1. Introduction
Retrosynthesis analysis is a strategy used in the design of organic synthesis that achieves synthesis by breaking down the target compound into smaller fragments and building these fragments step by step. And this method can help chemists more effectively plan the synthesis path of complex organic molecules and improve the synthesis efficiency. Also, this approach allows chemists to approach synthesis in a more methodical manner, breaking down seemingly arduous tasks into manageable parts. This process involves breaking down the target molecule into simpler precursor structures through a series of logical steps, ultimately revealing a viable synthetic path.
At the same time, the proposed analytical method has effectively promoted the development of computer-aided organic synthesis. The computer-designed synthesis route is exactly the opposite of the laboratory experimental process, the laboratory synthesis is from the raw material to the product, and the computer design is from the product target molecule to find the raw material synthons called reverse search method, through multiple reverse searches to find the composition of the synthons. Moreover, the results of Retrosynthesis analysis are helpful to the study of chemical analysis methods and the cultivation of reverse thinking.
In this paper, the basic principles, guiding principles and application examples of Retrosynthesis analysis will be introduced. Retrosynthesis analysis was first proposed and developed by E.J.Corey. He provided a systematic approach to organic synthesis by breaking down target compounds into accessible starting materials. Corey and his colleagues developed a series of rules and strategies that have made Retrosynthesis analysis an important tool in organic synthesis. With the development of computer technology, Retrosynthesis analysis software has been widely used, which can quickly generate possible synthesis routes.
Retrosynthesis analysis can not only be used for combinations of known reactions, but can also inspire chemists to explore new types of reactions. By thinking about how to break down target molecules into specific building blocks, chemists may discover some new chemical reaction pathways that advance the field of organic chemistry.
Based on the development history of Retrosynthesis analysis, the application of Retrosynthesis analysis with the support of current technology and the existing application methods of Retrosynthesis analysis, this paper focuses on the six guiding principles of Retrosynthesis analysis, the types of two structural transformations and the application ideas and methods. The author uses the six guiding principles and Retrosynthesis ideas summarized in this paper, and shows the application of Retrosynthesis analysis method with the synthesis of indole as a practical case. This article is helpful to further promote the development of big data-assisted organic synthesis, which has practical significance.
2. The concept and history of retrosynthesis
The concept of retrosynthesis, developed by E.J. Corey [1], involves thinking backward from the target molecule to identify simpler compounds that can be used as building blocks. By repeatedly applying this strategy, chemists can trace a path from complex molecules to readily available starting materials. This technique is particularly useful for synthesizing complex natural products and pharmaceuticals.
Since this method was proposed, scientists have been using computer-aided organic synthesis to complete the inverse synthesis analysis process. Although this method can improve the synthesis efficiency, the traditional computer-aided method is still slow and provides uneven molecular mass. Humans still need to manually search databases of chemical reactions to find the best way to make molecules.
Until A new AI tools developed by Segler's team. Chemists see this development as a milestone in the field of drug synthesis, not only because it could speed up the drug discovery process, but also because it is one of the most efficient procedures currently available for using AI to mark potential reaction routes.
The new AI tool uses deep learning neural networks to learn all known single-step organic chemical reactions - about 12.4 million. This allows it to predict the chemical reactions that can be used in any single step. AI tools repeatedly apply these neural networks to plan multi-step synthesis, deconstructing the desired molecules until they finally get a usable reagent.
In March 2018, Grzybowski, a chemist at the National Institute of Science and Technology in Ulsan, South Korea, reported that he had tested eight chemical reaction pathways suggested by the new AI tool algorithm developed by Segler's team in the lab, and all were successful.
3. Guiding principle
1) Simplifications of the target molecule.[2]
2) Maximize convergency.
3) Minimize the complexity of forward reaction.
4) Priority is given to the formation of a carbon skeleton, as shown in Figure 1:The formation of carbon skeleton is given priority because the skeleton is the carrier of functional groups, and only when the skeleton is established first, the functional groups have a destination. When considering the formation of the skeleton, attention should be paid to the impact of functional groups on the skeleton.
5) The carbon-heteroatomic bond is preferentially disassembled, as shown in Figure 2:The chemical bond connecting heteroatoms is usually unstable, and it is easy to synthesize and break in the process of synthesis, so the carbon-heteroatom bond should be synthesized as far as possible in the process of synthesis, which is why the reverse synthesis gives priority to breaking the carbon-heteroatom bond.
