1. Introduction
1.1. Concept
Retrosynthetic analysis concept’s proposal has completely changed the way organic chemists design synthesis routes. It is not merely a simple reverse derivation method but a systematic strategy that helps chemists methodically dismantle structures and search for feasible starting materials and reaction steps when faced with complex molecular structures. This approach has greatly enhanced the efficiency of synthesis route design and reduced the number of blind attempts, playing an irreplaceable role in promoting the development of the field of organic chemical synthesis.
1.2. Historical review
E.J. Corey proposed the concept of retrosynthetic analysis in the 1960s, which brought a huge transformation to the field of organic synthesis. He not only put forward this concept but also developed and perfected the method system of retrosynthetic analysis through a large amount of research work. Corey’s work provided organic chemists with a brand-new thinking framework, making the synthesis of complex natural products and other molecules more methodical. His contributions are not only reflected in theoretical innovation but also in guiding numerous practical synthesis cases, setting an example for later generations and greatly promoting the transformation of organic synthesis chemistry from traditional empirical exploration to scientific and systematic design [1].
2. Basic concepts of retrosynthetic analysis
2.1. Disconnection
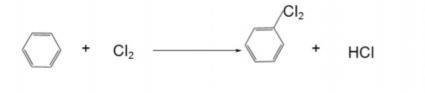
Bond disconnection in a molecule is the reverse process of bonding. Chemical bond disconnection is not arbitrary and requires comprehensive consideration of multiple factors such as molecular structure, reaction activity, and the feasibility of subsequent synthesis. For example, in some complex polycyclic systems, choosing to break a specific carbon - carbon bond may simplify subsequent synthesis steps because it may generate relatively stable and easily accessible synthons. At the same time, different types of chemical bonds, such as carbon - oxygen bonds and carbon - nitrogen bonds, have their own characteristics in terms of disconnection methods and conditions, and chemists need to make accurate judgments based on specific situations [2]. As shown in Figure 1, the process of bond disconnection can be visualized.

2.2. Synthon
A synthon is a hypothetical molecular fragment created when the chemical bonds of a target molecule are broken. It is an abstract concept in retrosynthetic analysis but has strong practicality. These fragments, although they may not exist independently in actual reactions, can simulate the reaction behavior of synthons by finding suitable reagents or reaction conditions, thereby realizing the construction of the target molecule. For example, when designing the synthesis route of some molecules with specific functional groups, identifying key synthons can guide chemists to select appropriate starting materials and reaction steps, making the synthesis process more efficient and targeted.As shown in Figure 2, the relationship between synthons and synthetic reactions is demonstrated[3].
2.3. Synthetic equivalent
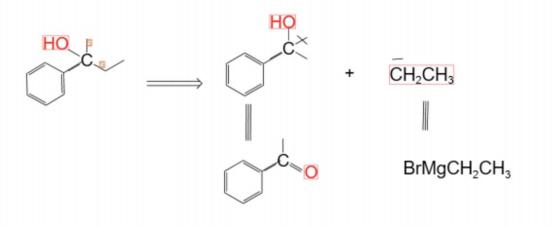
A corresponding reagent capable of acting as a synthon. Selecting a suitable synthetic equivalent is a crucial step in retrosynthetic analysis. In addition to matching the reactivity of the synthon, factors such as its stability, cost, availability, and adaptability to reaction conditions also need to be considered. For example, in industrial synthesis, synthetic equivalents that are inexpensive and easy to prepare are often preferred. Even if their reactivity may not be the highest, efficient conversion can still be achieved by optimizing the reaction conditions. At the same time, for some synthetic equivalents that are sensitive to reaction conditions, precise control of the reaction environment is required to ensure that they can react as expected to construct the target molecule.[4]As shown in Figure 3, an example of alcohol severing and its related synthetic equivalent is presented.
