1. Introduction
The uses of organic compounds are numerous and varied. They have a significant influence on society since they are utilized in all aspects of life, including food, cosmetics, medicine, polymers, dyes, clothes, paints, fragrances, and fuels. Synthesis is one of the key activities that organic chemists must do. Large-scale chemical research is occasionally sparked by the discovery of a novel molecule with peculiar features in order to create and develop sophisticated materials with desired qualities. To design their synthesis, the disconnection approach is a useful tool. Retrosynthetic analysis is yet another name for this. This is the opposing approach to examining molecules while keeping in mind how they were created. This is the synthetic route from a simpler starting molecule or molecule to the desired product (target molecule, TM). Nowadays, every organic synthesis course includes a substantial portion of this "disconnected" synthesis methodology [1,2].
2. History
The idea of retrosynthetic analysis was first developed by Harvard University professor Elias J. Corey, who was the winner of the 1990 Nobel Prize for Chemistry for his original contributions to the theory and methodology of organic synthesis, specifically retrosynthetic analysis.
Retrosynthetic analysis, Corey's method for organizing his many thoughts on chemical synthesis, was first developed in October 1957. At that time, the conventional approach to designing laboratory syntheses of complex organic molecules was to start with simple building blocks that could be assembled by a series of reactions to form the desired target molecule. This method was brilliantly used by many chemists around the world. Corey, on the other hand, conceptually planned his syntheses by theoretically deconstructing the target molecule into components from which it might probably be synthesized, continuing the process of disassembly until simple starting material were obtained. Through the use of retrosynthetic analysis, any target molecule could be broken down into a number of different synthetic schemes, each of which was based on well-known reactions of related molecules. In order to successfully synthesize the target molecule, a combination of deliberate choice and experimental trial and error could be used. Later, organic synthesis adopted this method as the standard practice.
3. Key Terms in retrosynthesis
Disconnection: an analytical operation, which breaks a bond and converts a molecule into a possible starting material. It is indicated by a wavy line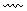
Retrosynthetic arrow: an open-ended arrow, ⇒, used to indicate the reverse of a synthetic reaction
Reagent: a compound which reacts to give an intermediate in the planned synthesis or to give the target molecule itself. The synthetic equivalent of a synthon.
Synthetic equivalent: a reagent carrying out the function of a synthon which cannot itself be used, often because it is too unstable.
Synthon: idealized fragment resulting directly from a disconnection (synthons need to be replaced by reagents in a suggested synthesis).
Target Molecule: the molecule whose synthesis is being planned.
4. What is Retrosynthetic analysis?
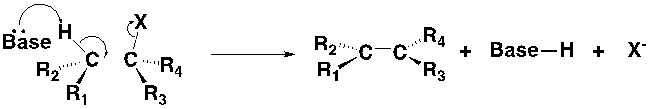
The retrosynthetic analysis is a technique for solving problems in the planning of organic synthesis. Normally speaking, people often start their synthetic plan from the simple molecules to produce a more complex molecule that you want to make, as shown in figure 1.
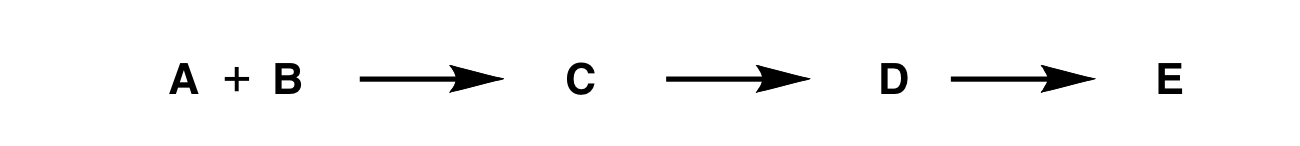
Figure 1. A simple schematic example of synthesis
Retrosynthesis is the process of deconstructing a target molecule (the molecule you want to make) into simpler molecules. This is accomplished by imagining the last reaction used to prepare the target molecule and take the molecule backward through a retrosynthetic scheme.
The point here is that disconnection should correspond to a known and reliable reaction and every complex molecule ultimately has an infinite number of solutions to its synthesis and retrosynthetic analysis is a way to explore some of those possible ways. To be more specific, people can disconnect the bond to change the target molecule from E to D with the help of their creativity, even though they have not yet learned the forward synthesis reaction from D to E and then they can find the corresponding reaction through the Internet or their coursebooks since there is a large number of synthesis reactions in the organic chemistry world.
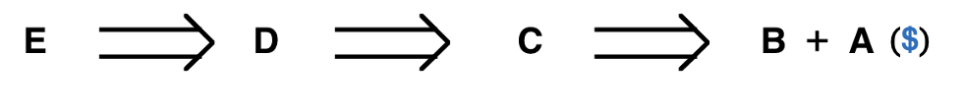
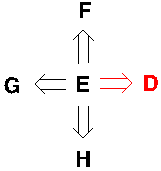
Figure 2. A retrosynthetic sequence and four ways to retrosynthesis for E
In the Figure 2, the first step an organic chemist should take is to think about what would be the last step to make molecule E. Probably, there are many ways to make it, and we need to decide which one is the most strategic way which is simpler, safer, etc. In this case, D would be the most strategic way, and D becomes the target molecule, then applying the same logical thought to molecule D. In the retrosynthetic analysis, people are constantly trying to simplify one step at a time, and finally, they will get back to molecules that people can purchase or are readily available. Furthermore, we can employ the retrosynthetic thought on things beyond chemistry. For instance, a triangle!