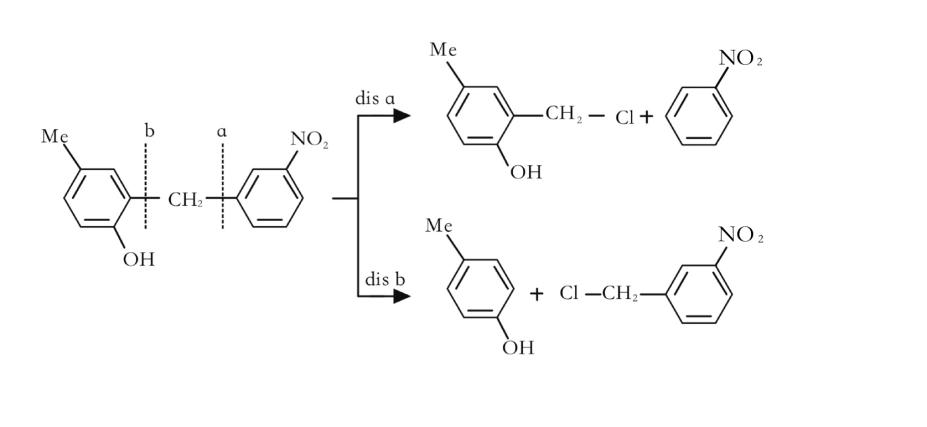
6) Disconnect preferentially at the functional group position, as shown in Figure 3:Functional groups, which are the most active part of the molecule and where bonds are prone to break, should also be given priority in inverse synthesis analysis.
4. Type of structural transformation
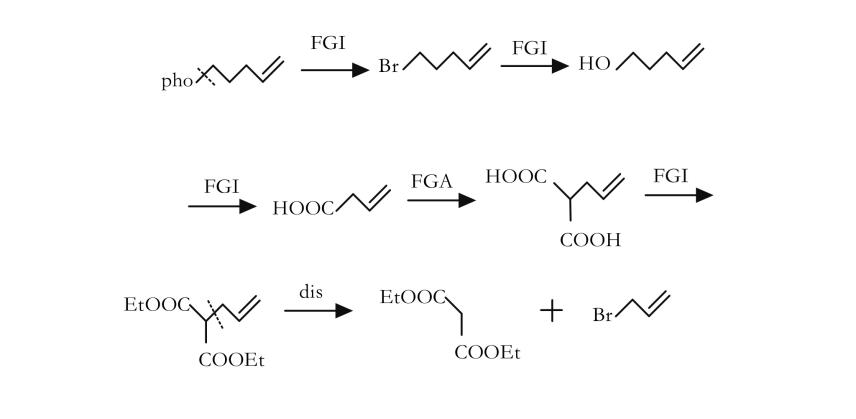
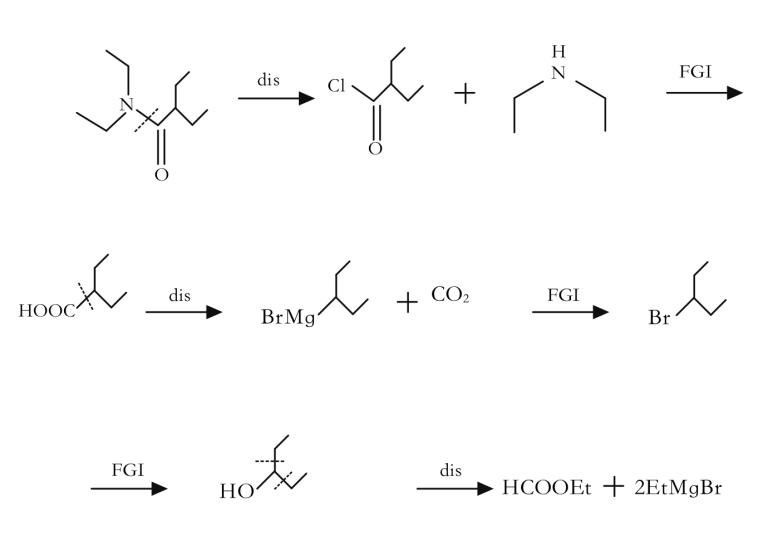
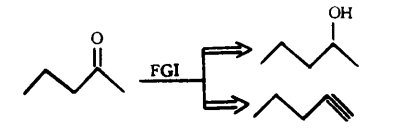
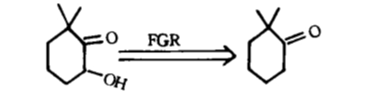
Fine tuning: the reverse transformation of functional groups is a method of changing the nature or position of functional groups by interchanging (as shown in Figure 4), adding (as shown in Figure 5), or removing (as shown in Figure 6) functional groups without changing the basic skeleton of the target molecule.
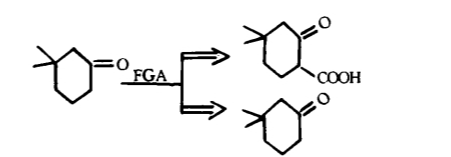
Reverse cutting / ligation (as shown in Figure 7) or rearrangement (as shown in Figure 8): In a synthesis design, reverse functional group conversion can be used to replace target molecules that are easier to prepare during synthesis. At the same time, in the reverse cutting, connection or rearrangement, it is necessary to convert the originally inappropriate functional groups on the target molecules into the desired form, or temporarily add some necessary functional groups. These added functional groups have the function of activation, protection or blocking, thereby enhancing the regioor stereoselectivity of the chemical reaction.
