1. Introduction
1.1. Background
1.1.1. Introduction to uvarialeptone B
Uvarialeptone B is an organic compound extracted from Uvaria leptopoda, which is a plant growing in the rainforest in Thailand. Uvaria has been used in medicine. For instance, the roots of U. cherrevensis were used to deal with urinary disorders. In addition, the stem of U. dulcis and the roots of U. rufa were used to deal with fever and kidney failure.
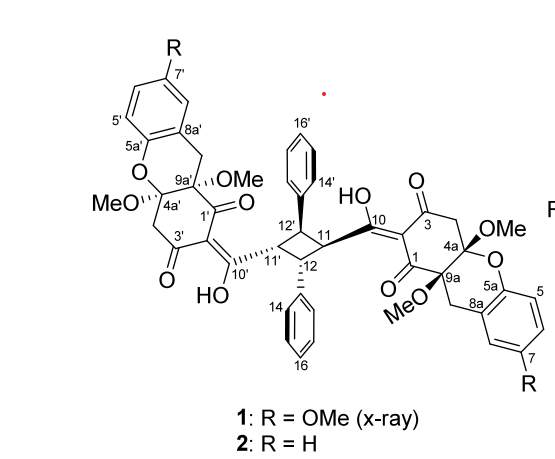
Specifically, uvarialeptone B (2) is a kind of yellow powder. Uvarialeptone B was found to be toxic to SNU-1 human gastric cancer cells and has the ability to perform anti-inflammatory activities [1]. The value of refractivity of uvarialeptone B is 219.11 m³·mol⁻¹, and the value of polarizability is 83.05 ų [2].
The chemical formula of uvarialeptone A (1) and B (2) is listed below. (F1)
1.1.2. Introduction to Fleming-Tamao Oxidation
A particular reaction named Fleming-Tamao Oxidation is going to be used in the rest of the study. It involves converting carbon-silicon bounds to carbon-oxygen bonds, using peroxy acid and hydrogen peroxide. In this part, this reaction mechanism of Fleming-Tamao Oxidatrion will be listed.
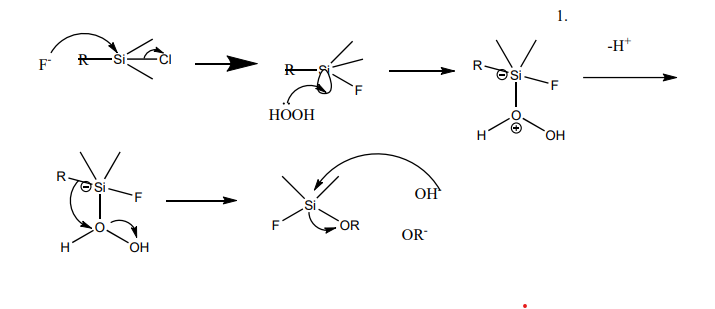
As Figure 2 indicates, a fluorine anion attacks the silicon connecting with F, Cl, OR, H, and NR2, forming a silicon anion. Then, hydrogen peroxide is added, and the lone pair electrons of hydrogen peroxide can get into the empty orbit of the silicon and be connected. Then, deprotonation takes place.
1.2. Objective
Uvarialeptone B was extracted from plants directly, which means the yield tends to be low and cannot be applied to the industry. In order to lower the cost of manufacturing uvarialeptone B and enhance productivity, the discovery of a chemical synthetic method is necessary.
