersomes when 1/2 ≤ p ≤ 1 [27].
In comparison to liposomes, polymersomes have superior mechanical characteristics. Early experiments confirmed the robustness of polymeric vesicles, demonstrating a ten-fold increase in critical areal strain prior to fracture compared to liposomes [25]. Their membranes surprisingly keep their elasticity even at their high molecular mass. It is also important to note that the flexibility membrane permeability of polymersomes is also improved when relative to their lipid counterparts, and this parameter is dependent on the membrane thickness and the relative polarity of the hydrophobic membrane. Polymersomes with extremely permeable and practically impermeable membranes can be made to serve certain functional needs [28]
One of the most surprising abilities of polymersomes is its ability to act as a nanoreactor in the biomanufacture of ATP as reported by Choi and Montemagno in 2005 [29]. Both bacteriorhodpsin and F0F1-ATP synthase enter the polymersome via light-driven transmembrane proton pumps were reconstituted within the copolymer vesicle membrane and shown to function in combination to effectively convert ADP into ATP when exposed to LED (5.04 W green LED, 14 570 nm) that activates the pump as in Figure 7.
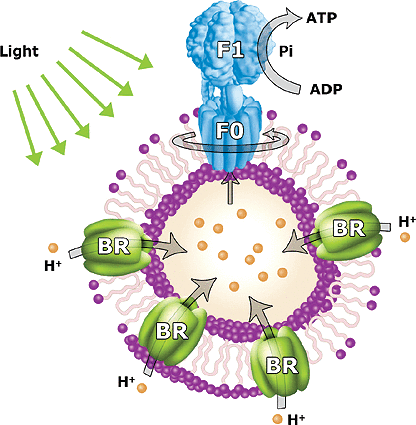
Figure 7. The conversion of ADP into ATP in a polymersome [29]
The inclusion of hydrophobic moieties in polymer vesicle membranes can create polymersomes of different medical purposes. such as the membrane loading of strongly linked porphyrin-based near-infrared (NIR) supramolecular fluorophores. The surface conjugation of such 'NIR-polymersomes' allowed for in vivo cell tracking and tissue imaging of dendritic cells to centimeter depths. Polymersome s with hydrophobic magnetic nanoparticle-loaded membranes might be employed as contrast agents in magnetic resonance imaging (MRI) and for radio frequency-induced local heating of malignant cells [30]. When placed within PEO-PBD vesicle membranes, paclitaxel (TAX), a hydrophobic anticancer medicine, was reported to exhibit long-term in vivo release with reduced multidrug resistance and improved effectiveness in MCF-7 breast cancer cell death.li [31]. However, these technologies are still a long way from mass production for the benefit of the general public.3. Conclusions
The development of physically/chemically linked hydrogels has diverged into two general paths due to their distinct physical properties. On one hand, physical supramolecular hydrogels formed by molecular self-assembly are sensitive to external stimuli such as temperature or pH, but are mechanically weak overall. These gels are gaining popularity as vehicles for drug delivery, and molecular control and discharge. Physically linked liquid crystalline hydrogel are much tougher mechanically and have better uses as soft actuators or possibly biotissues. Based on these properties, it is possible to suggest that these hydrogels can be used as materials for miniature microrobots operating inside the human body or other liquid environments for their versatility of adjusting sizes in different environments.
Self-assembled micelles, like hydrogels, are very flexible in their use as containers. Its components can be changed readily to suit different uses (such as switching the binding unit to the n-alkyl chain can change the FSR detector from Cu2+ to Hg+). Moreover, they are inexpensive and convenient to produce. Therefore, scientists are now also experimenting with micelles made of biologically compatible block-copolymers as a replacement for conventional surfactants in the self-assembly of multicomponent nanosized sensors for detecting other properties of atoms or molecules present in aqueous solutions. The next key steps that scientists need to take is to find nouvel binding units which can detect other types of ions or different properties of molecules via experimentation and comparison and find ways to keep the FSR operating in the presence of monodispersed surfactant monomers in the solution.