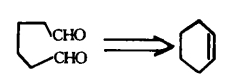
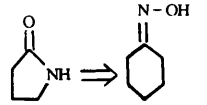
5. Application of retrosynthesis: the case of indole
To illustrate the application of retrosynthesis, let's consider the synthesis of indole, a core structure in many biologically active compounds and pharmaceuticals. Indole is an aromatic heterocycle containing a fused benzene and pyrrole ring. Its synthesis can be approached in various ways, but retrosynthetic analysis helps identify the most efficient route.
Step 1: Identifying Key Bonds
The first step in retrosynthesis involves identifying key bonds within the indole structure that can be broken to simplify the molecule. One such bond is the C2-N1 bond in the pyrrole ring.
Step 2: Disconnection to Simplify
By breaking the C2-N1 bond, we can conceptually divide the indole into a benzene derivative and a pyrrole derivative. This disconnection suggests that the synthesis can proceed by forming the pyrrole ring onto a benzene derivative.
Step 3: Synthesizing Precursors
Next, we consider how to synthesize the identified precursors. One common approach is to start with o-nitrotoluene as the benzene derivative. This compound is commercially available and can be reduced to o-toluidine, which is a precursor for forming the pyrrole ring.
Step 4: Constructing the Pyrrole Ring
To form the pyrrole ring, we can use the Fischer indole synthesis [3]. In this method, phenylhydrazine reacts with a ketone or aldehyde to form an intermediate, which undergoes cyclization to produce the indole structure. In our case, o-toluidine can react with a suitable aldehyde to form an intermediate that cyclizes to yield indole.
Example Synthesis Route for Indole
1) Starting Material: o-Nitrotoluene
2) Reduction: Reduce o-nitrotoluene to o-toluidine.
3) Formation of Hydrazone: React o-toluidine with a suitable aldehyde, such as acetaldehyde, to form a hydrazone intermediate.
4) Cyclization: Heat the hydrazone intermediate to induce cyclization, forming the indole ring system.
Practical Applications of the Fischer Indole Synthesis
Total Synthesis of (+-)-Deethylibophyllidine: J. Bonjoch and co-workers used a regioselective Fischer indole synthesis to obtain octahydropyrrolo[3,2-c] carbazoles, followed by a Pummerer rearrangement-thionium ion cyclization to generate a quaternary spiro stereocenter.
6. Application of retrosynthesis: synthesis of ibuprofen
Ibuprofen is a widely used nonsteroidal anti-inflammatory drug (NSAID). Retrosynthetic analysis helps design an efficient route for its synthesis by simplifying its complex structure into smaller and more easily accessible starting materials.
Step 1: Identifying Key Bonds
In the ibuprofen structure, one key disconnection involves the Cα-Cβ bond in the alkyl chain connected to the aromatic ring. This disconnection helps break down the molecule into two components: a substituted benzene derivative and an aliphatic side chain.
Step 2: Disconnection
By breaking the Cα-Cβ bond, we can identify two simpler components: isobutylbenzene and acetic acid derivatives. This approach suggests that the synthesis can begin with the formation of the carboxyl group and the construction of the side chain.
Step 3: Synthesizing Precursors
The isobutylbenzene can be synthesized through Friedel-Crafts alkylation using benzene and isobutylene as starting materials. The carboxyl group can be introduced using a Grignard reaction, where the side chain is further functionalized with a carboxyl group from acetic acid.
Example Synthesis Route for Ibuprofen[4]
1) Starting Material: Isobutylbenzene
2) Friedel-Crafts Alkylation: Benzene reacts with isobutylene in the presence of a catalyst (AlCl₃) to form isobutylbenzene.
3) Grignard Reaction: The isobutyl group is functionalized to introduce a carboxyl group.
4) Oxidation: Finally, oxidation of the alcohol intermediate leads to the formation of ibuprofen.
7. Application of retrosynthesis: synthesis of penicillin V
Penicillin V is an important antibiotic in the β-lactam family. Retrosynthesis can be used to simplify its synthesis by breaking down its structure into key intermediates, such as the thiazolidine ring and β-lactam ring.
Step 1: Identifying Key Bonds
The critical bond in penicillin V is the amide bond that connects the thiazolidine and β-lactam rings. Disconnection of this bond allows the retrosynthetic pathway to focus on forming these two rings separately before coupling them.
Step 2: Disconnection
By disconnecting the amide bond, the synthesis is divided into the formation of the β-lactam ring and the synthesis of the thiazolidine ring. The disconnection reveals that the thiazolidine ring can be formed from L-cysteine and the β-lactam from a dipeptide precursor.