2. Synthetic method
2.1. Retrosynthesis of uvarialeptone B
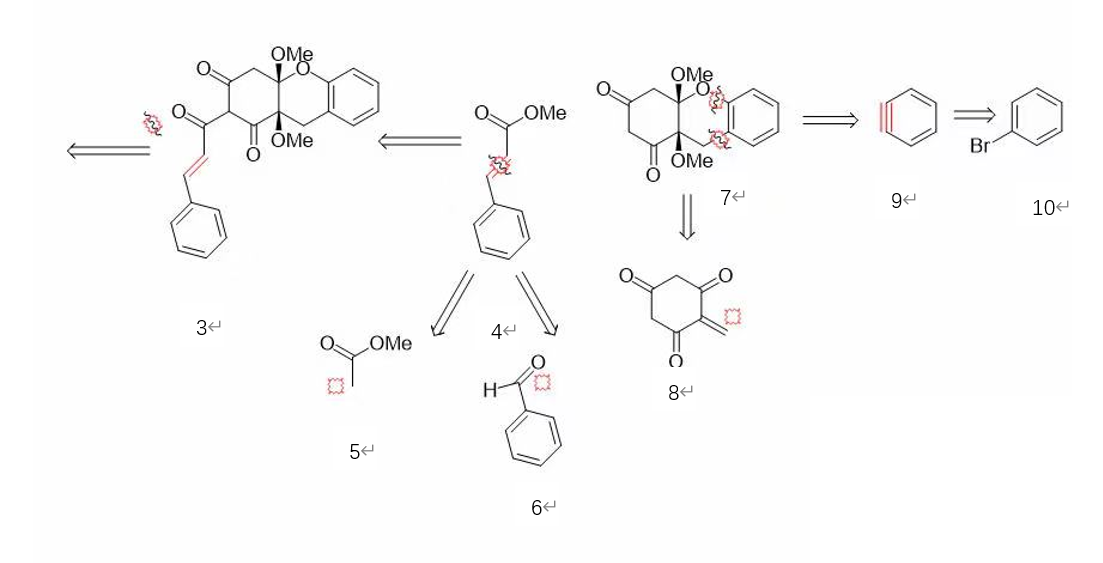
The chart (F3) above illustrates the process of the retrosynthesis of uvarialeptone B. By observing the structure of the molecule; it is found that the molecule is symmetry about the four-element ring at the middle, so the four-element ring can disconnect the whole molecule, while a double bound is obtained at the middle of the molecule 3. After the disconnection, molecule 3 can also be disconnected into two molecules, which are 4 and 7. The molecule 4 is possible to be broken into molecule 5 and 6. For molecule 7, it is able to be disconnected into a four elements ring, connecting with three carbonyl groups and a carbon connected with a double bound, which is molecule 8, and a benzene with a triple bound, which is molecule 9. The molecule 9 can be obtained from a benzene connected with a leaving group.
