1. Introduction
The continuous progression and development of modern human society demand significant energy, i.e., the world’s energy demand is expected to double by 2050 from 23.23 trillion kWh to 60 trillion kWh [1]. Conventionally, energy is derived from fossil fuels (coal, petroleum, and natural gas), and significant consumption of fossil fuels in human history has resulted in serious environmental impacts such as global warming and climate change, jeopardizing the sustainable development of human society. Hence, the global community urges a paradigm shift in the energy landscape, iconized by the 2015 United Nations Climate Change Conference (COP 21) in Paris, which reached a landmark agreement to combat climate change and to accelerate and intensify the actions and investments needed for a sustainable low carbon future [2].
A crucial strategy for minimizing the carbon footprint associated with energy is the replacement of fossil fuels with sustainable sources (such as wind, hydro and solar energy). However, due to the intermittent nature of these renewables, influenced by seasonal and geographical constraints, the application scope of renewables is rather limited [3]. Consequently, the development of appropriate energy carriers is essential. Hydrogen (H2), produced via electrolysis powered by green electricity from renewables, presents a promising solution. With a high energy density of 33.3 kWh/kg, H2 can be directly used as the fuel in various applications, including fuel cell and heating [4]. Importantly, H2 combustion produces only water (i.e., zero carbon emission), making it a potentially transformative clean energy carrier.
Green H2 production via water electrolysis includes two key reactions: the oxygen evolution reaction (OER) and the hydrogen evolution reaction (HER). The OER is particularly challenging because it is both thermodynamically demanding, requiring a potential of E = 1.23 − 0.059 × pH V versus the normal hydrogen electrode (NHE), and kinetically sluggish due to the involvement of four electrons and four protons (2H2O → 4H+ + 4e− + O2) [5]. These factors significantly hinder the overall efficiency of water splitting and its practical applications. In nature, the OER occurs efficiently during photosynthesis in plants, driven directly by the tyrosine/tyrosine radical pair, which operates at a redox potential of only 1.2 eV [6]. This natural system achieves turnover frequencies (TOFs) of 100 − 400 s-1, vastly outperforming industrial catalysts such as RuO2 and IrO2 (Ibid). The catalytic center of photosynthesis features a multinuclear metal cluster (Mn4CaO5), which serves as a model for designing artificial catalysts. Efforts to replicate its structure and function have focused on developing dual-site and multi-site catalysts, with a particular emphasis on understanding their catalytic mechanisms at the molecular level. To progress the field, this paper reviews the recent advances in the design and mechanistic understanding of the multi-site water oxidation catalyst, highlighting key strategies and outlining the challenges for future development. Homogeneous ruthenium-based water oxidation catalysts (Ru-WOCs) have demonstrated superior performance in enhancing reaction rates, making them a key focus of research. This review therefore concentrates on Ru-based catalysts.
2. Water oxidation mechanisms