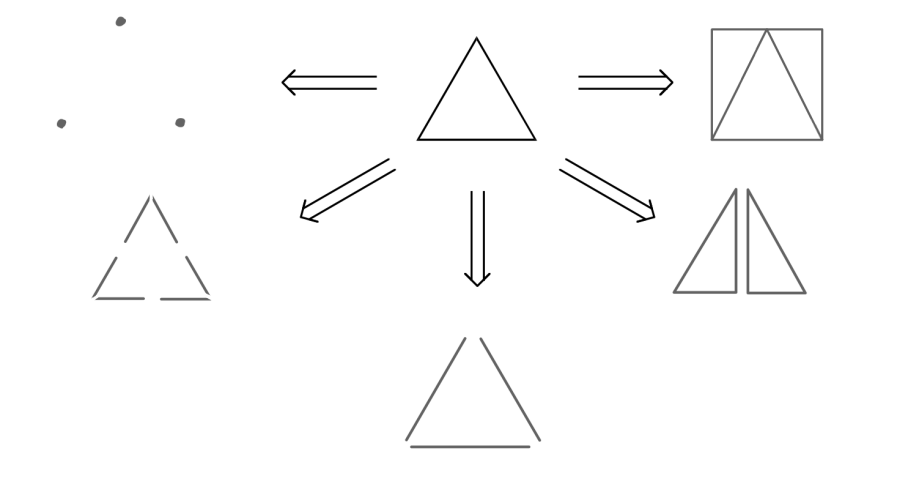
Figure 3. Retrosynthesis for a triangle
In the figure 3, there are five possible ways (just based on your imagination) for a triangle to be analyzed by retrosynthesis. The triangle can consist of three dots, six short sticks, three long sticks, two right triangles, or a square.

Figure 4. Structural consequences and the structure for cyclopropane
It could be quite different when the triangle becomes cyclopropane. Regarding chemistry, the prominent rule people have to follow is that we need to focus on breaking the bond (in cyclopropane, we can break either the C-C bond or the C-H bond). We may just concentrate on the C-C bond since we can gain a simpler molecule after breaking that bond.
The figure 4 shows the structural consequences of breaking one bond at a time, two bonds at a time and three bonds at a time.
When breaking one bond at a time, you will gain the linear structure.
When breaking two bonds at a time, you will gain a two-carbon unit plus one-carbon unit.
When breaking three bonds at time, you will gain three separate one-carbon unit.
These can all achieved by the cycloaddition reaction.
5. Consequences of Breaking the Bond
When a bond is broken, the bonding electrons will not disappear and will move to synthons. There are three possible consequences of the electrons moving after breaking the bond:
All bonding electrons flow to the right:
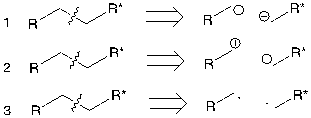
Figure 5. Three possible consequences
Seen from figure 5, bonding electrons in the first one all flow to the left, all flow to the right in the second one, and in the third one, each side has one electron.
The first one and the second one depends on polarization mechanism and the third one is in radical mechanism. The following below will focus on the polarization mechanism, in which the direction of electrons will depend on functional groups.
6. Disconnection Methods
6.1 One Group Disconnection
There is only one functional group in an organic compound to decide the electrons direction.

Figure 6. Example of alcohol
In alcohol, we should focus on OH group since there are two lone pairs in oxygen. When one lone pair moves to the central carbon forming a new C-O single bond, the carbon will have too many electrons, which causes one of the three bonds around the central carbon to break and brings the carbon with it. Therefore, there are three ways (1, 2, 3) in total with only one electrons flow direction, as shown in the figure 6.
6.2 Two Group Disconnection
There are two functional groups in an organic compound that decides the electrons direction.
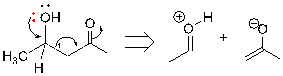
Figure 7. Example of the organic compound that both has alcohol and carbonyl group
We can see is figure 7, what makes the two groups' disconnection similar to the one group disconnection is that both of them focus on the functional group first, and the lone pair will push the electrons to move. The difference is that there is only one way and such a step happens three times, the double bond driven away and forming a negative charge. The anion resigned on oxygen is better due to its strong electronegativity of oxygen and resonance structure.
6.3 Electrocyclic Disconnection
The synthesis reaction is called the Diels-Alder reaction, the most common reaction for connecting a six-membered ring.
The reaction happens between a diene which has two double bonds, and a dienophile which has one double bond, and these two molecules will form a cyclohexane derivative [3].
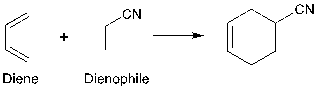
Figure 8. Diels-Alder reaction
In figure 8, based on the number of carbons, the reaction is called a [4+2] cycloaddition reaction.
The Electrocyclic disconnection is based on the Diels Alder reaction. This is often best found by drawing the reverse reaction mechanism when cyclohexene (1) with at least one electron-withdrawing group (Z) on the far side of the ring is made, three arrows around the ring are made in either direction, starting from the double bond [4], as shown is figure 9.
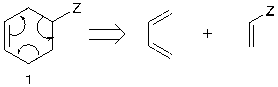
Figure 9. Electrocyclic disconnection
In addition, the synthons and the synthetic equivalents are the same in this example, which means the reaction is quite stable.