2.2. Synthesis of uvarialeptone B
There are three main stages in the process.
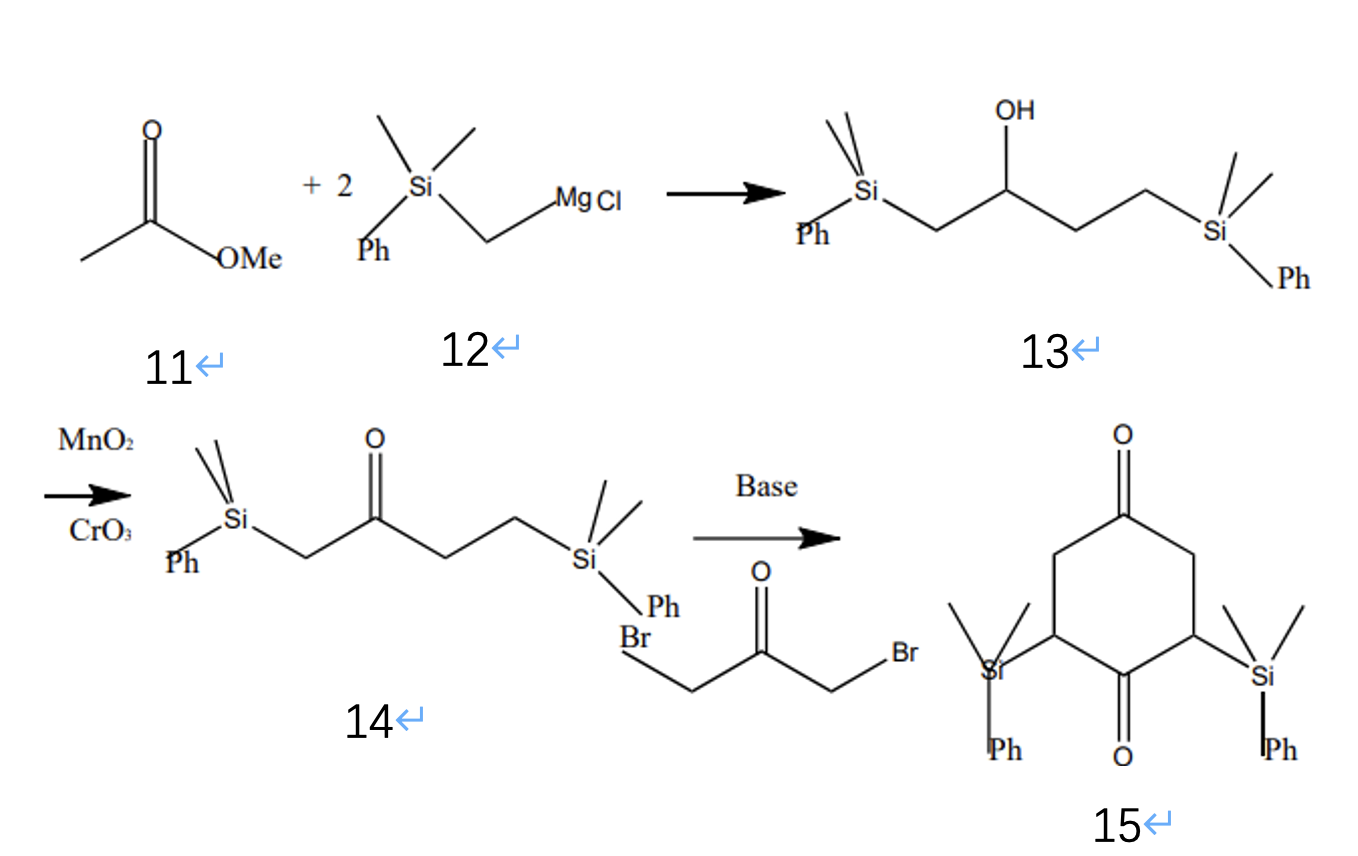
In the first stage of the process, as figure 4 indicates, the first step of this reaction is that molecule 11 can react with molecule 12 and form 13. After the reaction, the molecule can be oxidized using manganese dioxide and chromium dioxide as oxidizing agents, while a base and a ketone connecting with two leaving groups (bromine) are added and form the molecule 15.
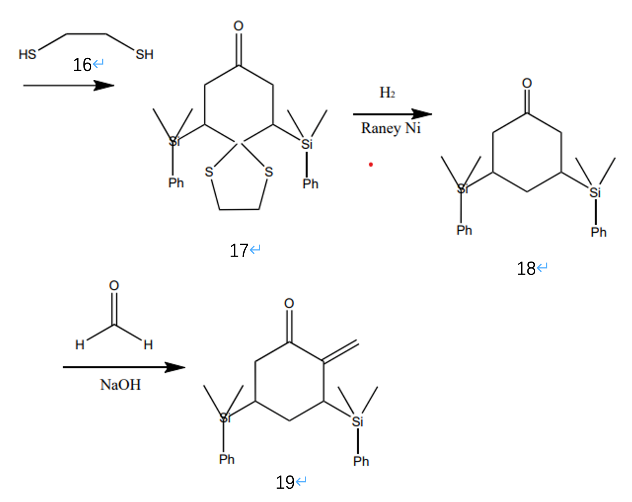
The bottom of molecule 15 has a larger hindrance than the top. As Figure 5 illustrates, to remove the carbonyl group, molecule 16 can be used to protect the carbonyl at the bottom of molecule 15 and form molecule 17. After the deprotection using hydrogen and Raney Ni, a carbon with a double-bound can be added to the α carbon on molecule 18 and form molecule 19 by reacting with formaldehyde and sodium hydroxide.
