1. Introduction
Molecular dynamics (MD) is a deterministic simulation method that models the gradual equilibrium of atoms in a system based on a microscopic description of the existence of a determined physical system [1–5]. MD is ideally suited to the study of microscopic carbon nanomaterial systems [6–11].
In cyclic voltammetry, the current through carbon nanotube electrodes is measured and clearly analyzed as a function of potential. In general, the flat panel capacitor model can be used for ideal equivalent treatment of electrodes with electric double-layer capacitors, and the capacitance calculation formula is:
c=\frac{s ξ}{d}
c means capacitance ( F ); ξ means permittivity ( F/m ); S is Equivalent effective area of double electric layer ( { m^{2}} ); d is Equivalent double electric layer thickness ( m ).
However, it is difficult to actually test the parameters, so we can replace them with the equivalent:
C =\frac{c}{m}=\frac{I Δt}{m ΔU}
where “ m ” is the mass of the electrode material. The unit of “ C ” is in F/g .
It is precisely because of the existence of defects and dislocation structures that the application prospects of carbon nanotubes in various aspects such as optoelectronics are seriously affected, so thorough investigation into them is likely to encourage the widespread application of carbon nanotubes in a variety of fields in the future society.
This paper provides a summary of the interaction of carbon-carbon double bonds, an analysis of the electrochemical properties of single-wall carbon nanotubes with various chiral graphene defects, and an investigation of the electrochemical properties of double-walled carbon nanotubes with blade dislocation, spiral dislocation, and their composite structures, all based on molecular dynamics simulation.
2. Study of Capacitive properties of nanotube materials
Carbon nanotubes of diameter 50 nm were successfully prepared by vapor deposition method by J.H. Chen et al. [12], and cyclic voltammetry tests revealed that carbon nanotube electrodes exhibited typical electric double layer capacitor characteristics.
Well-aligned CNT Arrays Catalyzed with Porous Anodic Aluminum Oxide Template were prepared by He Chunjian et al., [13] following in the footsteps of J.H.Chen. Carbon nanotubes are assembled into a supercapacitor electrode and the whole thing is put together. Outstanding electrochemical properties are demonstrated by the results, indicating that the electrode is highly effective.
Afterwards, the carbon nanotube and KOH mixture was subjected to a high-temperature activation treatment for 90 minutes, as described by K. Jurewicz et al. [14]. (multi-walled carbon nanotubes and K0H mixed in the ratio of 4:1 mass ratio). High-temperature activation experiments reveal a dramatic increase in carbon nanotube functional groups, suggesting that this approach is clearly conducive to boosting the specific capacity of carbon nanotube supercapacitors.
Just a small number of studies and analyses have looked at how carbon nanotube defects affect their electrochemical properties; most of the existing research and analysis is based on different electrode materials or a mixture of carbon nanotubes and other materials.
3. Defects of carbon nanotube materials
During production and preparation, the preparation of carbon nanotubes is imperfect, and it is inevitable that certain defects will be generated in the tube wall of carbon nanotubes, which will have a certain degree of impact on the physical and chemical properties of carbon nanotubes (such as stability, diffusion coefficient, and material transport performance, etc.).
