1. Introduction
The synthesis of complex natural products remains one of the most challenging yet rewarding endeavors in organic chemistry. Discovering a simpler way to synthesize a globally used compound such as Penicillin V or morphine would save the drug industry millions of dollars, while making these compounds more accessible to everyone who needs them.
Peniapyrone E was one of 9 “Five cyclopenta[d]pyrano[4,3-b]pyran-1,7(6H)-dione 6/6/5-fused tricyclic ring system containing metabolites” isolated from the fungus Penicillium brefeldianum [1]. Peniapyrone E, among others, showed “cytotoxic activity against AsPC-1, CRL-2234, and MCF-7 cancer cell lines”, giving reason for a simplistic synthesis pathway to be developed for it [2].
The retrosynthetic steps in this paper are taken after careful consideration of oxidation states, dioxygenation patterns, and the resulting products(or rather, potential reactants) [3-5].
2. Retrosynthetic analysis
To begin the retrosynthetic process, we first examine the target molecule, Peniapyrone E, shown in Figure 1.
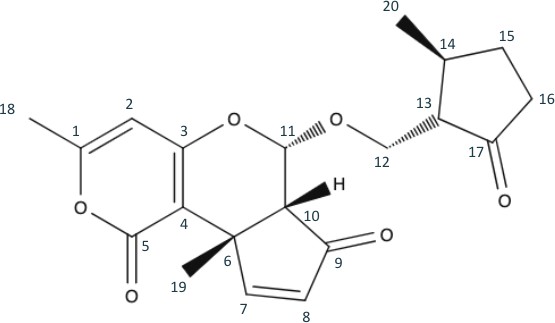
Upon first observation, we can easily see that Peniapyrone E consists of two main parts: the complex triple-ring structure to the left, and the 3,methylcyclopentanone structure to the right. The logical retrosynthetic step is to separate these structures from each other, and it is best done by breaking the hash wedged C-11 bond. Since C-11 was originally in the aldehyde oxidation level, its bond could be broken into two alcohols with an aldehyde left over. It will be simple for these pieces to be synthetically reattached. The resulting products are seen in figure 2.
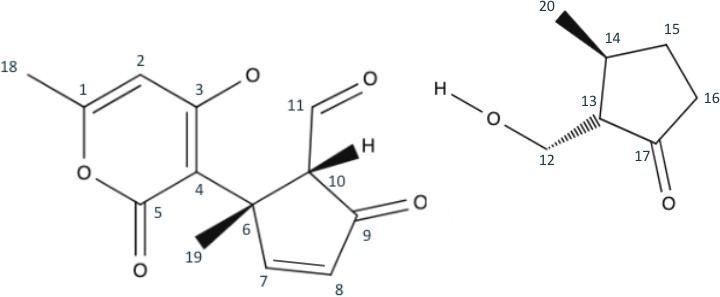
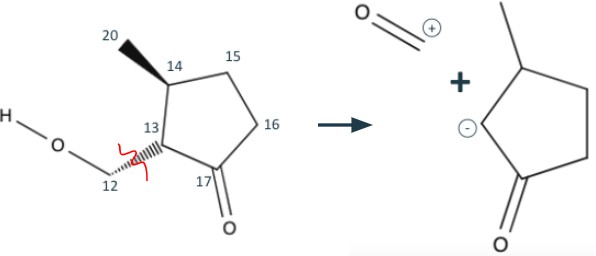
Continuing the retrosynthesis, we shift our attention to the cyclopentane structure to the right. Taking advantage of the 1,3 dioxygenation pattern in carbons 12, 13, and 17, we can break bond C12-C13. By disconnecting this bond, the alcohol tail is turned into a formaldehyde and the cyclopentane becomes 3,methylcyclopentanone with an anion at carbon 13(shown in Figure 3). A synthetic equivalent would be required to represent the anion, but formaldehyde already has a partial positive charge at its carbon.
Now we continue to retrosynthesize what remains of the triple-ring system (on the left of Figure 2). It now consists of a benzene-like and a cyclopentene structure, connected by bond
C4-C6. We could disconnect this bond right here, but the resulting methylcyclopentenedione will be a difficult molecule to simplify. Instead, we can opt to do some fine tuning instead. First, we do a functional group removal and remove the olefin from the cyclopentene structure(Figure 4).