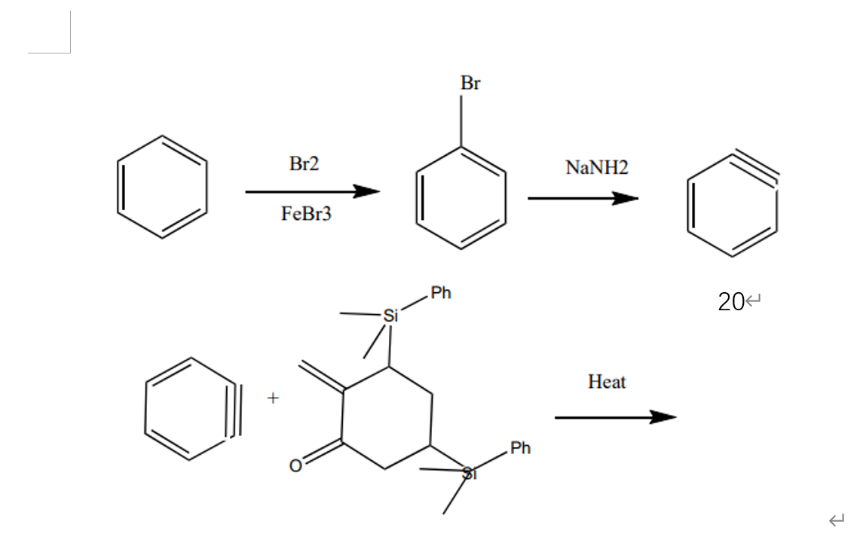
Enter the second stage, it begins with the benzene reacting with iron (III) bromide and bromine and bromine, as a leaving group is connected on the benzene. Then, the benzene connected with a bromine can do elimination and form a triple bound in the basic environment. The last step of this stage is that molecules 20 and 19 do Diels-Alder Reaction and form a six elements ring. (F6, F7) [3]
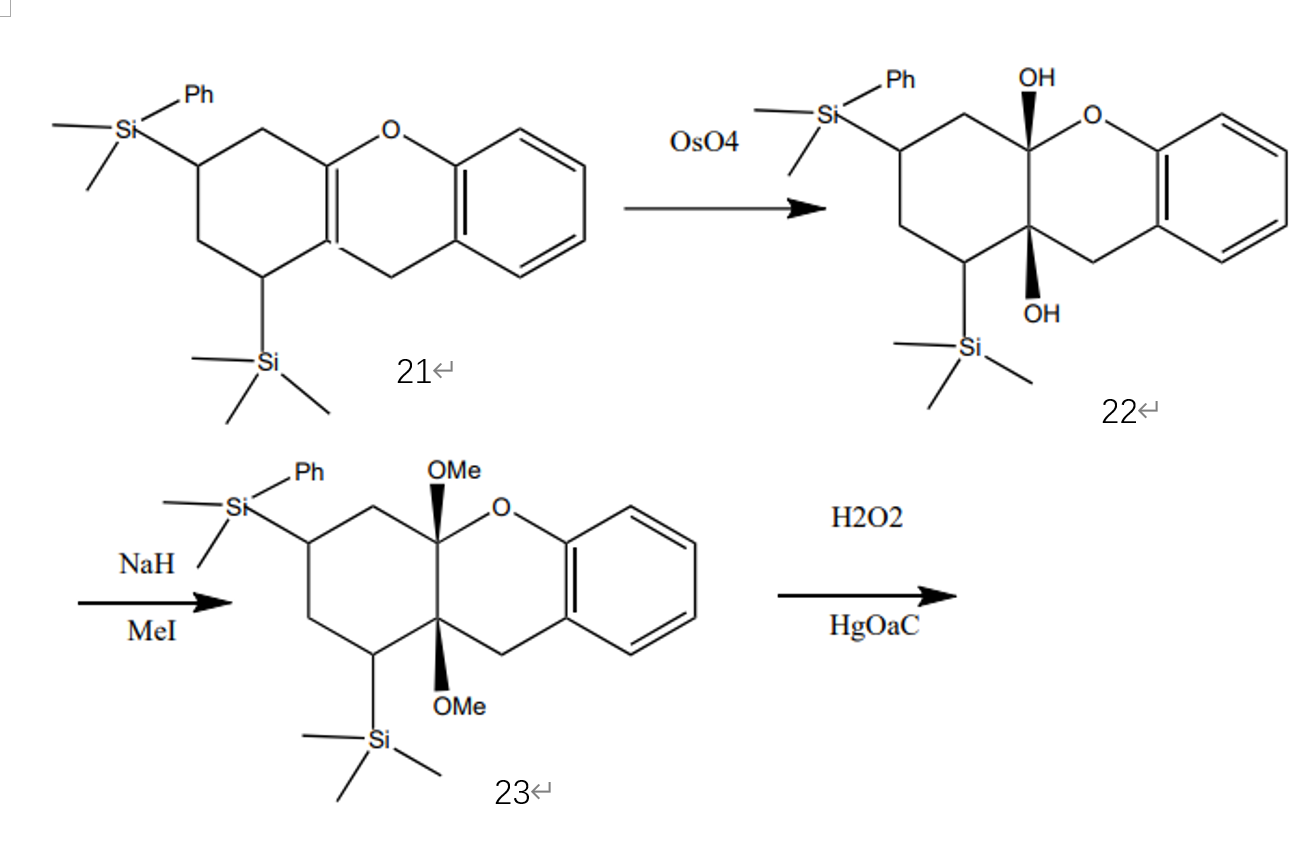
In the final stage, as Figure 7 indicates, molecule 21 reacts with osmium (VIII) oxide and connects