Step 3: Synthesizing Precursors
L-cysteine serves as a precursor for the thiazolidine ring, while a properly protected dipeptide precursor can be used to form the β-lactam ring.
Example Synthesis Route for Penicillin V [5]
1) Starting Material: L-cysteine
2) Thiazolidine Formation: L-cysteine reacts with a thioester to form the thiazolidine ring.
3) β-lactam Formation: A dipeptide is cyclized to form the β-lactam ring.
4) Coupling: The thiazolidine and β-lactam rings are then coupled to form penicillin V.
8. Application of retrosynthesis: synthesis of morphine
Morphine is a complex alkaloid used in pain management. The retrosynthetic analysis of morphine involves identifying strategic disconnections that break it into simpler subunits.
Step 1: Identifying Key Bonds
Morphine has a polycyclic structure, with the key disconnection being the C4-C5 bond between the isoquinoline and cyclohexene rings. This allows for the molecule to be divided into simpler aromatic and aliphatic units.
Step 2: Disconnection
The C4-C5 disconnection breaks morphine into a benzylic precursor and a cyclohexene derivative. These two parts can be synthesized separately and then joined to form the desired polycyclic structure.
Step 3: Synthesizing Precursors
The benzyl component can be derived from simple aromatic precursors such as phenol derivatives, while the cyclohexene component can be built from an enol or enone system.
Example Synthesis Route for Morphine [6]
1) Starting Material: Phenol derivative
2) Aromatic Precursors: The aromatic ring is functionalized to introduce the required substituents.
3) Cyclohexene Formation: The aliphatic part is synthesized through cyclization of an enone.
4) Coupling and Cyclization: The two fragments are coupled, followed by cyclization to yield the morphine structure.
9. Conclusion
Retrosynthesis provides a strategic framework for designing synthetic routes to complex molecules. By breaking down a target molecule like indole into simpler precursors and constructing a logical synthetic pathway, chemists can efficiently achieve their synthetic goals. The Fischer indole synthesis, in particular, highlights the ingenuity and enduring relevance of classical synthetic methods in modern chemistry.
Retrosynthesis analysis is a powerful tool, which is of great significance to organic synthesis chemists. It can help us better understand the nature of organic reactions, improve the efficiency of synthesis, and expand the possibilities of organic synthesis. Through continuous learning and practice, we can gradually master the skills of Retrosynthesis analysis and make greater contributions to the research of organic synthesis.
References
[1]. Corey E J, Cheng Xue-Min, The logic of chemical synthesis.John Wiley & Son, 1989.
[2]. Kurti, Laszlo; Czako, Barbara. Strategic Applications of Named Reactions in Organic Synthesis. Academic Press, 2005-3-18
[3]. Zhu Guanhua, Yu Liangmin, Zhang Qi. Investigation on the mechanism of Fischer index synthesis method and its application progress. Guangzhou Chemical Industry-2012, Issue 017
[4]. Richard A Kjonaas, Peggy E Williams, David A Counce, Lindsey R Crawley. Synthesis of ibuprofen in the introductory organic laboratory. Journal of Chemical Education 88 (6), 825-828, 2011
[5]. Ch L Cooney, F Acevedo. Theoretical conversion yields for penicillin synthesis. Biotechnology and bioengineering 19 (10), 1449-1462, 1977
[6]. Kenji Uchida, Satoshi Yokoshima, Toshiyuki Kan, Tohru Fukuyama. Total synthesis of (±)-morphine. Organic letters 8 (23), 5311-5313, 2006
Cite this article
Fu,Q. (2025). Retrosynthesis: A Strategy for Organic Synthesis. Applied and Computational Engineering,208,32-39.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Corey E J, Cheng Xue-Min, The logic of chemical synthesis.John Wiley & Son, 1989.
[2]. Kurti, Laszlo; Czako, Barbara. Strategic Applications of Named Reactions in Organic Synthesis. Academic Press, 2005-3-18
[3]. Zhu Guanhua, Yu Liangmin, Zhang Qi. Investigation on the mechanism of Fischer index synthesis method and its application progress. Guangzhou Chemical Industry-2012, Issue 017
[4]. Richard A Kjonaas, Peggy E Williams, David A Counce, Lindsey R Crawley. Synthesis of ibuprofen in the introductory organic laboratory. Journal of Chemical Education 88 (6), 825-828, 2011
[5]. Ch L Cooney, F Acevedo. Theoretical conversion yields for penicillin synthesis. Biotechnology and bioengineering 19 (10), 1449-1462, 1977
[6]. Kenji Uchida, Satoshi Yokoshima, Toshiyuki Kan, Tohru Fukuyama. Total synthesis of (±)-morphine. Organic letters 8 (23), 5311-5313, 2006