Polymersome, like the other colloids presented in this paper, have great flexibility of component choice and are very versatile to adjust to different functions. With high load capacity, customizable release patterns, and the capacity to attach ligands for guiding drug delivery, polymersomes demonstrated potential to not only fulfill the tasks of therapeutic delivery, but also act as nanoreactors at the reaction sites for the conversion of biological molecules. The most exciting potential of polymersomes comes from the treatment of cancer. Paclitaxel can be delivered into the tumor by polymersomes to have more significant effects on treating tumors from MCF-7 breast cancer cells. Other anticancer drugs featuring polymersome vessels such as AZD2811 (a PLA-PEG polymersome containing aurora B kinase inhibitor) [32] and polyethylenimine nanoparticles encapsulating siRNA against eIF5A (a translational initiation factor) [33] are already in stage I of clinical trials.
The next generation of nanoparticle revolution will be spearheaded by self-assembled structures due to their many advantages compared to their traditional counterparts. Many times, addition of these new structures do not completely change the entire conventional process of treating diseases but alleviate the effect of the drugs which are already currently in use. Other times, the mechanical properties of self-assembled colloids make them great candidates for construction of nouvel nanomachines. There are no doubts that more work will be done into research aiding the realization of the fascinating potential of self-assembled colloids.
References
[1]. Wang, L., , & Wang, J., (2019). Self-assembly of colloids based on microfluidics. Nanoscale, 11(36), 16708–16722. https://doi.org/10.1039/c9nr06817a
[2]. Yadav, S., Sharma, A. K., & Kumar, P. (2020). Nanoscale Self-Assembly for Therapeutic Delivery. Frontiers in bioengineering and biotechnology, 8, 127. https://doi.org/10.3389/fbioe.2020.00127
[3]. Bangs, L.B.; Kenny, M.T. Old, new, borrowed, blue. In d. Re s., 1976, 18(8), 46-49.
[4]. International Union of Pure and Applied Chemistry., Jones, R. G., & International Union of Pure and Applied Chemistry. (2009). Compendium of polymer terminology and nomenclature: IUPAC recommendations, 2008. Cambridge: Royal Society of Chemistry, 211-236
[5]. Stepto, R. (2009). Dispersity in polymer science (IUPAC Recommendations 2009). Pure and Applied Chemistry, 81(2), 351-353. https://doi.org/10.1351/PAC-REC-08-05-02
[6]. Ahmed, E. M. (2015). Hydrogel: Preparation, characterization, and applications: A review. Journal of advanced research, 6(2), 105–121. https://doi.org/10.1016/j.jare.2013.07.006
[7]. Gulrez, S. K. H. , Al-Assaf, S., & Phillips, G. (2011). Hydrogels: Methods of Preparation, Characterisation and Applications. In (Ed.), Progress in Molecular and Environmental Bioengineering - From Analysis and Modeling to Technology Applications. IntechOpen. https://doi.org/10.5772/24553
[8]. Fajardo, André & Pereira, Antonio & Rubira, Adley & Valente, Artur & Muniz, Edvani. (2015). Stimuli-Responsive Polysaccharide-Based Hydrogels. 10.1201/b19751-10.
[9]. Le, H. (2018). Hydrogels and their life-saving capabilities. Medium. Retrieved July 29, 2022, from https://medium.com/@hannah.lgbhan/alginate-hydrogels-and-their-life-saving-capabilities-c08016aea192
[10]. Kim, T. G., Shin, H., & Lim, D. W. (2012). Advanced Function Mater., 22, 2146
[11]. Cong Truc Huynh, Doo Sung Lee, Controlling the properties of poly(amino ester urethane)–poly(ethylene glycol)–poly(amino ester urethane) triblock copolymer pH/temperature-sensitive hydrogel, Colloid Polym. Sci. 290 (11) (2012) 1077–1086, https://doi.org/10.1007/s00396-012-2624-z
[12]. A.L. Lakes, C.T. Jordan, P.T. Gupta, D.A. Puleo, J.Z. Hilt, T.D. Dziubla, (2018) Reducible disulfide poly (beta-amino ester) hydrogels for antioxidant delivery, Acta Biomater. 68 178–189, https://doi.org/10.1016/j.actbio.2017.12.030.
[13]. Le, H. (2018, July 31). Hydrogels and their life-saving capabilities. Medium. Retrieved July 29, 2022, from https://medium.com/@hannah.lgbhan/alginate-hydrogels-and-their-life-saving-capabilities-c08016aea192
[14]. Dai Phu Huynh, Guang Jin Im, Su Young Chae, Kang Choon Lee, Doo Sung Lee, Controlled release of insulin from pH/temperature-sensitive injectable pentablock copolymer hydrogel, J. Control. Release 137 (1) (2009) 20–24, https://doi.org/10.1016/j.jconrel.2009.02.021.