with two hydroxyl groups. Then, molecule 22 can react with sodium hydride and methyl iodide and remove the two hydroxyls with two methoxy groups. Next, molecule 23 can do Tamao-Fleming oxidation and form molecule 24. (F7, F8) [4]
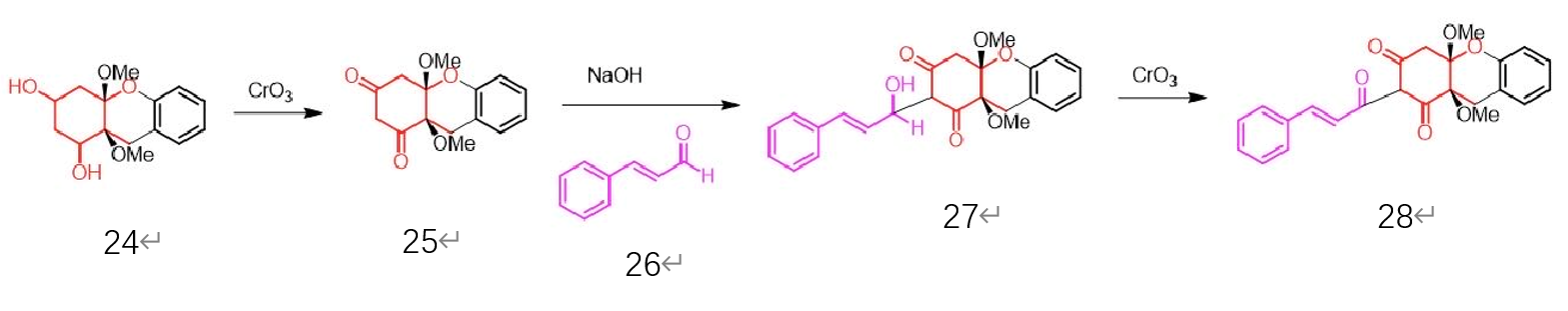
The two hydroxyl groups in molecule 24 can then be oxidized using chromium (VI) oxide and be replaced by two carbonyl groups. In the basic condition, molecule 26 can then react with molecule 25 and be connected together. After the oxidation using chromium (VI) oxide, the molecule 28 is formed.