2.4. Function Group Intercharge (FGI)
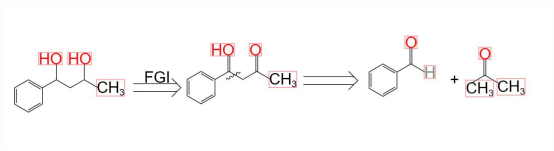
Function group intercharge plays an important role in retrosynthetic analysis. It provides chemists with a flexible means to adjust molecular structures. By converting one functional group into another, the synthesis route can be optimized and diversified. For example, in some cases, converting a difficult - to - react functional group into a more reactive one can make subsequent synthesis steps easier. Functional group conversion can also be used to introduce or remove specific functional groups to meet the structural requirements of the target molecule or improve its physical and chemical properties, thereby enhancing the feasibility and efficiency of synthesis.As shown in Figure 4, an example of FGI is illustrated [5].
2.5. Function Group Addition (FGA)
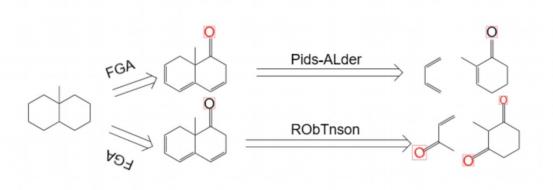
Function group addition is an important strategy for constructing complex molecules. Taking the synthesis of 9 - methyl - octahydronaphthalene as an example, through appropriate functional group addition steps, key methyl and other functional groups can be introduced, laying the foundation for subsequent cyclization reactions and other complex structure constructions. Functional group addition can not only change the chemical properties of molecules but also affect their stereochemical characteristics, which is of great significance for synthesizing complex molecules with specific stereoconfigurations. In the field of drug synthesis, precisely controlling the position and type of functional group addition can achieve the regulation of drug activity and selectivity, improving the efficacy and safety of drugs. As shown in Figure 5, the synthesis of 9 - methyl - octahydronaphthalene with function group addition is shown [6].
2.6. Reconnection
Reconnection involves joining two appropriate carbon atoms in the target molecule to form a new chemical bond, resulting in a synth that is easy to further split.As shown in Figure 6, the reconnection of adipic aldehyde is demonstrated.
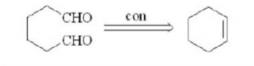
3. Steps of retrosynthetic analysis
1. According to the structural characteristics of the molecule, a chemical bond is cut to produce synthons
2. Find the reagent or synthetic equivalent corresponding to the synthon
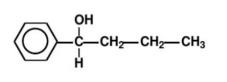
3. Write the synthesis route and the synthesis reaction conditions of each step according to the retrosynthetic analysis.As shown in Figure 7, for the molecule 1- phenyl -1- butanol, a chemical bond is cut to produce synthons.
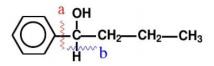
(1):According to the structural characteristics of the molecule, a chemical bond is cut to produce synthons.As shown in Figure 8.
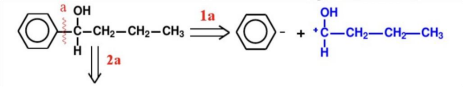
(2):Find the reagent or synthetic equivalent corresponding to the synthon
Method A: Breaking of the Ph-C bond.As shown in Figure 9, the synthetic route of Ph-C bond break is presented.
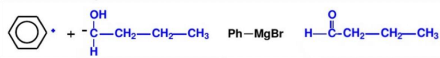
Method B: Breaking of the C-H bond. As shown in Figure 10, the synthetic route of C - H bond break is shown.
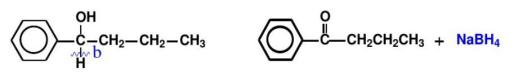
Phenylbutanone is not a direct raw material, and its retrosynthetic analysis is as follows(as shown in Figure 11):
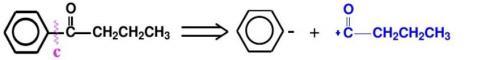
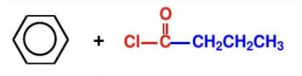
(3):Write the synthesis route and the synthesis reaction conditions of each step according to the retrosynthetic analysis
Method A: Breaking of the Ph-C bond.As shown in Figure 12, the synthesis route of Ph - C bond break with reaction conditions is provided.