6.4 Illogical Disconnection
This disconnection is illogical because it is not really a disconnection process rather than making bonds retrosynthetically.
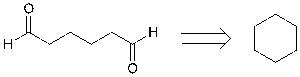
Figure 10. Illogical disconnection
In figure 10, the linear structure ends with aldehydes which can be tied up together and makes into a six-membered ring structure.
7. Functional Group Manipulation (also called Fine-Tuning)
Whenever disconnection does not lead to a proper precursor with reliable forward synthesis reaction, the functional group manipulation has to be carried out.
For example, the disconnection between compound A and B is not possible because there is no corresponding reaction to introduce compound B directly to compound A. Hence, it has to be converted to compound C by FGI, FGA, or FGR since compound C can be disconnected, which has an appropriate synthesis reaction from compound B to compound C, as shown is figure 11.

Figure 11. Why do we use functional group manipulation
Here are three types of functional group manipulation:
7.1 Functional Group Interconversion (FGI)
A functional group interconversion is where you have a target molecule that has a functional group. And if you perform an FGI, it's just converting that functional group into a different functional group.
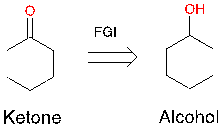
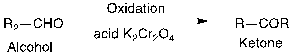
Figure 12. Example of FGI and a synthesis reaction
In figure 12, there is a ketone and retrosynthetically converting it to alcohol, which is helpful as you can use one-group disconnection. However, when you think about it from a strategic level, it has not done anything. This cyclohexane is the major complexity component in this molecule, and it is still there. The retrosynthetic product will not change. It is just being tweaked. There is a synthesis reaction from alcohol to ketone. (See Appendix A for more examples of FGI)
7.2 Functional Group Addition (FGA)
It is used when the target molecule does not have functionality in it. Shown below is the example of FGA.
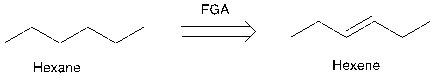
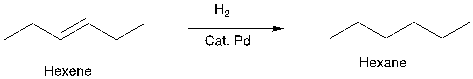
Figure 13. Example of FGA and a synthesis reaction
The hexane does not have a functional group, so maybe it is hard to think of a way to disconnect hexane, and adding a double bond maybe provide some thought for the disconnection process.
People can add some functional group retrosynthetically, as long as they know how to get rid of that group in the forward direction. In figure 13, people just need to add hydrogen and a catalyst. (See Appendix B for more examples of FGA)
7.3 Functional Group Removal (FGR)
It can be used when the target molecule has multiple functional groups, people can remove a functional group to simplify, which is FGR. Figure 14 is an example.
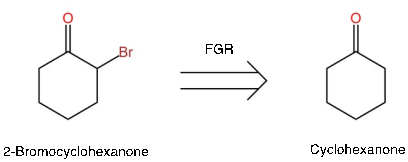
Figure 14. Example of FGR
You can see that the new target molecule is simpler and may use one-group disconnection. For simple functional groups, there are ways to introduce them into molecules. In this case, cyclohexanone would treat with base plus bromine to form 2-Bromocyclohexanone. (See Appendix C for more examples of FGR)
8. Guideline in Retrosynthetic analysis
8.1 Focus on Simplicity
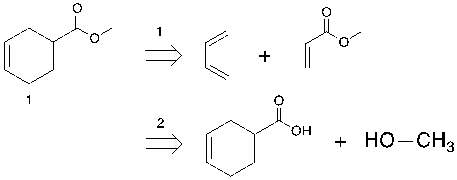
Figure 15. Example of simplicity
In the retrosynthetic analysis of molecule 1 that shows in figure 15, electrocyclic disconnection is a strategic method as the synthons on the right have more simplification of the target molecule.
By contrast, the second one is an example that does not simplify the molecule sufficiently, as the next step may also focus on the six-membered ring.
8.2 Maximize Convergence
The molecule should disconnect at roughly the middle of the molecule at the beginning of the retrosynthetic analysis.
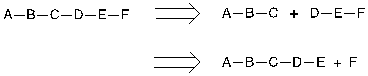
Figure 16. Example of maximizing convergence
In figure 16, the first disconnection is convergency, and the second one is linear, which has a lower yield than convergency.
Assume the yield of each step are all 80%.
The overall yield of linear synthesis:
>B->C->D->E->F: (0.8*0.8*0.8*0.8*0.8) *100= 32.77%
The overall yield of convergent synthesis:
A->B->C: (0.8*0.8) *100=64%
D->E->F: (0.8*0.8) *100 = 64%
C+F = 40.96%
8.3 Two Group Disconnections Are Better Than One Group Disconnections
It is better to focus on two functional groups at once if there is more than one functional group in the molecule as it can tend to simplify more parts of the molecule.
8.4 Minimize The Use of Functional Group Manipulation (FGI, FGA, FGR)
The more functional group manipulation is used, the more retrosynthetic steps will be required. One of the aims in the retrosynthetic analysis is to reduce the number of steps. Therefore, minimizing the use of functional group manipulation can decrease the number of steps [2].
8.5 Look for Symmetry and Exploit it

Figure 17. Example of symmetry
For instance, people can break the acetic acid on its two sides and leave with an alcohol as shown in figure 17. To be more specific, you only need one forward synthesis reaction instead of two steps, which could save the step count.