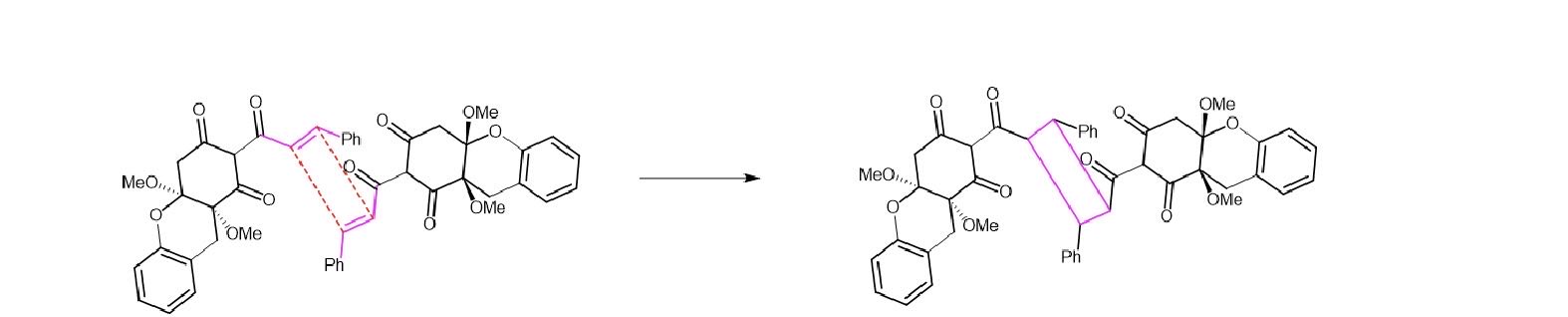
Two molecules 8 are taken, and the double bound at the middle can do Diels-Alder reaction, while the target molecule uvarialeptone B is formed.
3. Conclusion
In this study, a retrosynthetic analysis and a synthetic method are found and listed in the paper. Some traditional reactions, such as Diels–Alder reaction and Fleming-Tamao Oxidation are involved in this study. Comparing to the existing extractive technique, the synthetic method listed in this paper does not involve the use of the plant Uvaria leptopoda. The synthetic method in this study can be applied in the medicine industry to improve the productivity of uvarialeptone B. The weakness of the synthetic method is that there are too many steps, so the yield might not be very high. In the future studies, synthetic methods with less steps should be invited.
References
[1]. Tetrahydroxanthene-1, 3(2H)diones and Oxidized Hexadiene Derivatives from Uvaria leptopoda and Their Biological Activities https: //pubmed.ncbi.nlm.nih.gov/38805684/
[2]. NP-MRD Showing NP-Card of Uvarialeptone B (NP0333241) https: //np-mrd.org/natural_products/NP0333241
[3]. Wikipedia, Diels–Alder reaction https: //en.wikipedia.org/wiki/Diels%E2%80%93Alder_reaction
[4]. Wikipedia, Fleming-Tamao Oxidationhttps: //en.wikipedia.org/wiki/Fleming%E2%80%93Tamao_oxidation
Cite this article
Liu,J. (2025). Chemical Synthesis of Uvarialeptone B. Applied and Computational Engineering,208,48-53.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Tetrahydroxanthene-1, 3(2H)diones and Oxidized Hexadiene Derivatives from Uvaria leptopoda and Their Biological Activities https: //pubmed.ncbi.nlm.nih.gov/38805684/
[2]. NP-MRD Showing NP-Card of Uvarialeptone B (NP0333241) https: //np-mrd.org/natural_products/NP0333241
[3]. Wikipedia, Diels–Alder reaction https: //en.wikipedia.org/wiki/Diels%E2%80%93Alder_reaction
[4]. Wikipedia, Fleming-Tamao Oxidationhttps: //en.wikipedia.org/wiki/Fleming%E2%80%93Tamao_oxidation