This review will focus on elucidating the role of multi-site strategies from the perspective of catalytic mechanisms. Water oxidation mechanisms can be broadly categorized into two: water nucleophilic attack (WNA) and interaction of two metal-oxo entities (I2M) [7]. In the WNA mechanism, water molecules act as nucleophiles, attacking high-valent metal-oxo intermediates (e.g., M=O) to form O-O bonds. This process begins with the metal center generating high-valent metal-oxo intermediates through multiple proton and electron transfer steps, which involves water binding and water activation. Subsequently, a water molecule nucleophilically attacks the M=O species to form O-O bonds, followed by peroxide activation and oxygen release to complete the catalytic cycle (left side, Figure 1). In contrast, the I2M mechanism (right side, Figure 1) involves the coupling of two metal-oxo intermediates with radical character to form O-O bonds. This is followed by the disproportionation of the bridged metal peroxo species and the release of oxygen, completing the catalytic cycle.
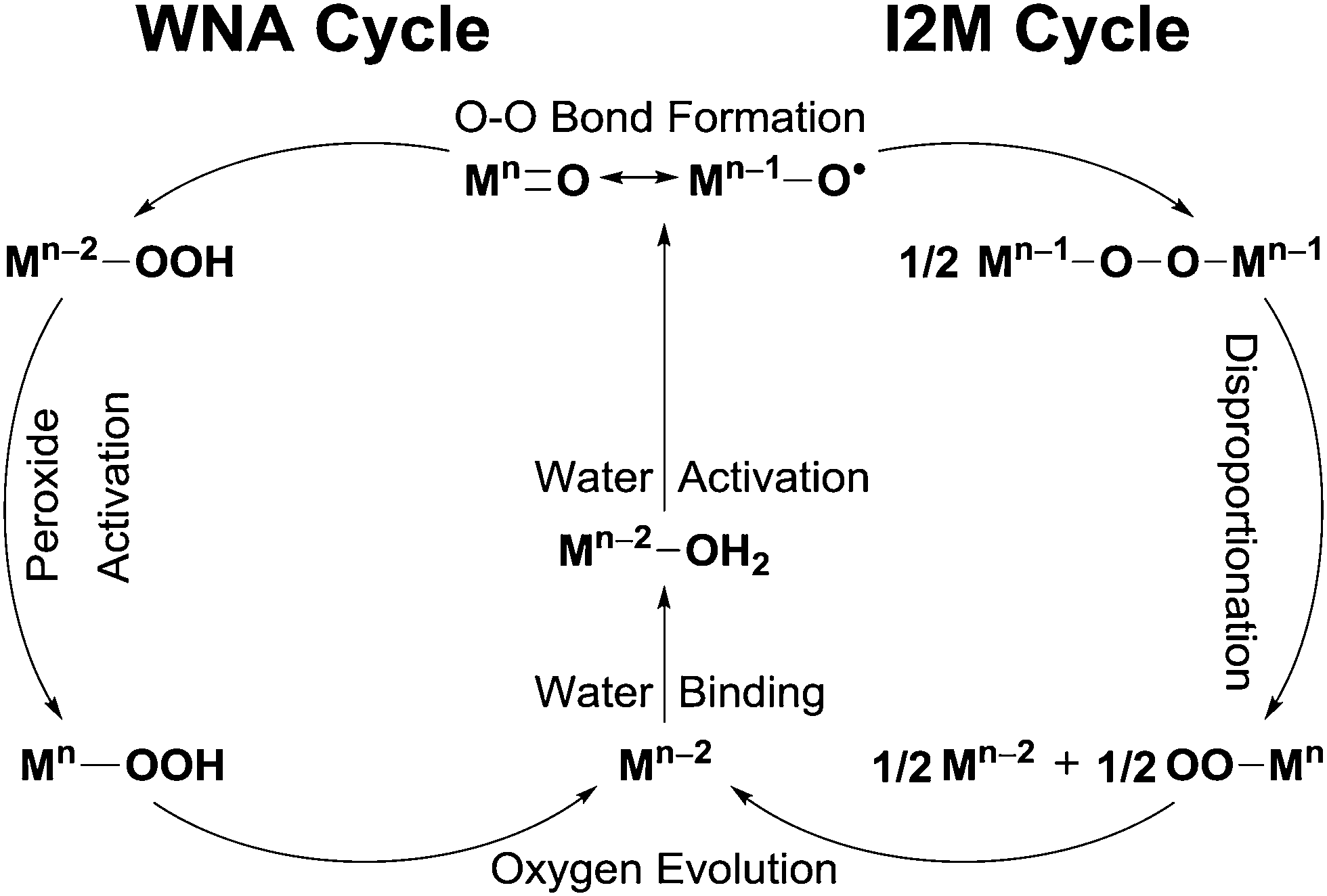
In terms of reaction kinetics, the WNA involves a nucleophilic attack by water molecules on a single metal-oxo intermediate, whereas the I2M mechanism involves radical coupling between two metal-oxo intermediates. Since the O-O bond formation is generally considered the rate-determining step (RDS) in most water oxidation reactions, their reaction rates differ significantly as shown in the following equations.
The TOF of I2M exhibits catalyst concentration ([cat]) dependence, leading to faster kinetics at high [cat] compared to WNA. However, when homogeneous catalysts are immobilized on an electrode, diffusion limitations can hinder O-O bond formation, particularly for the I2M mechanism. Under such conditions, catalysts following the WNA mechanism are more advantageous. Consequently, the rational design of catalysts that operate via either WNA or I2M mechanisms is essential for advancing water oxidation catalysis.
3. Ru(bda) water oxidation catalyst
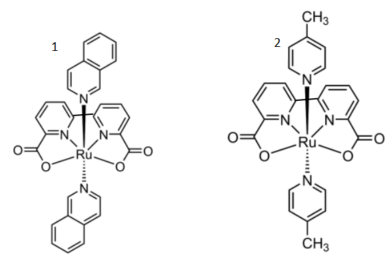
Ru(bda) is recognized as one of the most effective water oxidation catalysts. The monomeric Ru(bda) forms O−O bonds through a dual-site I2M mechanism. As shown in Figure 2, kinetic studies using 2 provide a clearcut insight into the mechanism of the O–O bond formation and reveal a radical coupling of RuV=O species as a key process, where two RuV=O intermediates couple to form a peroxo dimer, followed by rapid oxygen release. Isoquinolines were introduced as the axial ligand in 1 based on the idea that the barrier for the radical coupling of the Ru-O species could become lower due to non-covalent attractive interactions between isoquinolines. The catalyst’s exceptional activity stems from its unique design, combining a bipyridine-dicarboxylate ligand motif with isoquinoline axial ligands. the bda ligand provides a stable equatorial coordination environment with negative charges, thereby enriching the electron density of the Ru center and facilitating water activation at lower potentials. The isoquinoline ligands facilitate O−O bond formation through non-covalent π-π stacking interactions, lowering the energy barrier for radical coupling of RuV-O species. Catalyst 1 displayed a remarkable turnover frequency (TOF) of 303 s-1, which outperforms many previously reported water oxidation catalysts, including mono- and dinuclear ruthenium, iridium, manganese, cobalt, and iron complexes, which typically exhibit TOFs below 5 s-1 [8].
4. Ru(bda) dimer
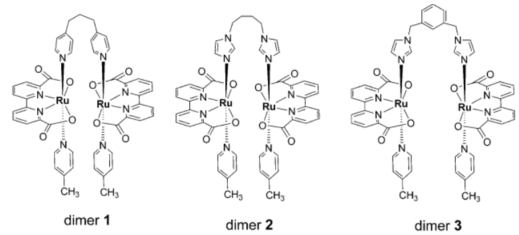
However, the reactivity of the above catalysts was found to significantly depend on the catalyst concentration due to the inherent bimolecular coupling of RuV=O active species during the O−O bond formation. This limitation poses a challenge for their practical application, whether in solution or on the electrode surface of a water-splitting device. It was proposed that by appropriately bridging two Ru(bda) units, the concentration constraint might be overcome by promoting easy intramolecular radical coupling of the oxidative intermediate RuV=O. A series of dimeric catalysts were synthesized based on Ru(bda), demonstrating markedly improved catalytic activity compared to their monomeric counterparts in a homogeneous solution.