Commonly observed defects include substitution defects caused by vacancy defects and impurity intrusion, and vacancy defects include single atom vacancy defects and diatomic vacancy defects. Furthermore, thermal vibration caused by high temperature or tensile multi-directional stress can affect carbon nanotubes and cause them to form a graphene (Stone-Wales) defect [15], so that carbon nanotubes at high temperatures and under certain conditions, easily open at the p-n junction. [16]
4. Electrochemical properties of defective carbon nanotubes
4.1. Capacitive properties of different types of defective carbon nanotubes
Li Zhongqiu et al. examined the capacitance properties of handrail faulty carbon nanotubes and jagged defective carbon nanotubes using them as examples [17]. First, the dislocation structure of the double-walled carbon nanotubes structured like a handrail is produced. The carbon nanotubes are staggered from left to right and spaced 1.23 Å apart from top to bottom, so that the inner tube of the upper tube and the outer tube of the lower tube, as well as the outer tube of the upper tube and the inner tube of the lower tube, are aligned precisely. After a 100 ps delay at room temperature, edge dislocations and screw dislocations of armrest-type double-walled carbon nanotubes and their composite structures were formed. Similarly created in this manner is the composite dislocation structure of sawtooth double-walled carbon nanotubes. Since the theoretical radius of the handrail type carbon nanotube (n, n) is {R_{A}}=\frac{3{na_{0}}}{2π}=0.678n Subject to the above qualifications, we can conclude that the diameter relationship of coaxial handrail type double-walled carbon nanotubes should be satisfied |{R_{A1}}- {R_{A2}}|=0.678|{n_{1}}-{n_{2}}|≥3.4Å . In other words, Δn =|{n_{1}}-{n_{2}}|=5.1 . That is to say, because n is an integer, the model parameter difference between the two walls of the handrail type double-walled carbon tube should be 5 or 6, so they simulated the handrail type dislocation double-walled carbon nanotubes of different diameters.
4.2. Electroconversion properties of different types of defective carbon nanotubes
Using the aforementioned armrest dislocation carbon nanotube method, two sawtooth double walled carbon nanotubes of the same model are placed staggered with a distance of 1.42 Å from top to bottom, with the inner tube of the upper tube aligned with the outer tube of the lower tube, and the outer tube of the upper tube aligned with the inner tube of the lower tube. Delaying 100000 steps (100 ps ) at room temperature yielded the edge dislocation and screw dislocation