[15]. Razavi, Z., & Ahmadi, M. (2011). Efficacy of Thrice-daily versus Twice-daily Insulin Regimens on Glycohemoglobin (Hb A1c) in Type 1 Diabetes Mellitus: A Randomized Controlled Trial. Oman medical journal, 26(1), 10–13. https://doi.org/10.5001/omj.2011.03
[16]. Iqbal, S., Qu, Y., Dong, Z., Zhao, J., Rauf Khan, A., Rehman, S., & Zhao, Z. (2020). Poly (β‐amino esters) based potential drug delivery and targeting polymer; an overview and perspectives (review). European Polymer Journal, 141, 110097. https://doi.org/10.1016/j.eurpolymj.2020.110097
[17]. Liu, Y., Li, Y., Keskin, D., & Shi, L. (2018). Poly(β‐amino esters): Synthesis, formulations, and their biomedical applications. Advanced Healthcare Materials, 1801359. https://doi.org/10.1002/adhm.201801359
[18]. Wu, Z., & Gong, J. (2011). Hydrogels with self-assembling ordered structures and their functions. NPG Asia Material, 3. 57-64. https://doi.org/10.1038/asiamat.2010.200
[19]. Kaneko, T., Yamaoka, K., Gong, J. P. & Osada, Y. (2000) Liquid-crystalline hydrogels. 1. Enhanced effects of incorporation of acrylic acid units on the liquid-crystalline ordering. Macromolecules 33, 412–418.
[20]. Mary, J., Trinh, H., & Mitra, A. (2017). Peptide and Protein-Based Therapeutic Agents. 145-147. https://doi.org/10.1016/B978-0-323-42978-8.00007-3.
[21]. File:Micelle scheme-en.svg. (2020, September 12). Wikimedia Commons, the free media repository. Retrieved 05:36, August 4, 2022 from https://commons.wikimedia.org/w/index.php?title=File:Micelle_scheme-en.svg&oldid=456559908.
[22]. Y. Diaz-Fernandez, A. Perez-Gramatges, V. Amendola, F. Foti, C. Mangano, P. Pallavicini, S. Patroni, Chem. Commun. (2004) 1650
[23]. Piersandro Pallavicini, Yuri Antonio Diaz-Fernandez, Luca Pasotti, Micelles as nanosized containers for the self-assembly of multicomponent fluorescent sensors, Coordination Chemistry Reviews, Volume 253, Issues 17–18, 2009, 2226-2240, https://doi.org/10.1016/j.ccr.2008.11.010.
[24]. K. Niikura, E.V. Anslyn, J. Org. Chem. 68 (2003) 10156.
[25]. Discher B M, Bermudez H, Hammer D A, Discher D E, Won Y-Y, Bates F S Journal of Physical Chemistry B (2002), 106(11), 2848-2854
[26]. Buckiova, Daniela & Ranjan, Sanjeev & Newman, Tracey & Johnston, Alexander & Sood, Rohit & Kinnunen, Paavo & Popelar, Jiri & Chumak, Tetyana & Syka, Josef. (2012). Minimally invasive drug delivery to the cochlea through application of nanoparticles to the round window membrane. Nanomedicine . 7. 1339-54. 10.2217/nnm.12.5.
[27]. Aslani Zakariya, Alireza. (2012). Organic Materials in Nanochemistry, 240-241
[28]. G. Battaglia, A. J. Ryan, J. Am. Chem. Soc. 2005, 127, 8757.
[29]. Choi H., & Montemagno C. D.(2005) Nano Letters 5 (12), 2538-2542 DOI: 10.1021/nl051896e
[30]. N. A. Christian, M. C. Milone, S. S. Ranka, G. Li, P. R. Frail, K. P. Davis, F. S. Bates, M. J. Therien, P. P. Ghoroghchian, C. H. June, D. A. Hammer, Bioconjugate Chem. 2007, 18, 31.
[31]. S. Li, B. Byrne, J. Welsh, A. F. Palmer, Biotechnol. Prog. 2007, 23, 278.