The development of bridged dinuclear ruthenium complexes, such as dimer 1, dimer 2, and dimer 3, has significantly advanced water oxidation catalysis, as shown in Figure 3. These catalysts, derived from the Ru(bda) monomer, feature flexible bridging spacers that facilitate intramolecular radical coupling, thereby overcoming the concentration dependence typical of bimolecular O−O bond formation. Dimer 1, linked by three methylene linkers, achieved a turnover number (TON) of 20,780 (10,390 per Ru center) , significantly outperforming its monomeric precursor (TON = 1,550) [8]. Dimer 2, with four methylene linkers, showed slightly lower TON of 16,690 but maintained high efficiency The most active catalyst, dimer 3, incorporated a rigid -CH2PhCH2- spacer and achieved a remarkable TON of 42,840 (21,420 per Ru center) under optimized conditions, with an initial TOF of 40 s-1 [8]. This exceptional performance highlights the critical role of spacer flexibility in enhancing catalytic activity. The dinuclear design not only improves efficiency at low catalyst concentrations but also mimics the cooperative interactions seen in natural systems like the oxygen-evolving complex (OEC) of photosystem II [9-11].
5. Ru(bda) macrocycle
Inspired by nature’s strategy of enhancing enzyme catalysis through complex supramolecular matrices in the second coordination sphere where weak non-covalent interactions lower activation barriers or facilitate substrate transport, supramolecular catalysts are designed to improve the OER by fine-tuning the second coordination sphere of the active center. This is accomplished by providing specific substrate binding sites or creating defined cavities that mimic enzymatic pockets.
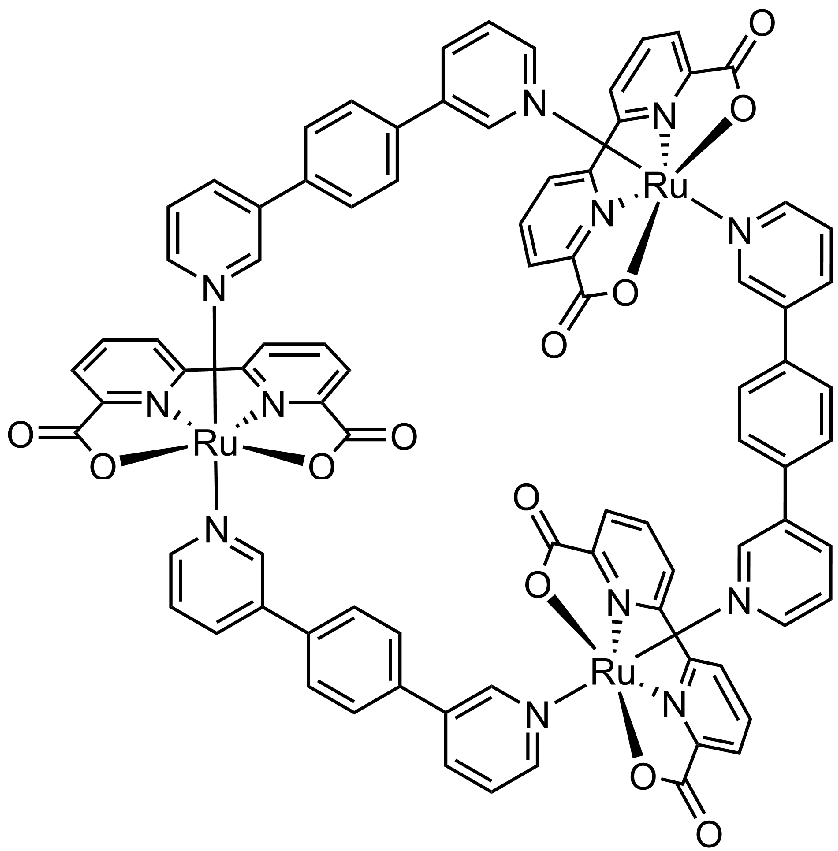
In contrast to the Ru(bda) monomer that achieve high TOFs over 300 s-1 via the I2M mechanism, catalysts following the WNA mechanism are less active. So here is a strategy to achieve OEC-PSII-like catalytic activity via the WNA pathway by embedding Ru(bda) into a supramolecular architecture. The catalyst, a metallosupramolecular macrocycle [Ru(bda)bpb]3 in Figure 4, positions three Ru(bda) subunits close together through axial coordination of 1,4-bis(pyrid-3-yl) benzene (bpb) linkers. Computational studies suggest that this arrangement preorganizes up to ten water molecules within the cavity, facilitating proton transfer during water oxidation via the hydrogen-bonded network. This design conceptually mimics the second coordination sphere of natural OEC-PSII, highlighting the importance of multinuclear catalysts for efficient oxygen evolution.
The incorporation of Ru(bda) into macrocycles has proven effective for creating highly active multi-site water oxidation catalysts. Precise control over cavity size and geometry is crucial for facilitating substrate water binding and PCET processes, which drive water activation and O-O bond formation. There are a series of macrocyclic catalysts (MC1–MC4) as shown in Figure 5, and researchers have investigated the impact of cavity size on catalytic activity. Molecular dynamics simulations reveal that medium-sized catalysts, such as MC3, accommodate an extensive hydrogen-bonded water network, enhancing interactions between substrate water molecules and coordinated aqua/hydroxide ligands. This results in elongated RuO–H and shortened Ru–OH bonds, improving catalytic efficiency. Experimental results show a clear size dependence, with MC3 exhibiting the highest activity (TOF = 7.9 s-1), while the smallest macrocycle, MC1, shows the lowest (TOF = 0.3 s-1) [12]. The O-O bond formation proceeds via the WNA mechanism, and catalytic activity correlates with the hydrogen/deuterium (H/D) kinetic isotope effect (KIE). MC3 displays a KIE of 2.8, indicating a strong proton-coupled rate-determining step, while MC1 shows a negligible KIE of 1.2 [12-17].