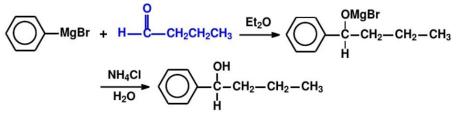
Method B: Breaking of the C-H bond.As shown in Figure 13, the synthesis route of C - H bond break with reaction conditions is presented.
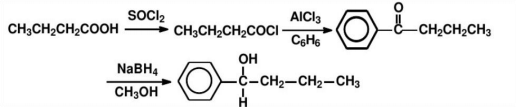
4. Severing chemical bonds follows the main principle
4.1. Cut at the functional group
Function group addition is an important strategy for constructing complex molecules. Taking the synthesis of 9 - methyl - octahydronaphthalene as an example, through appropriate functional group addition steps, key methyl and other functional groups can be introduced, laying the foundation for subsequent cyclization reactions and other complex structure constructions. Functional group addition can not only change the chemical properties of molecules but also affect their stereochemical characteristics, which is of great significance for synthesizing complex molecules with specific stereoconfigurations. In the field of drug synthesis, precisely controlling the position and type of functional group addition can achieve the regulation of drug activity and selectivity, improving the efficacy and safety of drugs[7].As shown in Figure 14, using the severing of the Diels - Alder reaction at the functional group is demonstrated, and Figure 15 shows the corresponding synthetic route.
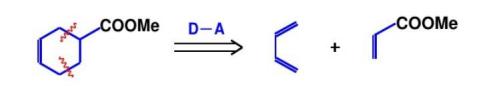
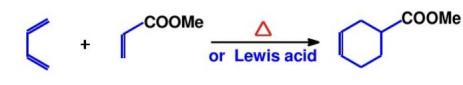
4.2. Cut at the heteroatom
Cutting chemical bonds at the heteroatom is a common strategy with certain advantages. The electron cloud distribution around the heteroatom is usually different from that of carbon atoms, which gives the chemical bond at the heteroatom unique reactivity. For example, in the synthesis of some nitrogen - containing heterocyclic compounds, cutting the chemical bond near the nitrogen atom can conveniently introduce or construct other functional groups, thus realizing the diversification of molecular structures. However, cutting chemical bonds at the heteroatom may also face some limitations. For example, the heteroatom may participate in other side reactions, or special protection and deprotection treatments may be required for the heteroatom in subsequent synthesis steps, increasing the complexity of the synthesis. Therefore, when choosing to cut the chemical bond at the heteroatom, various factors need to be comprehensively considered to weigh the pros and cons.As shown in Figure 16, an example of cutting at the heteroatom is presented.
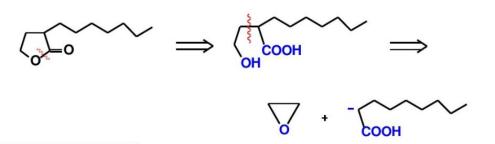
4.3. The asymmetric molecules are changed to symmetrical molecules, and the molecular symmetry is cut off
Transforming an asymmetric molecule into a symmetric molecule and cutting off the molecular symmetry is a clever synthesis strategy. Taking the synthesis of tropinone as an example, by simultaneously cutting multiple chemical bonds, the asymmetric structure is converted into a symmetric precursor molecule, and then subsequent synthesis steps are carried out, which can simplify the synthesis route and improve the synthesis efficiency. However, this strategy also faces some challenges in practical applications. For example, the synthesis of the symmetric precursor molecule may require specific reaction conditions and starting materials, and in the process of converting the symmetric precursor molecule into the target asymmetric molecule, precise control of the reaction selectivity is required to avoid the production of unwanted isomers. Chemists need to skillfully apply this strategy according to the specific molecular structure and synthesis requirements and overcome the possible difficulties.As shown in Figure 17, the synthesis of tropinone with multiple bond severing is illustrated.
