1. Introduction
Battery researchers are racing to solve a tough puzzle. Lithium metal acts like the holy grail for energy storage - it's incredibly energy-dense and efficient [1, 2]. But there's a dark side. When batteries charge and discharge, lithium doesn't always behave. It forms branch-like structures that can pierce through battery components [1]. These dendrites not only cause short circuits but also constantly consume the battery's liquid ingredients [1, 3]. To make matters worse, lithium expands and contracts dramatically with each cycle, tearing apart its protective layers [1, 3, 4].
Scientists are fighting back with clever designs. The battle plan includes: (i) creating microscopic homes for lithium to live in [4-7], (ii) building better armor for lithium surfaces [8], and (iii) teaching lithium where to settle [4, 7]. This review examines how graphene helps implement these strategies and explores what's still holding lithium batteries back [9].
2. Three-dimensional graphene structures for lithium anode applications
The dendrite problem keeps engineers awake at night [1]. Imagine a microscopic sponge that can control lithium growth - that's what 3D graphene offers [5, 6]. Researchers create these porous structures using various methods, including chemical vapor deposition and freeze-drying. One team led by Yun engineered a collector full of tiny holes [5]. This graphene sponge provides enormous surface area - think of a golf ball-sized material that could cover a football field if unfolded. All this space gives lithium plenty of room to settle, preventing crowded conditions that lead to dendrites [5, 6]. Their design maintained stable performance for an impressive 1000 hours [5].
The experimental data reveals clear advantages of 3D graphene structures. First, Yun's basic porous current collector operated for 1000 hours at 1 mA cm⁻². This is much longer than copper collectors, which typically fail after 200-300 hours [5]. Then Chen's team made it better. They used nitrogen doping and reached 2000 hours. This method improved how graphene interacts with lithium ions [10].
For fast charging, Zhang's conductive network worked well. Its pore structure helped electrons move quickly and reduced resistance by 60% [11]. And Wang's cobalt-graphene hybrid was even better. It kept 85% capacity at 10C rates because it had very high conductivity of 2500 S/m [6].
Another test looked at heavy usage. Jin's lithiophilic matrix survived 500 cycles at 5 mAh cm⁻². It only swelled 8% [8]. Liu's natural-inspired design also did well. It expanded just 5% during cycling. The special pore structure helped spread out stress and keep the electrode stable [4]. All these results show 3D graphene hosts can solve many lithium metal anode problems.
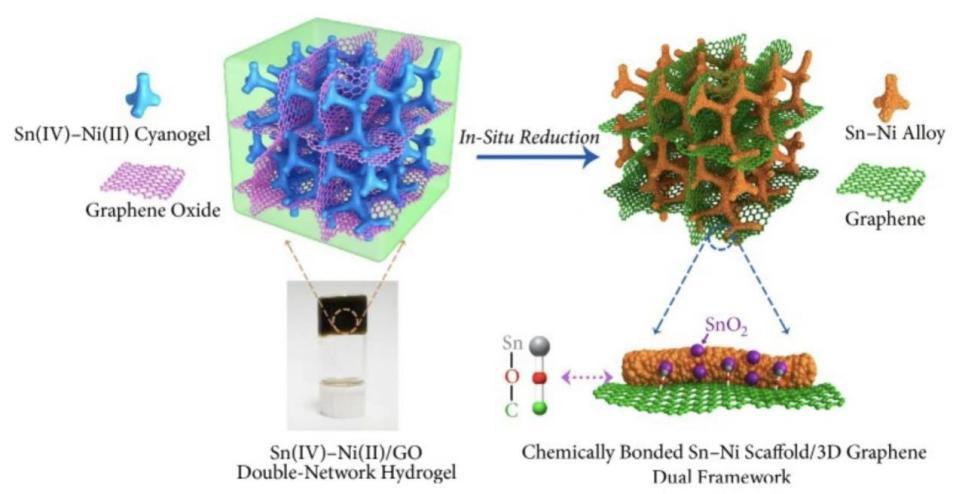
![Figure 1. Building a graphene-metal hybrid scaffold through a multi-step process [12]](https://file.ewadirect.com/press/media/markdown/document-image2_RS47fj2.jpeg)
Figure 1. Building a graphene-metal hybrid scaffold through a multi-step process [12]
Graphene's conductivity acts like a superhighway for electrons [11]. Recent breakthroughs with cobalt-graphene composites achieved unprecedented conductivity [6]. This means electrons can race through the material, enabling incredibly fast charging.
The material's flexibility is equally impressive. Jin's matrix expanded only 8% after 500 cycles, while raw lithium balloons by 300% [8]. Liu's biological approach maintained structural integrity through 1000 cycles [4].