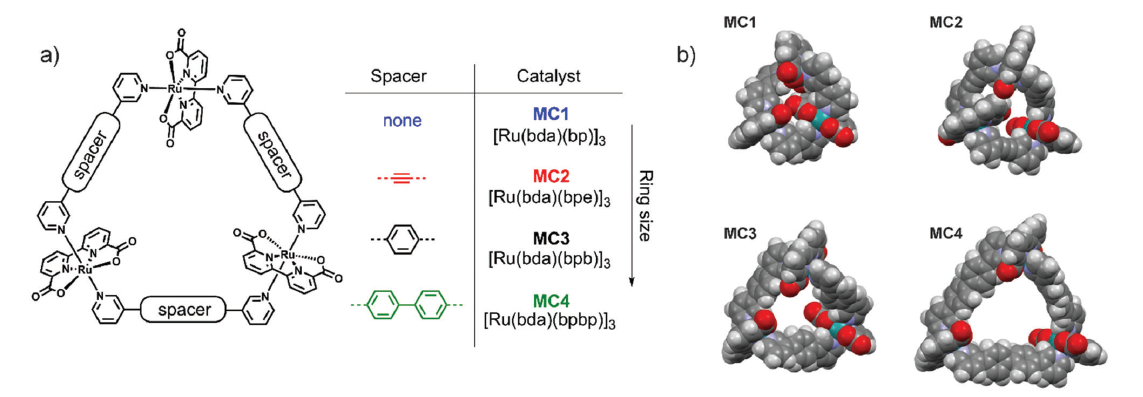
To address the issue of poor water solubility of [Ru(bda)bpb]3, which requires 50% (v/v) acetonitrile for catalysis, water-soluble derivatives were developed. By introducing triethylene glycol chains (MC2’) (Figure 6) or protonable tertiary amines (MC3’) into the bridging ligands, water solubility was significantly improved. MC2’ reduced the acetonitrile requirement by half, while MC3’ achieved full solubility in pure water under acidic conditions [18-24]. This enhanced solubility not only facilitated catalytic performance but also enabled stability verification via 1H NMR under harsh conditions (pH=1, up to 355 K), avoiding acetonitrile-induced line broadening. These modifications provide crucial support for achieving eco-friendly, organic solvent-free water oxidation, laying the foundation for the development of more sustainable catalytic systems [12].
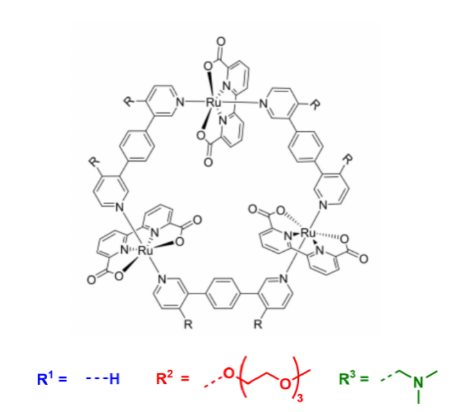
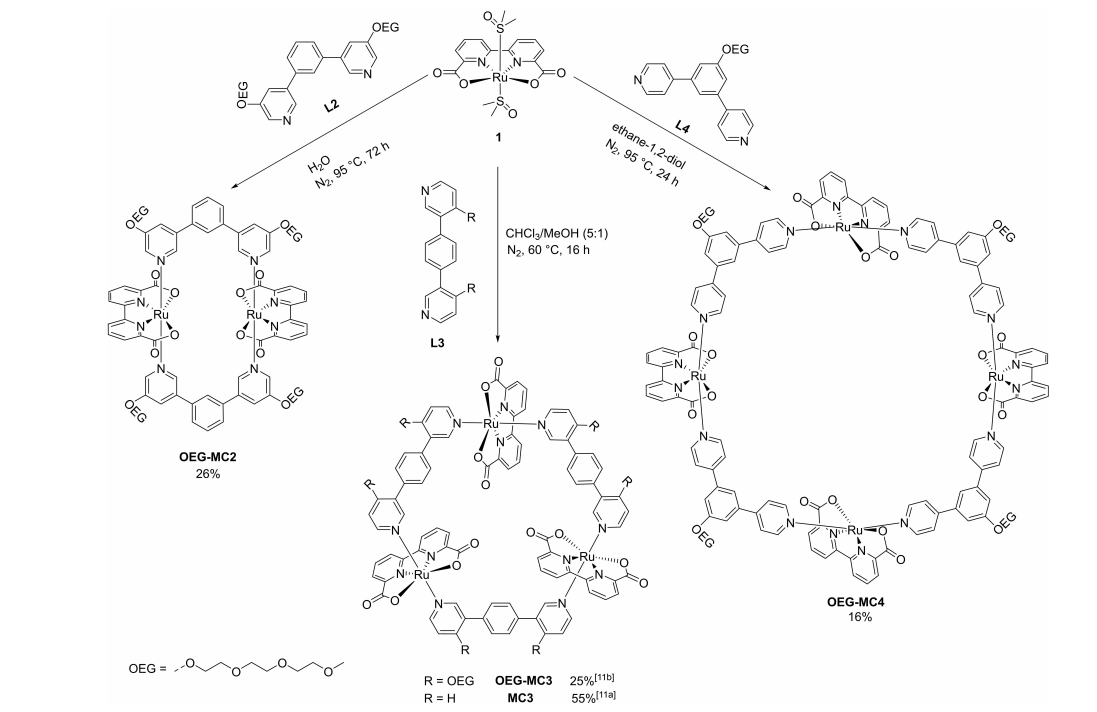
The catalytic efficiency of Ru(bda) macrocycles also depends on their nuclearity. The tetranuclear complex OEG-MC4 (Figure 7), with a TOF of 42±3 s-1, outperforms trimeric oligoethylene glycol-MC3 (OEG-MC3, TOF = 26 s-1) and dimeric OEG-MC2 (TOF = 12±2 s-1) [25], demonstrating the positive correlation between macrocycle size and catalytic activity. The incorporation of OEG chains enhances solubility and catalytic performance, with OEG-MC4 showing exceptional activity even at very low concentrations (6 nM). Further analysis suggests that synergistic interactions between multiple Ru centers in OEG-MC4 enhance catalytic turnover. The multiple Ru centers in OEG-MC4 cooperate to accelerate O-O bond formation via the I2M mechanism, while flexible linkers stabilize reactive intermediates, boosting catalytic efficiency, making it a promising candidate for sustainable energy applications.