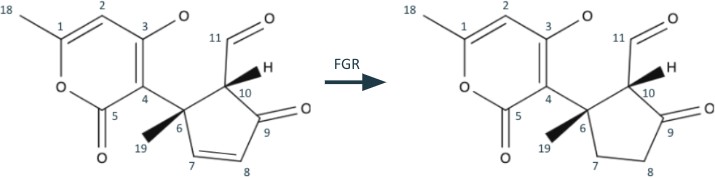
In a synthesis, this would be accommodated by a desaturation. Now, we can utilize the 1,5 dioxygenation pattern at C5, C4, C6, C10, and C11 to justify the C4-C6 disconnection, shown in Figure 5.
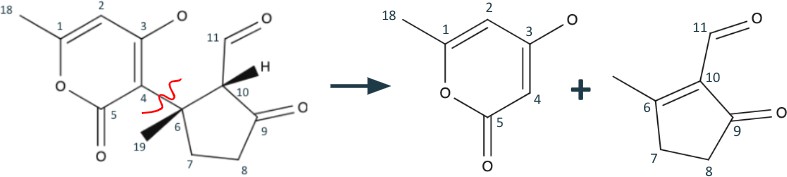
The rightward cyclopentane molecule might look a bit familiar. This is because it’s almost identical to the cyclopentane in figure 2. Having two identical molecules to work for at different stages of a synthesis would be very helpful in reducing the complexity of the synthesis process.
Therefore, using functional group interconversion, we can convert the aldehyde at C11 into an alcohol to take advantage of the potential 1,3 dioxygenation pattern at C9-11. Then, we use functional group removal to take care of the olefin, similar to what we did for C7=C8.
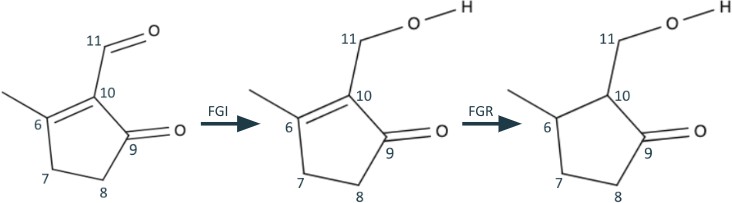
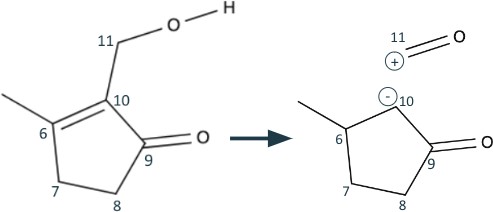
Finally, we use the resulting 1,3 dioxygenation pattern to break C10-C11, much like we broke C12-C13. We end with the synthons identical to those in Figure 3: formaldehyde and a 3,methylcyclopentanone enolate.
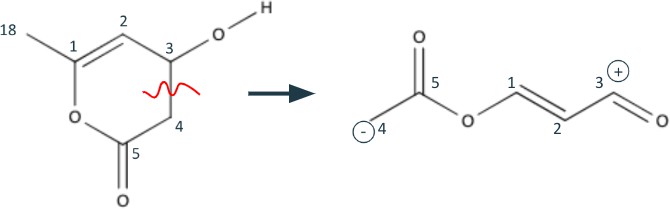
The last steps of this retrosynthetic process involves the quasi-benzene structure in the middle of Figure 5. Although a retrosynthetic step might not immediately be apparent, carbons 3-5 are very close to being a 1,3 dioxygenation pattern. Therefore, we do two functional group interconversions on the neighboring bonds of carbon 3. The first is a hydrogen shift from C3-O to C3=C3, removing the olefin and forming an aldehyde. The second is an interconversion from the resulting aldehyde into an alcohol.
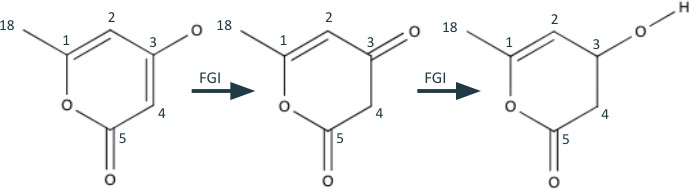
Finally, we can use the newly formed 1,3 dioxygenation pattern to break bond C3-C4 and turn the 6-membered ring into a carbon and oxygen chain.