9. Real Examples Using Retrosynthetic Analysis
9.1 Compound One with No Biological Activity
The following figure 18 is the retrosynthetic analysis of this compound.

Figure 18. Retrosynthetic analysis of the compound one
The first step employs functional group interconversion (FGI) in order to turn amine into amide.
The second step disconnects the carboxamide into two substances, one of which, phenylacetyl chloride, can be purchased.
The third step employing functional group interconversion (FGI) turns amine into carbonyl.
The fourth step disconnects the phenyl and carbonyl, leading to two simple structures, benzene and acetylchloride.
The synthetic process of this compound is shown in figure 19.
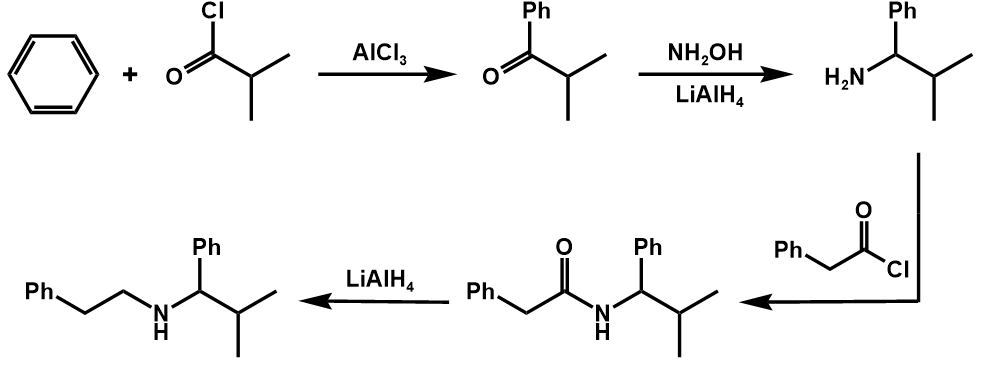
Figure 19. Synthesis of the compound one
9.2 Compound Two with No Biological Activity
The following figure 20 is the retrosynthetic analysis of this compound.
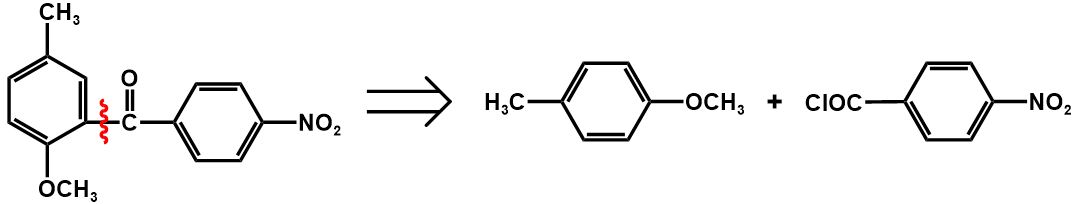
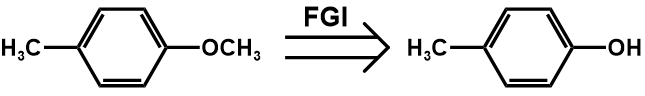

Figure 20. Retrosynthetic analysis of the compound two
The first step disconnects this compound into two substances, p-methyl anisole and para-nitrobenzyl chloride.
The second step employs functional group interconversion (FGI), and turns ether into hydroxyl.
The third step employing functional group interconversion (FGI) turns the acyl into carboxyl, which then can be further converted into p-nitrotoluene, which makes the molecule more accessible.
The synthetic process of this compound is shown in figure 21.

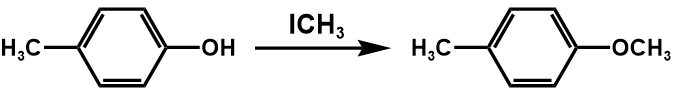
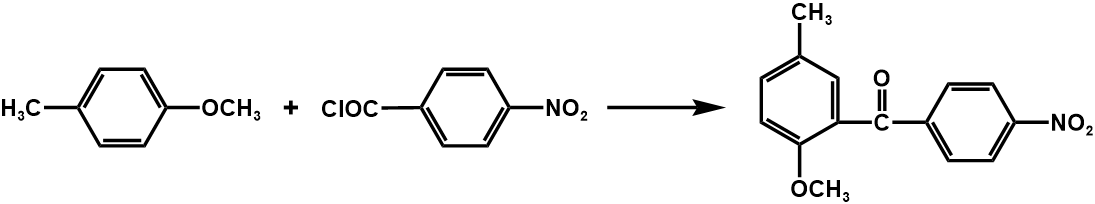
Figure 21. Synthesis of the compound two
9.3 Aspirin
Aspirin is used to treat mild to moderate discomfort from ailments like headaches, the common cold, and toothaches as well as to lower temperature. It can also be used to treat illnesses like arthritis by lowering pain and swelling. Aspirin is known as a salicylate and a nonsteroidal anti-inflammatory drug (NSAID).
The following is the retrosynthetic analysis of Aspirin.