The advancements in the development of efficient water oxidation catalysts (WOCs) have highlighted the potential of trinuclear Ru(bda) macrocyclic complexes for heterogeneous catalysis. A notable study demonstrated the successful immobilization of a trinuclear Ru(bda) macrocycle (MC3) on multi-walled carbon nanotubes (MWCNTs) through non-covalent π–π interactions, enabling its application in electrochemical water oxidation (Figure 8). The immobilized catalyst exhibited exceptional performance, achieving a current density of 186 mA cm-2 at 1.45 V versus NHE, with an onset overpotential of 330 mV [26]. Remarkably, the catalyst demonstrated high stability, sustaining 1.8 million turnovers without degradation, as confirmed by X-ray absorption spectroscopy and electrochemical analyses.
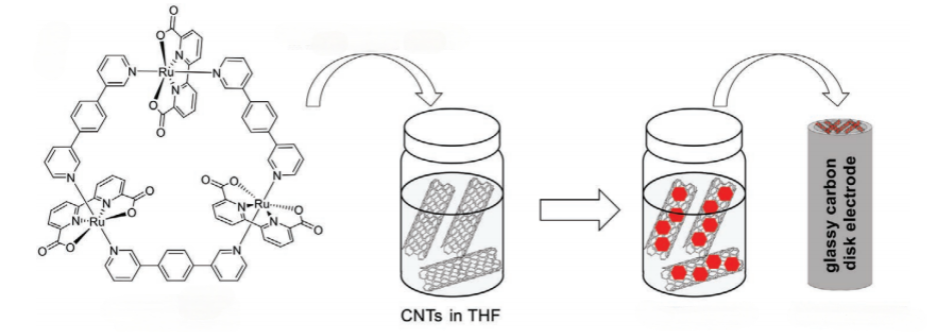
The study revealed that the catalytic activity of MC3’@CNT (MC3’ anchored on CNTs) could be further enhanced through an electrochemical activation process involving repetitive cyclic voltammetry scans. This activation led to the formation of a more active species, likely due to the coordination of additional water molecules to the Ru centers, as supported by semiempirical calculations. Foot-of-the-wave analysis indicated a WNA mechanism with a maximum turnover frequency (TOFmax) of 3,200 s-1, one of the highest reported for Ru-based WOCs [26]. The high Faraday efficiency of 99% further confirmed the catalyst's efficiency in producing oxygen without significant side reactions. These findings underscore the potential of supramolecular Ru(bda) macrocycles for heterogeneous water oxidation, particularly when immobilized on conductive supports like CNTs. The study provides valuable insights into the design of efficient and stable WOCs for integration into water-splitting devices, paving the way for practical applications in renewable energy technologies. Future research could explore the immobilization of similar catalysts on other conductive surfaces or the development of hybrid materials to further enhance catalytic performance and stability.
6. Ru(bda) covalent organic framework
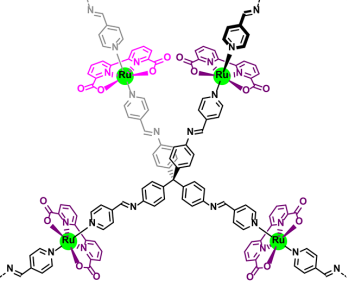
The synthesis of a highly crystalline covalent organic framework (COF) was pursued to enhance catalyst stability for chemical and photochemical water oxidation applications. The interpenetrated structure of this framework effectively preserved catalytic performance across multiple reaction cycles.
Integration of Ru(bda) catalysts into 3D imine polymers (Figure 9) yielded either amorphous Ru(bda)-polymer or crystalline Ru(bda)-COF, depending on the solvent conditions during synthesis. The amorphous variant displayed restricted catalytic activity, attributed to the disordered arrangement of Ru active sites, which favored the slower WNA pathway. In contrast, the crystalline CO’s well-defined framework promoted cooperative interactions among multiple active sites, enabling efficient water oxidation through the I2M mechanism.