3. Conclusion
This paper presents a detailed retrosynthetic analysis of Peniapyrone E, a complex natural product with promising cytotoxic activity. The process of retrosynthesis involved careful consideration of oxidation states and dioxygenation patterns, leading to a series of manageable synthetic intermediates. These results should provide a framework that would aid in a possible synthetic process of Peniapyrone E. For example, many retrosynthetic steps involving a 1,3 dioxygenation pattern could be reversed with an aldol addition reaction, while 1,5 disconnections could be reversed with a michael addition process [2]. As shown in this paper, retrosynthetic analysis is a technique that will help many synthetic chemists devise a synthetic pathway to their target molecules.
References
[1]. Bai, Yan; Ma, Xiaodong; Ren, Duo; Yu, Guoqing; Hu, Jiangchun; Hua, Huiming; et al. (2024). Peniapyrones A–I, Cytotoxic Tricyclic-Fused α‑Pyrone Derivatives from an Endophytic Penicillium brefeldianum F4a. ACS Publications. Collection. https: //doi.org/10.1021/acs.jnatprod.4c00383
[2]. Ye, Y. (2023). Introductory to Organic Synthesis and Retrosynthesis Analysis. In MedScien (Vol. 1, Issue 1). Dean & Francis Press. https: //doi.org/10.61173/5s5brt52
[3]. Guo J , Yu C , Li K , et al.Retrosynthesis Zero: Self-Improving Global Synthesis Planning Using Reinforcement Learning [J].Journal of Chemical Theory and Computation, 2024, 20(11): 18.DOI: 10.1021/acs.jctc.4c00071.
[4]. El-Mernissi R , Alaqarbeh M , Khaldan A , et al.3D-QSAR, molecular docking, ADMET, simulation dynamic, and retrosynthesis studies on new styrylquinolines derivatives against breast cancer [J].OPEN CHEMISTRY, 2024, 22(1).DOI: 10.1515/chem-2024-0041.
[5]. Zhong Z , Song J , Zunlei FengTiantao LiuLingxiang JiaShaolun YaoTingjun HouMingli Song.Recent advances in deep learning for retrosynthesis [J].Wiley Interdisciplinary Reviews. Computational Molecular Science, 2024, 14(1): n/a-n/a.DOI: 10.1002/wcms.1694.
Cite this article
Wang,E. (2025). Retrosynthetic Analysis of Peniapyrone E. Applied and Computational Engineering,208,27-31.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Bai, Yan; Ma, Xiaodong; Ren, Duo; Yu, Guoqing; Hu, Jiangchun; Hua, Huiming; et al. (2024). Peniapyrones A–I, Cytotoxic Tricyclic-Fused α‑Pyrone Derivatives from an Endophytic Penicillium brefeldianum F4a. ACS Publications. Collection. https: //doi.org/10.1021/acs.jnatprod.4c00383
[2]. Ye, Y. (2023). Introductory to Organic Synthesis and Retrosynthesis Analysis. In MedScien (Vol. 1, Issue 1). Dean & Francis Press. https: //doi.org/10.61173/5s5brt52
[3]. Guo J , Yu C , Li K , et al.Retrosynthesis Zero: Self-Improving Global Synthesis Planning Using Reinforcement Learning [J].Journal of Chemical Theory and Computation, 2024, 20(11): 18.DOI: 10.1021/acs.jctc.4c00071.
[4]. El-Mernissi R , Alaqarbeh M , Khaldan A , et al.3D-QSAR, molecular docking, ADMET, simulation dynamic, and retrosynthesis studies on new styrylquinolines derivatives against breast cancer [J].OPEN CHEMISTRY, 2024, 22(1).DOI: 10.1515/chem-2024-0041.
[5]. Zhong Z , Song J , Zunlei FengTiantao LiuLingxiang JiaShaolun YaoTingjun HouMingli Song.Recent advances in deep learning for retrosynthesis [J].Wiley Interdisciplinary Reviews. Computational Molecular Science, 2024, 14(1): n/a-n/a.DOI: 10.1002/wcms.1694.