of sawtooth dislocation double walled carbon nanotube, as well as the composite structure of these two dislocations. Since the diameter coefficient of the inner tube and the outer tube, Δn=|{n_{1}}-{n_{2}}|=8.7 , must be an integer, the difference between their pipe diameter coefficients must be 8 or 9, and the pipe diameter coefficient difference of 10 is also made as an integer. Bi-walled carbon nanotubes with a serrated dislocation and a range of coefficient differences were thus obtained.
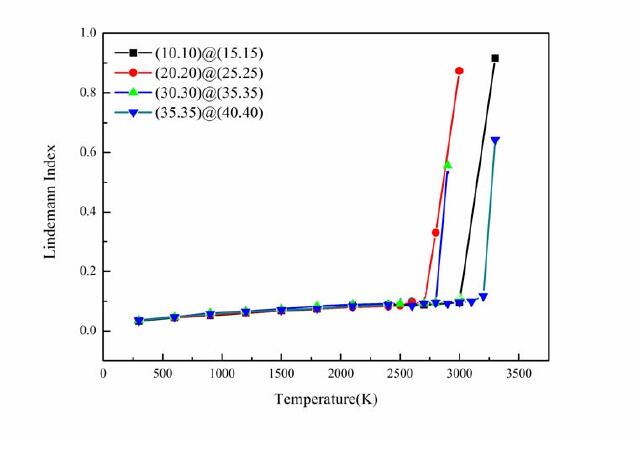
Figure 1. Thermal stability curve of a handrail type dislocation double-walled carbon nanotube with a tubular type parameter difference of 5 [17]
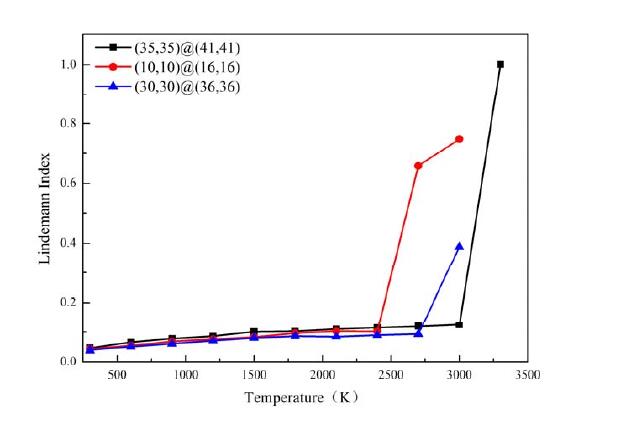
Figure 2. Thermal stability curve of a handrail type dislocation double-walled carbon nanotube with a tubular type parameter difference of 6 [17]
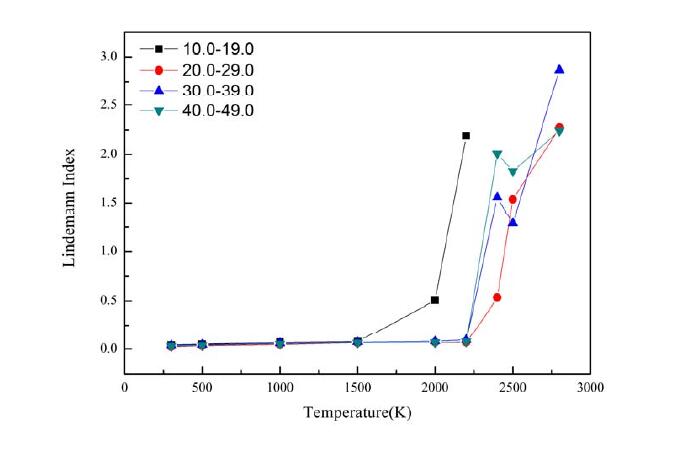
Figure 3. Thermal stability curve of double-walled carbon nanotubes with serrated dislocation deviation 9 [17]
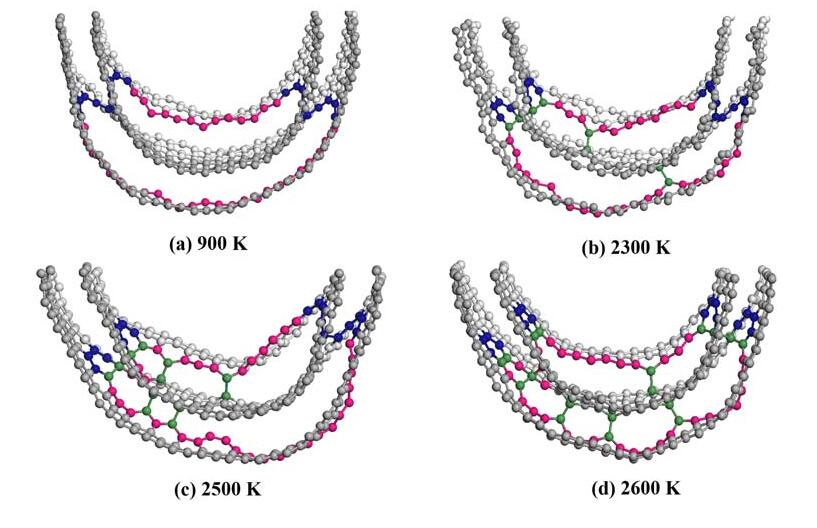
Figure 4. Experimental trend of cross-movement of serrated dislocation carbon nanotube blade dislocation and spiral dislocation [17]
As shown in Figure 1-4, analysis revealed that handrail type and serrated double-walled carbon nanotubes can generate some relatively stable and symmetrical composite dislocation structures at the joint; after the occurrence of stratigraphic dislocation, the nanotubes will form four screw dislocation structures; and through these four screw dislocations, the outermost layer of the blade dislocation and the innermost layer of the blade dislocation can be connected. The inner tube of a handrail-type dislocation double-walled carbon tube tends to expand outward, which can effectively increase its capacitance, the electrical conversion efficiency is reduced, and the conductivity deficiency is significantly reduced; the inner pipe of a jagged inner dislocation double-walled carbon tube tends to converge inward, which results in a gradual decrease in capacitance, an increase in electrical conversion efficiency, and a steady conductivity. This demonstrates that the electrochemical properties of the electrode material may be altered by introducing defects into the crystalline material at the right places. The efficient electrocatalytic performance is a result of the metal particles being effectively fixed on the carrier, which is made possible by the enhanced interaction between the metal and the oxide carrier made possible by the unique electronic structure of the defect structure itself and the coordination environment around it.