The structural robustness of the material was further enhanced by a 5-fold interpenetrated dia-network, which contributed to exceptional stability. This design allowed the heterogeneous catalyst to be recycled repeatedly without significant degradation in activity. The modular nature of this approach suggests potential applicability for immobilizing other molecular catalysts, offering a promising strategy to combine molecular-level precision with enhanced durability and processability.
This study highlights the importance of crystalline frameworks in optimizing catalyst performance and stability, providing insights for the development of advanced catalytic systems for sustainable energy applications.
7. Conclusion
Recent advances in Ru(bda)-based water oxidation catalysts demonstrate that precise structural control through dimerization, macrocyclization, and framework immobilization can simultaneously enhance activity, stability, and mechanistic efficiency. The field has evolved from studying discrete molecular catalysts to developing hierarchical architectures (COFs, CNT hybrids) that combine the benefits of homogeneous and heterogeneous systems. Key breakthroughs include achieving TOFs exceeding 3000 s-1 through optimized I2M pathways in crystalline frameworks, while maintaining stability via interpenetrated networks. However, challenges remain in bridging lab-scale performance with industrial requirements, particularly regarding long-term stability under high current densities and economic viability. Future research should focus on establishing universal design principles for earth-abundant alternatives, developing standardized device integration protocols, and employing computational tools to navigate the complex multi-site catalysts. These developments position molecular water oxidation catalysis as a maturing field transitioning from fundamental understanding to practical implementation in renewable energy systems.
References
[1]. Goss, L. (2024) Global electricity demand to double by 2050, IEA’s World Energy Outlook says. MarketWatch. Retrieved from marketwatch.com/story/global-electricity-demand-to-double-by-2050-ieas-world-energy-outlook-says-ededbb1d.
[2]. C2ES (2015) 21stSession of the Conference of the Parties to the United Nations Framework Convention on Climate Change. Retrieved from https: //www.c2es.org/content/cop-21-paris/.
[3]. Iychettira, K.K. (2021) Lessons for renewable integration in developing countries: The importance of cost recovery and distributional justice. Energy Research & Social Science, 77, 102069.
[4]. Yue, M., Lambert, H., Pahon, E., Roche, R., Jemei, S., and Hissel, D. (2021) Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renewable and Sustainable Energy Reviews, 146, 111180.
[5]. Pizzolato, E., Stener, M. and Prato, M. (2016) New Molecules and Nano-materials for Artificial Photosynthesis. Università degli Studi di Trieste. Retrieved from https: //arts.units.it/retrieve/e2913fdb-84c6-f688-e053-3705fe0a67e0/Tesi%20dottorato%20-%20Erica%20Pizzolato.pdf.
[6]. Duan, L., Bozoglian, F., Mandal, S., Stewart, B., Privalov, T., Llobet, A., and Sun, L. (2012) A molecular ruthenium catalyst with water-oxidation activity comparable to that of photosystem II. Nature Chemistry, 4, 418–423.
[7]. Shaffer, D.W., Xie, Y., and Concepcion, J. (2017) O–O bond formation in ruthenium-catalyzed water oxidation: single-site nucleophilic attack vs. O–O radical coupling. Chemical Society Reviews, 46, 6170-6193.
[8]. Jiang, Y., Li, F., Zhang, B., Li, X., Wang, X., Huang, F., and Sun, L. (2013) Promoting the Activity of Catalysts for the Oxidation of Water with Bridged Dinuclear Ruthenium Complexes. Angewandte Chemie, 125, 3482-3485.
[9]. Betley, T.A., Wu, Q., Voorhis, T.V., and Nocera, D.G. (2008) Electronic design criteria for O-O bond formation via metal-oxo complexes. Inorganic Chemistry, 47, 1849-1861.
[10]. Wasylenko, D.J., Ganesamoorthy, C., Koivisto, B.D., and Berlinguette, C.P. (2010) Examination of water oxidation by catalysts containing cofacial metal sites. European Journal of Inorganic Chemistry, 20, 3135-3142.
[11]. Yoshida, M., Masaoka, S., and Sakai, K. (2009) Oxygen evolution from water catalyzed by mononuclear ruthenium complexes with a triazamacrocyclic ligand in a facial fashion. Chemistry Letters, 38, 702-703.
[12]. Kunz, V., Lindner, J.O., Schulze, M., Röhr, M.I.S., Schmidt, D., Mitrić, R., and Würthner, F. (2017) Cooperative water oxidation catalysis in a series of trinuclear metallosupramolecular ruthenium macrocycles. Energy & Environmental Science, 10, 2137-2153.
[13]. Capello, C., Fischer, U., and Hungerbuhler, K. (2007) What is a Green Solvent? A Comprehensive Framework for the Environmental Assessment of Solvents. Green Chemistry, 9, 927-934.
[14]. Wang, L., Duan, L., Wang, Y., Ahlquist, M.S.G., and Sun, L. (2014) Highly Efficient and Robust Molecular Water Oxidation Catalysts Based on Ruthenium Complexes. Chemical Communications, 50, 12947-12950.
[15]. Song, N., Concepcion, J.J., Binstead, R.A., Rudd, J.A., Vannucci, A.K., Dares, C.J., Coggins, M.K., and Meyer, T.J. (2015) Base Enhanced Catalytic Water Oxidation by a Carboxylate−bipyridine Ru(II) Complex. Proceedings of the National Academy of Sciences, 112, 4935-4940.
[16]. Duan, L., Wang, L., Inge, A.K., Fischer, A., Zou, X., and Sun, L. (2013) Insights into Ru-Based Molecular Water Oxidation Catalysts: Electronic and Noncovalent-Interaction Effects on Their Catalytic Activities. Inorganic Chemistry, 52, 7844-7852.