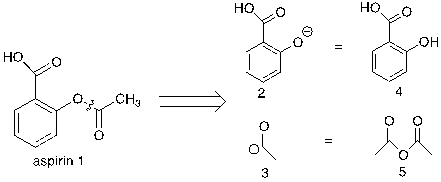
Figure 22. First step of retrosynthetic analysis of aspirin
As shown in figure 22, aspirin 1 can be ruptured into synthons 2 and 3. The reason for this bond breaking is that the equivalent molecules 4 and 5 can react with each other in a nucleophilic addition elimination reaction.
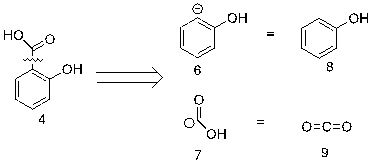
Figure 23. Second step of retrosynthetic analysis of aspirin
In figure 23, Salicylic acid 4 can be ruptured further into synthons 6 and 7. The reason for the direction of electrons flow is that equivalent molecules 8 and 9 can react with each other through electrophilic substitution.
This retrosynthetic analysis can be proved by the synthesis process of Aspirin starting from 8 as shown in figure 24.
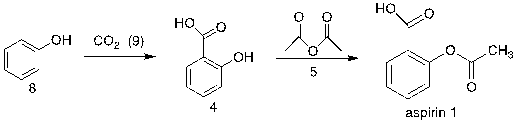
Figure 24. Synthesis of aspirin
9.4 Ibuprofen
Ibuprofen is an antipyretic analgesic, non-steroidal anti-inflammatory drug. It can be used for the relief of mild to moderate pain, such as headache, arthralgia, migraine, toothache, muscle pain, neuralgia, dysmenorrhea, and also for fever caused by the common cold or flu [5].
Figure 25 is the retrosynthetic analysis of ibuprofen.

Figure 25. Retrosynthetic analysis of ibuprofen
The first step employs functional group interconversion (FGI) in order to turn the carboxylic acid group into the ketone group.
The second step disconnects the carbon-oxygen double bond and phenyl group, leading to two substances, isobutylbenzene, which can be purchased easily, and acetylchloride.
The entire synthetic process of ibuprofen is shown in figure 26.

Figure 26. Synthesis of ibuprofen
9.5 Diclofenac sodium
Diclofenac sodium, a non-steroidal anti-inflammatory drug, is one of the typical anti-inflammatory analgesic drugs, which is often used in the treatment of mild and moderate, acute, and chronic pain in orthopedics, such as osteoarthritis, rheumatoid arthritis, ankylosing spondylitis, etc. [6].
The following figure 27 is the retrosynthetic analysis of diclofenac sodium.
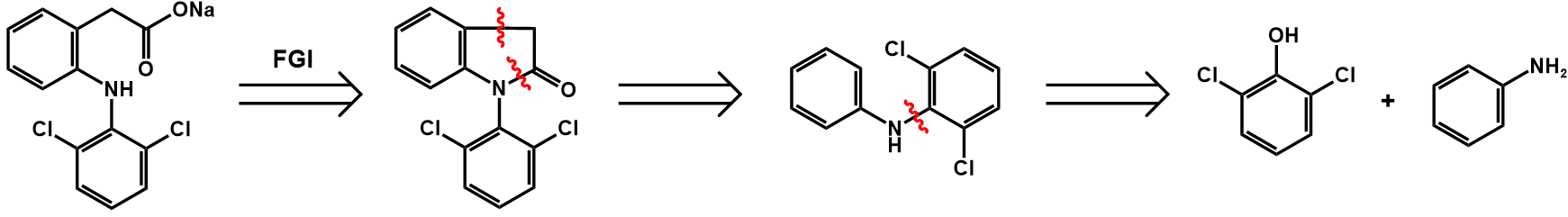
Figure 27. Retrosynthetic analysis of diclofenac sodium
The first step employing functional group interconversion (FGI) leads to cyclization and turns the ester group into the ketone bond.
The second step disconnects the five-membered ring.
The third step disconnects the two-part containing phenyl group, leading to two substances, 2,6-dichlorophenol and phenylamine, both of which can be purchased.
The entire synthetic process of diclofenac sodium is shown in figure 28.
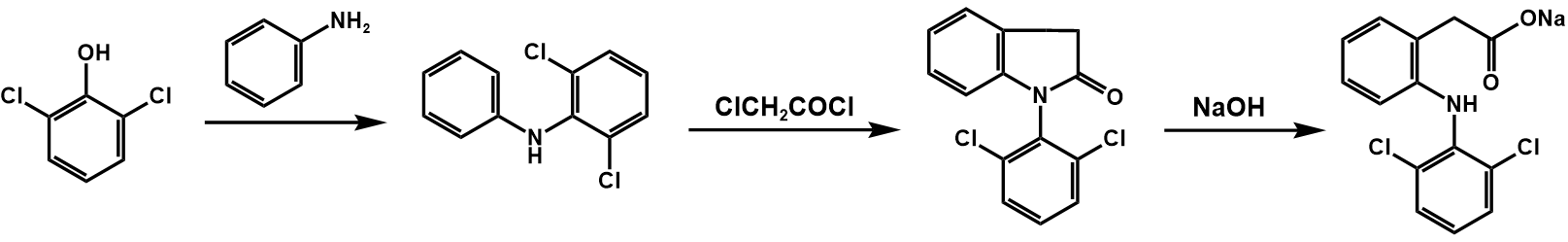
Figure 28. Synthesis of diclofenac sodium
9.6 Penicillin V
Penicillin is an important antibiotic with high efficiency, low toxicity and wide clinical application. Its use greatly enhances the ability of human to resist bacterial infections, and can effectively treat pneumonia, meningitis, endocarditis, diphtheria, anthrax and other diseases [7].