5. Conclusion
Carbon nanotubes are a great electrode material for supercapacitors because of their low equivalent series resistance and high power characteristics, thanks to their unique hollow structure and high electrical conductivity. Varying flaws have different impacts on the conductivity, electrical conversion efficiency, capacitance performance, and other features of carbon nanotubes, and these influences have led to various variations in the electrochemical properties of carbon nanotubes and their use as electrode materials. The effect of composite materials on the electrochemical characteristics of carbon nanotubes with different flaws is not investigated in this paper, but it will be in the future.
References
[1]. Zhang Xiaofeng, Li Daguang. Treatment and electrochemical properties of carbon nanotubes[D]. Guangdong University of Technology. 2012. I
[2]. Zhang Yiqiong, Wang Shuangyin. Synthesis of defect-assisted nanomaterials and their electrochemical properties[D]. Hunan University. 2019. II
[3]. Chen Shunlin. Computational Materials Science[M]. Beijing: Electrochemical Industry Press, 2005.
[4]. Zhang Yue, Gu Jinghua, Shang Jiaxiang et al. Fundamentals of Computational Materials Science[M]. Beijing: Beihang University Press, 2007
[5]. Ma Wenjin. Computational Physics [M]. Hefei: University of Science and Technology of China, 2001.
[6]. Kowaki Y, Harada A, Shimojo F. Radius dependence of the melting temperature of single-walled carbon nanotubes: molecular-dynamics simulations [J]. J Phys: Condens Matter, 2007, 19: 436224.
[7]. Tang C, Guo W, Chen C. Molecular dynamics simulation of tensile elongation of carbon nanotubes: Temperature and size effects [J]. Phys Rev B, 2009, 79(15): 155436-155444.
[8]. Wen Y-H, Fang H, Zhu Z-Z et al. A molecular dynamics study of shape transformation and melting of tetrahexahedral platinum nanoparticle [J]. Chem Phys Lett, 2009, 471(4-6): 295-299.
[9]. Bao Wenxing, Zhu Changchun. Molecular dynamics simulation of heat transfer in carbon nanotubes [J]. Acta Physica Sinica, 2006, 55(007): 3552-3557.
[10]. Xie Fang, Zhu Yabo, Zhang Zhaohui et al. Molecular dynamics simulation of carbon nanotube oscillation [J]. Acta Physica Sinica, 2008, 57(009): 5833-5837.
[11]. Zhang Kaiwang, Meng Lijun, Li Jun, et al. Structure and thermal stability of gold nanowires in carbon nanotubes [J]. Acta Physica Sinica, 2008, 57(007): 4347-4355.
[12]. J.H.Chen, Li WZ,Wang DZ, et al. Electrochemical characterization of carbon nanotubes as electrode in electrochemical double layer capacitors[J]. Carbon, 2002,(40): 1193-1197.
[13]. He Chunjian, Xue Kuanhong, Chen Qiaoling, et al. Cyclic voltammetry behavior of carbon nanotube array electrode [J]. Chemical Research, 2003, 15(5): 628-629.
[14]. Jurewicz K,Bable K,P ietrzak R,et al. Capacitance properties of multi walledcarbon nanotubes modified by activation and ammoxidation[J]. Carbon, 2006,(44): 2368 -2375. .
[15]. Ebbesen T, Takada T. Topological and sp3 defect structures in nanotubes [J]. Carbon, 1995, 33(7): 973-978.
[16]. Menon M. Carbon nanotube "T Junctions" Nanoscale metal-semiconductor-metal contact devices [J]. Phys Rev Lett, 1997, 79(22): 4453-4456.