[17]. Jiang, Y., Li, F., Huang, F., Zhang, B., and Sun, L. (2013) Chemical and Photocatalytic Water Oxidation by Mononuclear Ru Catalysts. Chinese Journal of Catalysis, 34, 1489-1495.
[18]. Vigara, L., Ertem, M.Z., Planas, N., Bozoglian, F., Leidel, N., Dau, H., Haumann, M., Gagliardi, L., Cramer, C.J., and Llobet, A. (2012) Experimental and quantum chemical characterization of the water oxidation cycle catalysed by [RuII(damp)(bpy)(H2O)]2+. Chemical Science, 3, 2576-2586.
[19]. Duffy, E.M., Marsh, B.M., and Garand, E. (2015) Probing the Hydrogen-Bonded Water Network at the Active Site of a Water Oxidation Catalyst: [Ru(bpy)(tpy)(H2O)]2+·(H2O)0–4. The Journal of Physical Chemistry A, 119, 6326-6332.
[20]. Xu, S., Smith, J.E.T., and Weber, J.M. (2016) Hydration of a binding site with restricted solvent access: solvatochromic shift of the electronic spectrum of a ruthenium polypyridine complex, one molecule at a time. The Journal of Physical Chemistry A, 120, 7650-7658.
[21]. Bianco, R., Hay, P.J., and Hynes, J.T. (2013) Theoretical Study of Water Oxidation by the Ruthenium Blue Dimer. II. Proton Relay Chain Mechanism for the Step [bpy2(HOO)RuIVORuIV(OH)bpy2]4+→ [bpy2(O2–)RuIVORuIII(OH2)bpy2]4+. The Journal of Physical Chemistry B, 117, 15761-15773.
[22]. Bianco, R., Hay, P.J., and Hynes, J.T. (2011) Theoretical Study of O–O Single Bond Formation in the Oxidation of Water by the Ruthenium Blue Dimer. The Journal of Physical Chemistry A, 115, 8003-8016.
[23]. Bianco, R., Hay, P.J., and Hynes, J.T. (2012) Proton relay and electron flow in the O–O single bond formation in water oxidation by the ruthenium blue dimer. Energy & Environmental Science, 5, 7741-7746.
[24]. Tong, L., Wang, Y., Duan, L., Xu, Y., Cheng, X., Fischer, A., Ahlquist, M.S.G., and Sun, L. (2012) Water oxidation catalysis: influence of anionic ligands upon the redox properties and catalytic performance of mononuclear ruthenium complexes. Inorganic chemistry, 51, 3388-3398.
[25]. Schindler, D., Meza-Chincha, A.-L., Roth, M., and Würthner, F. (2021) Structure-Activity Relationship for Di- up to Tetranuclear Macrocyclic Ruthenium Catalysts in Homogeneous Water Oxidation. Chemistry A European Journal, 27, 16938-16946.
[26]. Schindler, D., Gil-Sepulcre, M., Lindner, J.O., Stepanenko, V., Moonshiram, D., Llobet, A., Würthner, F. (2020) Efficient Electrochemical Water Oxidation by a Trinuclear Ru(bda) Macrocycle Immobilized on Multi-Walled Carbon Nanotube Electrodes. Advanced Energy Materials, 10, 2002329.
Cite this article
Wang,Y. (2025). A Focused Review on Bio-inspired Multi-site Water Oxidation Catalysis by Ruthenium Complexes. Applied and Computational Engineering,156,104-114.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of CONF-FMCE 2025 Symposium: AI and Machine Learning Applications in Infrastructure Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Goss, L. (2024) Global electricity demand to double by 2050, IEA’s World Energy Outlook says. MarketWatch. Retrieved from marketwatch.com/story/global-electricity-demand-to-double-by-2050-ieas-world-energy-outlook-says-ededbb1d.
[2]. C2ES (2015) 21stSession of the Conference of the Parties to the United Nations Framework Convention on Climate Change. Retrieved from https: //www.c2es.org/content/cop-21-paris/.
[3]. Iychettira, K.K. (2021) Lessons for renewable integration in developing countries: The importance of cost recovery and distributional justice. Energy Research & Social Science, 77, 102069.
[4]. Yue, M., Lambert, H., Pahon, E., Roche, R., Jemei, S., and Hissel, D. (2021) Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renewable and Sustainable Energy Reviews, 146, 111180.
[5]. Pizzolato, E., Stener, M. and Prato, M. (2016) New Molecules and Nano-materials for Artificial Photosynthesis. Università degli Studi di Trieste. Retrieved from https: //arts.units.it/retrieve/e2913fdb-84c6-f688-e053-3705fe0a67e0/Tesi%20dottorato%20-%20Erica%20Pizzolato.pdf.
[6]. Duan, L., Bozoglian, F., Mandal, S., Stewart, B., Privalov, T., Llobet, A., and Sun, L. (2012) A molecular ruthenium catalyst with water-oxidation activity comparable to that of photosystem II. Nature Chemistry, 4, 418–423.
[7]. Shaffer, D.W., Xie, Y., and Concepcion, J. (2017) O–O bond formation in ruthenium-catalyzed water oxidation: single-site nucleophilic attack vs. O–O radical coupling. Chemical Society Reviews, 46, 6170-6193.
[8]. Jiang, Y., Li, F., Zhang, B., Li, X., Wang, X., Huang, F., and Sun, L. (2013) Promoting the Activity of Catalysts for the Oxidation of Water with Bridged Dinuclear Ruthenium Complexes. Angewandte Chemie, 125, 3482-3485.