Figure 29 shows the retrosynthetic analysis of penicillin V.
 Figure 29. Retrosynthetic analysis of penicillin V
Figure 29. Retrosynthetic analysis of penicillin V
The first step disconnects the four-membered ring.
The second step employs functional group interconversion (FGI), and turns the carboxyl into ester.
The third step disconnects the compound into two parts by breaking two bonds simultaneously.
The entire synthetic process of penicillin V potassium is shown in figure 30 [8].
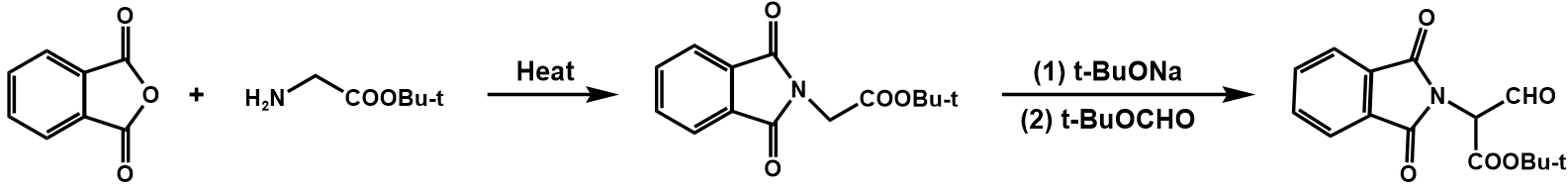
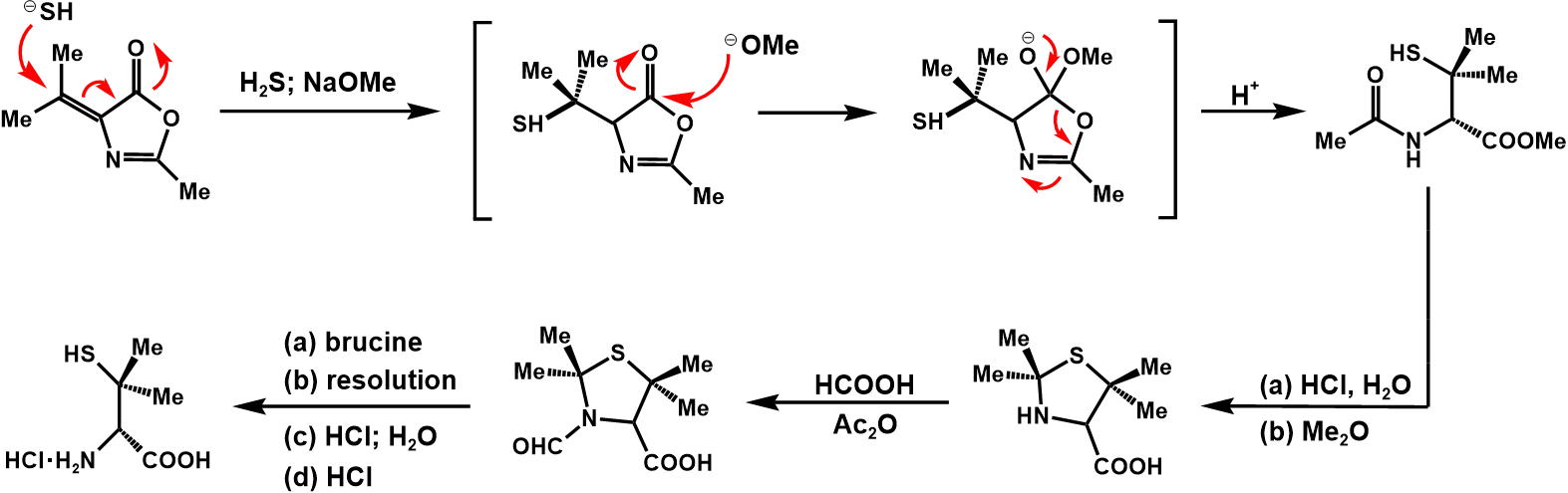
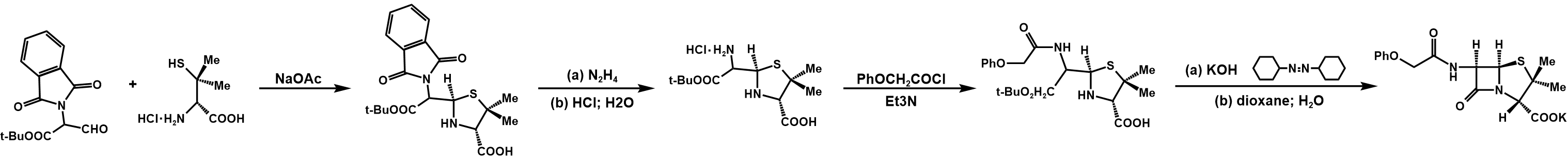
Figure 30. Synthesis of penicillin V
9.7 Baclofen
Baclofen is a drug used to help relax certain muscles in the human body. It relieves the spasms, cramping, and tightness of muscles caused by medical problems, including multiple sclerosis or certain injuries to the spine. Although baclofen does not treat these issues, it may make other treatments, such as physical therapy, more effective in helping to improve your condition, which makes it essential to the patient [9].