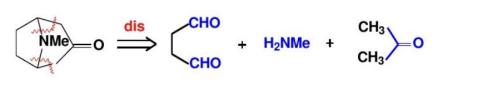
5. Latest progress and future direction
In the future, with advances in synthesis techniques, computational tools and machine learning methods, retrosynthetic analysis is expected to further improve its efficiency and reliability in organic synthesis. At the same time, interdisciplinary collaboration and innovation will drive the wide application of inverse synthetic analysis in drug design, materials science and the environment, providing new solutions and perspectives for solving complex chemical problems in the real world [8].
6. Conclusion
In summary, as a powerful organic synthesis design strategy, retrosynthetic analysis has demonstrated its unique value and broad application prospects in chemical research and application. As an important means of structure identification, the development and improvement of inverse synthesis analysis will continue to promote the progress in the field of analytical chemistry. In the future, through the innovation of technology and the integration of methods, the problem of structure confirmation of complex samples can be solved more effectively.
References
[1]. Corey, E. J. Retrosynthetic analysis: A powerful strategy for organic synthesis design [J]. Accounts of Chemical Research, 1989, 22(5): 455 - 461.
[2]. Smith, J. D. Strategies for Bond Disconnection in Organic Synthesis [M]. Springer, 2015.
[3]. Zhang Min, Guo Chun. Design Strategies of Organic Synthesis Based on Synthons [J]. Chinese Journal of Organic Chemistry, 2018, 38(12): 3191 - 3200.
[4]. Brown, A. L. Synthetic Equivalents: Selection and Application [J]. Journal of Organic Chemistry, 2017, 82(10): 5012 - 5021.
[5]. Li Hua, Wang Qiang, Zhao Gang. Research Progress on the Interconversion of Functional Groups in Organic Synthesis [J]. Progress in Chemistry, 2020, 32(3): 456 - 468.
[6]. Liu Mei, Sun Hao, Chen Jie. Application of Functional Group Addition in the Total Synthesis of Complex Natural Products [J]. Chemical Journal of Chinese Universities, 2019, 40(10): 1985 - 1992.
[7]. Wang Xia, Zhou Yong, Li Ming. Optimization of Organic Synthesis Strategies Based on the Diels - Alder Reaction [J]. Chemical Research and Application, 2021, 33(8): 1456 - 1462.
[8]. Williams, P. J. Retrosynthetic Analysis: Applications and Future Directions in Interdisciplinary Fields [J]. Advanced Synthesis & Catalysis, 2022, 364(1): 1 - 15.
Cite this article
Zhou,Z. (2025). Introduction to Retrosynthetic Analysis. Applied and Computational Engineering,208,40-47.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Corey, E. J. Retrosynthetic analysis: A powerful strategy for organic synthesis design [J]. Accounts of Chemical Research, 1989, 22(5): 455 - 461.
[2]. Smith, J. D. Strategies for Bond Disconnection in Organic Synthesis [M]. Springer, 2015.
[3]. Zhang Min, Guo Chun. Design Strategies of Organic Synthesis Based on Synthons [J]. Chinese Journal of Organic Chemistry, 2018, 38(12): 3191 - 3200.
[4]. Brown, A. L. Synthetic Equivalents: Selection and Application [J]. Journal of Organic Chemistry, 2017, 82(10): 5012 - 5021.
[5]. Li Hua, Wang Qiang, Zhao Gang. Research Progress on the Interconversion of Functional Groups in Organic Synthesis [J]. Progress in Chemistry, 2020, 32(3): 456 - 468.
[6]. Liu Mei, Sun Hao, Chen Jie. Application of Functional Group Addition in the Total Synthesis of Complex Natural Products [J]. Chemical Journal of Chinese Universities, 2019, 40(10): 1985 - 1992.
[7]. Wang Xia, Zhou Yong, Li Ming. Optimization of Organic Synthesis Strategies Based on the Diels - Alder Reaction [J]. Chemical Research and Application, 2021, 33(8): 1456 - 1462.
[8]. Williams, P. J. Retrosynthetic Analysis: Applications and Future Directions in Interdisciplinary Fields [J]. Advanced Synthesis & Catalysis, 2022, 364(1): 1 - 15.