[9]. Betley, T.A., Wu, Q., Voorhis, T.V., and Nocera, D.G. (2008) Electronic design criteria for O-O bond formation via metal-oxo complexes. Inorganic Chemistry, 47, 1849-1861.
[10]. Wasylenko, D.J., Ganesamoorthy, C., Koivisto, B.D., and Berlinguette, C.P. (2010) Examination of water oxidation by catalysts containing cofacial metal sites. European Journal of Inorganic Chemistry, 20, 3135-3142.
[11]. Yoshida, M., Masaoka, S., and Sakai, K. (2009) Oxygen evolution from water catalyzed by mononuclear ruthenium complexes with a triazamacrocyclic ligand in a facial fashion. Chemistry Letters, 38, 702-703.
[12]. Kunz, V., Lindner, J.O., Schulze, M., Röhr, M.I.S., Schmidt, D., Mitrić, R., and Würthner, F. (2017) Cooperative water oxidation catalysis in a series of trinuclear metallosupramolecular ruthenium macrocycles. Energy & Environmental Science, 10, 2137-2153.
[13]. Capello, C., Fischer, U., and Hungerbuhler, K. (2007) What is a Green Solvent? A Comprehensive Framework for the Environmental Assessment of Solvents. Green Chemistry, 9, 927-934.
[14]. Wang, L., Duan, L., Wang, Y., Ahlquist, M.S.G., and Sun, L. (2014) Highly Efficient and Robust Molecular Water Oxidation Catalysts Based on Ruthenium Complexes. Chemical Communications, 50, 12947-12950.
[15]. Song, N., Concepcion, J.J., Binstead, R.A., Rudd, J.A., Vannucci, A.K., Dares, C.J., Coggins, M.K., and Meyer, T.J. (2015) Base Enhanced Catalytic Water Oxidation by a Carboxylate−bipyridine Ru(II) Complex. Proceedings of the National Academy of Sciences, 112, 4935-4940.
[16]. Duan, L., Wang, L., Inge, A.K., Fischer, A., Zou, X., and Sun, L. (2013) Insights into Ru-Based Molecular Water Oxidation Catalysts: Electronic and Noncovalent-Interaction Effects on Their Catalytic Activities. Inorganic Chemistry, 52, 7844-7852.
[17]. Jiang, Y., Li, F., Huang, F., Zhang, B., and Sun, L. (2013) Chemical and Photocatalytic Water Oxidation by Mononuclear Ru Catalysts. Chinese Journal of Catalysis, 34, 1489-1495.
[18]. Vigara, L., Ertem, M.Z., Planas, N., Bozoglian, F., Leidel, N., Dau, H., Haumann, M., Gagliardi, L., Cramer, C.J., and Llobet, A. (2012) Experimental and quantum chemical characterization of the water oxidation cycle catalysed by [RuII(damp)(bpy)(H2O)]2+. Chemical Science, 3, 2576-2586.
[19]. Duffy, E.M., Marsh, B.M., and Garand, E. (2015) Probing the Hydrogen-Bonded Water Network at the Active Site of a Water Oxidation Catalyst: [Ru(bpy)(tpy)(H2O)]2+·(H2O)0–4. The Journal of Physical Chemistry A, 119, 6326-6332.
[20]. Xu, S., Smith, J.E.T., and Weber, J.M. (2016) Hydration of a binding site with restricted solvent access: solvatochromic shift of the electronic spectrum of a ruthenium polypyridine complex, one molecule at a time. The Journal of Physical Chemistry A, 120, 7650-7658.
[21]. Bianco, R., Hay, P.J., and Hynes, J.T. (2013) Theoretical Study of Water Oxidation by the Ruthenium Blue Dimer. II. Proton Relay Chain Mechanism for the Step [bpy2(HOO)RuIVORuIV(OH)bpy2]4+→ [bpy2(O2–)RuIVORuIII(OH2)bpy2]4+. The Journal of Physical Chemistry B, 117, 15761-15773.
[22]. Bianco, R., Hay, P.J., and Hynes, J.T. (2011) Theoretical Study of O–O Single Bond Formation in the Oxidation of Water by the Ruthenium Blue Dimer. The Journal of Physical Chemistry A, 115, 8003-8016.
[23]. Bianco, R., Hay, P.J., and Hynes, J.T. (2012) Proton relay and electron flow in the O–O single bond formation in water oxidation by the ruthenium blue dimer. Energy & Environmental Science, 5, 7741-7746.
[24]. Tong, L., Wang, Y., Duan, L., Xu, Y., Cheng, X., Fischer, A., Ahlquist, M.S.G., and Sun, L. (2012) Water oxidation catalysis: influence of anionic ligands upon the redox properties and catalytic performance of mononuclear ruthenium complexes. Inorganic chemistry, 51, 3388-3398.
[25]. Schindler, D., Meza-Chincha, A.-L., Roth, M., and Würthner, F. (2021) Structure-Activity Relationship for Di- up to Tetranuclear Macrocyclic Ruthenium Catalysts in Homogeneous Water Oxidation. Chemistry A European Journal, 27, 16938-16946.
[26]. Schindler, D., Gil-Sepulcre, M., Lindner, J.O., Stepanenko, V., Moonshiram, D., Llobet, A., Würthner, F. (2020) Efficient Electrochemical Water Oxidation by a Trinuclear Ru(bda) Macrocycle Immobilized on Multi-Walled Carbon Nanotube Electrodes. Advanced Energy Materials, 10, 2002329.