[17]. Li Zhongqiu, Zhang Kaiwang. Thermal stability of SW-deficient carbon nanotubes and interlayer coupling structure of double-walled carbon nanotubes [D]. Xiangtan University.2011 21. https://www.cnki.com.cn/Navi/B.htm
Cite this article
Chen,Z. (2023). Study on electrochemical effects of carbon nanotube material defects. Applied and Computational Engineering,7,99-103.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Materials Chemistry and Environmental Engineering (CONF-MCEE 2023), Part II
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Zhang Xiaofeng, Li Daguang. Treatment and electrochemical properties of carbon nanotubes[D]. Guangdong University of Technology. 2012. I
[2]. Zhang Yiqiong, Wang Shuangyin. Synthesis of defect-assisted nanomaterials and their electrochemical properties[D]. Hunan University. 2019. II
[3]. Chen Shunlin. Computational Materials Science[M]. Beijing: Electrochemical Industry Press, 2005.
[4]. Zhang Yue, Gu Jinghua, Shang Jiaxiang et al. Fundamentals of Computational Materials Science[M]. Beijing: Beihang University Press, 2007
[5]. Ma Wenjin. Computational Physics [M]. Hefei: University of Science and Technology of China, 2001.
[6]. Kowaki Y, Harada A, Shimojo F. Radius dependence of the melting temperature of single-walled carbon nanotubes: molecular-dynamics simulations [J]. J Phys: Condens Matter, 2007, 19: 436224.
[7]. Tang C, Guo W, Chen C. Molecular dynamics simulation of tensile elongation of carbon nanotubes: Temperature and size effects [J]. Phys Rev B, 2009, 79(15): 155436-155444.
[8]. Wen Y-H, Fang H, Zhu Z-Z et al. A molecular dynamics study of shape transformation and melting of tetrahexahedral platinum nanoparticle [J]. Chem Phys Lett, 2009, 471(4-6): 295-299.
[9]. Bao Wenxing, Zhu Changchun. Molecular dynamics simulation of heat transfer in carbon nanotubes [J]. Acta Physica Sinica, 2006, 55(007): 3552-3557.
[10]. Xie Fang, Zhu Yabo, Zhang Zhaohui et al. Molecular dynamics simulation of carbon nanotube oscillation [J]. Acta Physica Sinica, 2008, 57(009): 5833-5837.
[11]. Zhang Kaiwang, Meng Lijun, Li Jun, et al. Structure and thermal stability of gold nanowires in carbon nanotubes [J]. Acta Physica Sinica, 2008, 57(007): 4347-4355.
[12]. J.H.Chen, Li WZ,Wang DZ, et al. Electrochemical characterization of carbon nanotubes as electrode in electrochemical double layer capacitors[J]. Carbon, 2002,(40): 1193-1197.
[13]. He Chunjian, Xue Kuanhong, Chen Qiaoling, et al. Cyclic voltammetry behavior of carbon nanotube array electrode [J]. Chemical Research, 2003, 15(5): 628-629.
[14]. Jurewicz K,Bable K,P ietrzak R,et al. Capacitance properties of multi walledcarbon nanotubes modified by activation and ammoxidation[J]. Carbon, 2006,(44): 2368 -2375. .
[15]. Ebbesen T, Takada T. Topological and sp3 defect structures in nanotubes [J]. Carbon, 1995, 33(7): 973-978.
[16]. Menon M. Carbon nanotube "T Junctions" Nanoscale metal-semiconductor-metal contact devices [J]. Phys Rev Lett, 1997, 79(22): 4453-4456.
[17]. Li Zhongqiu, Zhang Kaiwang. Thermal stability of SW-deficient carbon nanotubes and interlayer coupling structure of double-walled carbon nanotubes [D]. Xiangtan University.2011 21. https://www.cnki.com.cn/Navi/B.htm