[32]. Merle, P., Camus, P., Abergel, A., Pageaux, G. P., Masliah, C., Bronowicki, J. P., ... & Attali, P. (2017). Safety and efficacy of intra-arterial hepatic chemotherapy with doxorubicin-loaded nanoparticles in hepatocellular carcinoma. ESMO open, 2(4), e000238.
[33]. U.N.L.o. Medicine, ClinicalTrials.gov, 2014.
Cite this article
Lu,Q. (2023). Review of self-assembly of nanoparticles and its applications in the manufacturing of different types of colloids. Applied and Computational Engineering,7,56-65.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Materials Chemistry and Environmental Engineering (CONF-MCEE 2023), Part II
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Wang, L., , & Wang, J., (2019). Self-assembly of colloids based on microfluidics. Nanoscale, 11(36), 16708–16722. https://doi.org/10.1039/c9nr06817a
[2]. Yadav, S., Sharma, A. K., & Kumar, P. (2020). Nanoscale Self-Assembly for Therapeutic Delivery. Frontiers in bioengineering and biotechnology, 8, 127. https://doi.org/10.3389/fbioe.2020.00127
[3]. Bangs, L.B.; Kenny, M.T. Old, new, borrowed, blue. In d. Re s., 1976, 18(8), 46-49.
[4]. International Union of Pure and Applied Chemistry., Jones, R. G., & International Union of Pure and Applied Chemistry. (2009). Compendium of polymer terminology and nomenclature: IUPAC recommendations, 2008. Cambridge: Royal Society of Chemistry, 211-236
[5]. Stepto, R. (2009). Dispersity in polymer science (IUPAC Recommendations 2009). Pure and Applied Chemistry, 81(2), 351-353. https://doi.org/10.1351/PAC-REC-08-05-02
[6]. Ahmed, E. M. (2015). Hydrogel: Preparation, characterization, and applications: A review. Journal of advanced research, 6(2), 105–121. https://doi.org/10.1016/j.jare.2013.07.006
[7]. Gulrez, S. K. H. , Al-Assaf, S., & Phillips, G. (2011). Hydrogels: Methods of Preparation, Characterisation and Applications. In (Ed.), Progress in Molecular and Environmental Bioengineering - From Analysis and Modeling to Technology Applications. IntechOpen. https://doi.org/10.5772/24553
[8]. Fajardo, André & Pereira, Antonio & Rubira, Adley & Valente, Artur & Muniz, Edvani. (2015). Stimuli-Responsive Polysaccharide-Based Hydrogels. 10.1201/b19751-10.
[9]. Le, H. (2018). Hydrogels and their life-saving capabilities. Medium. Retrieved July 29, 2022, from https://medium.com/@hannah.lgbhan/alginate-hydrogels-and-their-life-saving-capabilities-c08016aea192
[10]. Kim, T. G., Shin, H., & Lim, D. W. (2012). Advanced Function Mater., 22, 2146
[11]. Cong Truc Huynh, Doo Sung Lee, Controlling the properties of poly(amino ester urethane)–poly(ethylene glycol)–poly(amino ester urethane) triblock copolymer pH/temperature-sensitive hydrogel, Colloid Polym. Sci. 290 (11) (2012) 1077–1086, https://doi.org/10.1007/s00396-012-2624-z
[12]. A.L. Lakes, C.T. Jordan, P.T. Gupta, D.A. Puleo, J.Z. Hilt, T.D. Dziubla, (2018) Reducible disulfide poly (beta-amino ester) hydrogels for antioxidant delivery, Acta Biomater. 68 178–189, https://doi.org/10.1016/j.actbio.2017.12.030.
[13]. Le, H. (2018, July 31). Hydrogels and their life-saving capabilities. Medium. Retrieved July 29, 2022, from https://medium.com/@hannah.lgbhan/alginate-hydrogels-and-their-life-saving-capabilities-c08016aea192
[14]. Dai Phu Huynh, Guang Jin Im, Su Young Chae, Kang Choon Lee, Doo Sung Lee, Controlled release of insulin from pH/temperature-sensitive injectable pentablock copolymer hydrogel, J. Control. Release 137 (1) (2009) 20–24, https://doi.org/10.1016/j.jconrel.2009.02.021.