The following part focuses on the retrosynthetic analysis of Baclofen, as shown in figure 31.
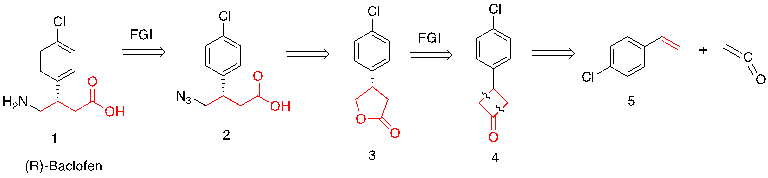
Figure 31. Retrosynthetic analysis of baclofen
The first step (1-2) focuses on the amino group and employs functional group interconversion (FGI) as the amino group in compound 1 can be made by the reduction of the Azides group.
The second step (2-3) disconnects the Azides group and carboxylic acid, and then the carboxylic acid becomes a lactone as the acid can be synthesized through the opening of a five-membered lactone (3), which is an illogical disconnection.
The third step (3-4) employs functional group interconversion (FGI). Lactones can be substituted by ketones in order to get a reliable synthesis reaction at the next step.
In the final step (4-5), the four-membered ring was broken based on the forward [2+2] cycloaddition reaction, which is an electrocyclic disconnection.
This retrosynthetic analysis can be proved by the synthesis process of Baclofen starting from 5, following with figure 32.
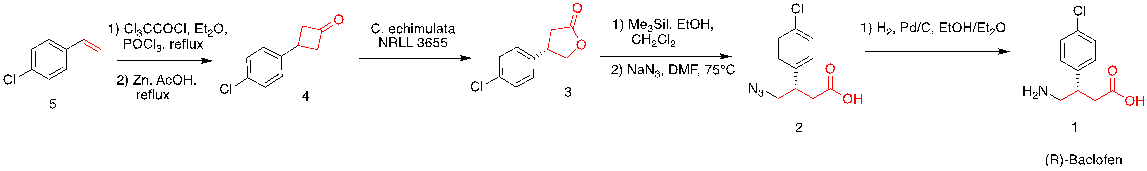
Figure 32. Synthesis of baclofen (Note: The fungus Cunninghamella echinulate NRLL 3655 used in the Baeyer-Villiger reaction converting ketones to lactones.)
10. Conclusion
The best synthetic route to a molecule cannot be predicted with certainty. For every given target molecule, retrosynthetic analysis provides a number of possible strategies. Thorough literature searches and laboratory experimentation will help you to narrow the possibilities down to the ones that are most likely to be successful. This means that the retrosynthetic analysis seeks to build an effective synthetic strategy for a complicated molecule by simplifying the process as well as assisting in the discovery of new synthesis routes and comparing them. The art of retrosynthetic analysis of a complex molecule is based on the human creativity. The methodology, like disconnection methods, functional group manipulation and the guides, can help to reduce the difficulties of planning the synthesis of a target molecule.
Appendix A
Examples of Functional Group Interconversion (FGI)
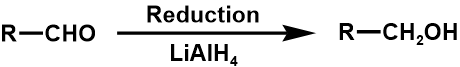
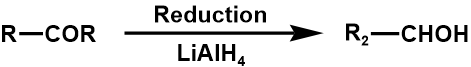
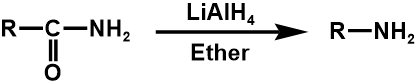
Numerous functional groups may be transformed into one another, for instance, the oxidation of alcohol produces an aldehyde, which can then be converted again to produce a carboxylic acid. To perform FGIs, several organic transformations might be applied. In this regard, carbonyl groups are very advantageous. When synthesizing, the carbonyl group's reactivity can be concealed by using dioxolane or a double bond (ozonolysis for FGI to produce an aldehyde) [1].
The list of alcohol, nitrile, alkyl halide as three examples are shown below [10].
1. Between primary alcohol and aldehyde

2. Between secondary alcohol and ketone

3. Between amide and 1° amine

4. Between Chlorine and alcohol
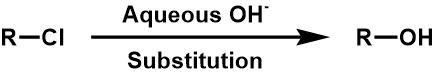

Appendix B
Examples of Functional Group Addition (FGA)
A chemical with "additional" functionality is produced through retrosynthesis. The use of FGA may be justified because a particular functional group is simpler to create or makes it possible to install other functional groups [1].
The list of more examples shown below.