[15]. Razavi, Z., & Ahmadi, M. (2011). Efficacy of Thrice-daily versus Twice-daily Insulin Regimens on Glycohemoglobin (Hb A1c) in Type 1 Diabetes Mellitus: A Randomized Controlled Trial. Oman medical journal, 26(1), 10–13. https://doi.org/10.5001/omj.2011.03
[16]. Iqbal, S., Qu, Y., Dong, Z., Zhao, J., Rauf Khan, A., Rehman, S., & Zhao, Z. (2020). Poly (β‐amino esters) based potential drug delivery and targeting polymer; an overview and perspectives (review). European Polymer Journal, 141, 110097. https://doi.org/10.1016/j.eurpolymj.2020.110097
[17]. Liu, Y., Li, Y., Keskin, D., & Shi, L. (2018). Poly(β‐amino esters): Synthesis, formulations, and their biomedical applications. Advanced Healthcare Materials, 1801359. https://doi.org/10.1002/adhm.201801359
[18]. Wu, Z., & Gong, J. (2011). Hydrogels with self-assembling ordered structures and their functions. NPG Asia Material, 3. 57-64. https://doi.org/10.1038/asiamat.2010.200
[19]. Kaneko, T., Yamaoka, K., Gong, J. P. & Osada, Y. (2000) Liquid-crystalline hydrogels. 1. Enhanced effects of incorporation of acrylic acid units on the liquid-crystalline ordering. Macromolecules 33, 412–418.
[20]. Mary, J., Trinh, H., & Mitra, A. (2017). Peptide and Protein-Based Therapeutic Agents. 145-147. https://doi.org/10.1016/B978-0-323-42978-8.00007-3.
[21]. File:Micelle scheme-en.svg. (2020, September 12). Wikimedia Commons, the free media repository. Retrieved 05:36, August 4, 2022 from https://commons.wikimedia.org/w/index.php?title=File:Micelle_scheme-en.svg&oldid=456559908.
[22]. Y. Diaz-Fernandez, A. Perez-Gramatges, V. Amendola, F. Foti, C. Mangano, P. Pallavicini, S. Patroni, Chem. Commun. (2004) 1650
[23]. Piersandro Pallavicini, Yuri Antonio Diaz-Fernandez, Luca Pasotti, Micelles as nanosized containers for the self-assembly of multicomponent fluorescent sensors, Coordination Chemistry Reviews, Volume 253, Issues 17–18, 2009, 2226-2240, https://doi.org/10.1016/j.ccr.2008.11.010.
[24]. K. Niikura, E.V. Anslyn, J. Org. Chem. 68 (2003) 10156.
[25]. Discher B M, Bermudez H, Hammer D A, Discher D E, Won Y-Y, Bates F S Journal of Physical Chemistry B (2002), 106(11), 2848-2854
[26]. Buckiova, Daniela & Ranjan, Sanjeev & Newman, Tracey & Johnston, Alexander & Sood, Rohit & Kinnunen, Paavo & Popelar, Jiri & Chumak, Tetyana & Syka, Josef. (2012). Minimally invasive drug delivery to the cochlea through application of nanoparticles to the round window membrane. Nanomedicine . 7. 1339-54. 10.2217/nnm.12.5.
[27]. Aslani Zakariya, Alireza. (2012). Organic Materials in Nanochemistry, 240-241
[28]. G. Battaglia, A. J. Ryan, J. Am. Chem. Soc. 2005, 127, 8757.
[29]. Choi H., & Montemagno C. D.(2005) Nano Letters 5 (12), 2538-2542 DOI: 10.1021/nl051896e
[30]. N. A. Christian, M. C. Milone, S. S. Ranka, G. Li, P. R. Frail, K. P. Davis, F. S. Bates, M. J. Therien, P. P. Ghoroghchian, C. H. June, D. A. Hammer, Bioconjugate Chem. 2007, 18, 31.
[31]. S. Li, B. Byrne, J. Welsh, A. F. Palmer, Biotechnol. Prog. 2007, 23, 278.
[32]. Merle, P., Camus, P., Abergel, A., Pageaux, G. P., Masliah, C., Bronowicki, J. P., ... & Attali, P. (2017). Safety and efficacy of intra-arterial hepatic chemotherapy with doxorubicin-loaded nanoparticles in hepatocellular carcinoma. ESMO open, 2(4), e000238.
[33]. U.N.L.o. Medicine, ClinicalTrials.gov, 2014.