Get rid of C-C double bond in the synthesis reaction
1. Bromine addition
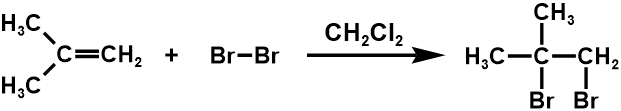
2. Oxymercuration
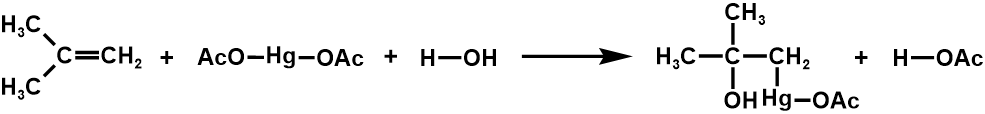
3. Hydroboration
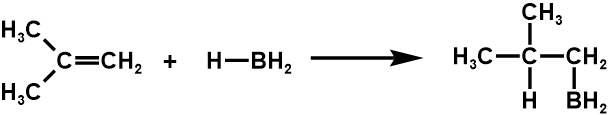
Carbocation Intermediates in Hydrogen Halide addition
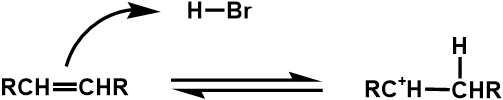
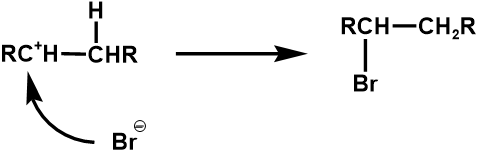
Appendix C
Examples of Functional Group Removal (FGR)
During the retrosynthesis, a certain functional group is eliminated in this transition. For instance, we can create cyclohexanone by removing the alcoholic functional group from 6-hydroxycyclohex-2-enone. Additionally, the hydroxyl functional group is removed.
The list of more examples shown below [1].
Removing alcohol
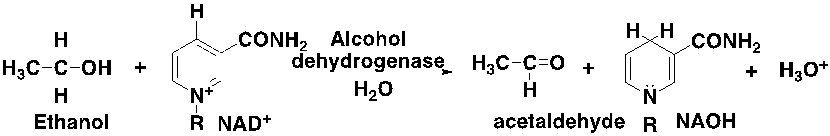
Removing X functional group
1. Anti-elimination:
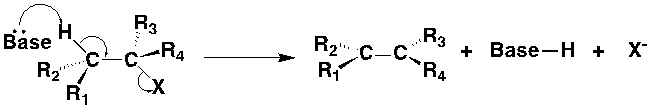
2. Syn-elimination:
References
[1]. A.K.Bakhshi, Vimal Rarh, Ram Singh, Varun Kumar Sharma; Organic Chemistry -IV (Advance Organic Synthesis and Supramolecular Chemistry and carbocyclic rings)
[2]. Kloetzel, M. C. The Diels-Alder Reaction with Maleic Anhydride. Organic Reactions 2011, 1–59.
[3]. Badami, B. V. Retrosynthetic Analysis. Resonance 2019, 24 (10), 1071–1086.
[4]. Warren, S. G.; Wyatt, P. Workbook for organic synthesis: The disconnection approach, Second edition; Wiley: Chichester, West Sussex, 2009.
[5]. Ibuprofen. The American Society of Health-System Pharmacists.
[6]. Roy Altman; Bill Bosch; Kay Brune; Paola Patrignani; Clarence Young. Advances in NSAID Development: Evolution of Diclofenac Products Using Pharmaceutical Technology. Drugs 2015, 75(8), 829-877.
[7]. Petzold, D.; Giedyk, M.; Chatterjee, A.; König, B. A Retrosynthetic Approach for Photocatalysis. European Journal of Organic Chemistry 2020, 2020 (10), 1193–1244.
[8]. Vardanyan, Ruben, and Victor Hruby. Synthesis of essential drugs. Elsevier, 2006.
[9]. Petzold, D.; Giedyk, M.; Chatterjee, A.; König, B. A Retrosynthetic Approach for Photocatalysis. European Journal of Organic Chemistry 2020, 2020 (10), 1193–1244.
[10]. Sarah Pierce Functional Groups in Organic Synthesis & Analysis. Study.com, 4 May 2017.
Cite this article
Hu,B.;Bao,Z.;Jiang,R.;Zhang,Y. (2023). Introduction to organic synthetic method retrosynthetic analysis. Applied and Computational Engineering,7,546-561.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Materials Chemistry and Environmental Engineering (CONF-MCEE 2023), Part II
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. A.K.Bakhshi, Vimal Rarh, Ram Singh, Varun Kumar Sharma; Organic Chemistry -IV (Advance Organic Synthesis and Supramolecular Chemistry and carbocyclic rings)
[2]. Kloetzel, M. C. The Diels-Alder Reaction with Maleic Anhydride. Organic Reactions 2011, 1–59.
[3]. Badami, B. V. Retrosynthetic Analysis. Resonance 2019, 24 (10), 1071–1086.
[4]. Warren, S. G.; Wyatt, P. Workbook for organic synthesis: The disconnection approach, Second edition; Wiley: Chichester, West Sussex, 2009.
[5]. Ibuprofen. The American Society of Health-System Pharmacists.
[6]. Roy Altman; Bill Bosch; Kay Brune; Paola Patrignani; Clarence Young. Advances in NSAID Development: Evolution of Diclofenac Products Using Pharmaceutical Technology. Drugs 2015, 75(8), 829-877.
[7]. Petzold, D.; Giedyk, M.; Chatterjee, A.; König, B. A Retrosynthetic Approach for Photocatalysis. European Journal of Organic Chemistry 2020, 2020 (10), 1193–1244.
[8]. Vardanyan, Ruben, and Victor Hruby. Synthesis of essential drugs. Elsevier, 2006.
[9]. Petzold, D.; Giedyk, M.; Chatterjee, A.; König, B. A Retrosynthetic Approach for Photocatalysis. European Journal of Organic Chemistry 2020, 2020 (10), 1193–1244.
[10]. Sarah Pierce Functional Groups in Organic Synthesis & Analysis. Study.com, 4 May 2017.