The innovation continues with composite materials. Huang demonstrated that adding gold nanoparticles creates perfect landing spots for lithium [13]. Other teams have achieved 99.8% efficiency using zinc oxide dots [7], while silicon-graphene hybrids maintained 90% capacity after 200 cycles [14].
3. Graphene as a 2D artificial SEI layer
The natural protective layer on lithium is notoriously unreliable. Graphene offers a man-made solution - an ultra-thin, incredibly strong coating that blocks dendrite formation [1, 8]. Think of it as bulletproof armor for lithium surfaces. This artificial layer resists damage during cycling [15], with some formulations reducing electrolyte consumption by 80% [16].
![Figure 2. How 3D structures promote healthier lithium growth [11]](https://file.ewadirect.com/press/media/markdown/document-image3_PKbTGxQ.jpeg)
![Figure 3. How 3D structures promote healthier lithium growth [11]](https://file.ewadirect.com/press/media/markdown/document-image4_MzsX3D4.jpeg)
raphene's secret weapon lies in its molecular channels that guide lithium ions into perfect formation [8, 13]. When combined with materials like lithium fluoride, the results are remarkable - improved efficiency and longer lifespan [13, 17].
The innovation continues with Janus films that manage ions differently on each side [9], vertical graphene channels that ease ion movement [18], and ceramic-polymer composites that excel in high-voltage conditions [19].
4. Graphene for nucleation control in lithium metal anodes
Where lithium first settles determines everything that follows. Graphene provides the perfect training ground [11, 12], lowering the energy needed for lithium to stick and spreading it evenly [8, 13].
![Figure 4. Material structure analysis through imaging techniques [5]](https://file.ewadirect.com/press/media/markdown/document-image5_Klrh4cw.png)
Graphene guides lithium through multiple mechanisms. Its natural defects and doped atoms act as preferred landing sites [12, 13]. Nitrogen-doped versions create particularly welcoming surfaces [12], while boron-doped materials perform even better [20]. The material's vast surface area prevents crowding [11, 5], while its conductivity ensures even energy distribution [11, 13].
Adding metal nanoparticles makes this effect even stronger [13]. For example, gold nanoparticles work as perfect starting points for lithium to grow, preventing dangerous dendrite formation [13]. Another good combination is silver-zinc, which shows remarkable performance [21].
The effectiveness of graphene in controlling lithium growth becomes clear when we examine the experimental results. When tested in half-cells, graphene-based materials consistently show excellent efficiency - above 99.5% for hundreds of cycles. One research team led by Zhou used boron-doped graphene and achieved 99.6% efficiency for more than 2000 cycles, even at practical current levels [20].
But what happens under more challenging conditions? Huang's team tested silver-zinc modified graphene at higher currents and capacities. The results were impressive - the material kept 99.3% efficiency after 300 cycles, proving its durability under stress [21].
The real test comes in complete battery cells. When paired with high-capacity cathodes, graphene-modified lithium anodes maintained 80% of their capacity after 500 cycles. This is a major improvement over plain lithium anodes, which typically last only about 150 cycles [13, 22].
If we look at the microscopic structure, the difference is striking. Lithium grown on graphene forms dense, uniform patches measuring 5-20 µm. On plain copper, it forms loose, needle-like structures over 100 µm long [20, 21]. This better structure means the interface between electrode and electrolyte stays more stable, and the battery consumes 70% less electrolyte during operation [16].
|
Materials |
Current (mA cm-2) |
Capacity (mA h cm-2) |
Cycle number |
Overpotential (mV) |
Refer. |
|
Li/CuCF |
1 |
2 |
120 |
20 |
[23] |
|
CC/CNT@Li |
2 |
1 |
500 |
23 |
[24] |
|
Li/C3N4/CC |
2 |
2 |
750 |
80 |
[25] |
|
Li/Co-CS |
1 |
1 |
400 |
50 |
[26] |
|
CFs@Au-Li |
1 |
2 |
700 |
60 |
[27] |
|
Li-3D Cu |
1 |
2 |
535 |
18 |
[28] |
|
CC@CN-Co@Li |
1 |
2 |
800 |
20 |
[20] |
Graphene's flexibility accommodates lithium's mood swings [4,19], while tunable pore structures optimize ion pathways [5,6]. The latest gradient designs guide lithium deposition from the bottom up, even under extreme conditions [26]. The development of 3D porous copper current collectors has provided additional options for enhanced lithium deposition [19].
5. Conclusion
The evidence is clear - graphene offers multiple paths to better lithium batteries. From 3D hosts to protective coatings and smart surfaces, this versatile material addresses lithium's worst behaviors.
Yet significant hurdles remain. Mass-producing perfect graphene structures challenges manufacturers, long-term reliability under extreme conditions needs verification, and costs must decrease for widespread adoption.
The road ahead demands smarter composites that solve multiple problems simultaneously. Advanced imaging techniques and computer simulations will reveal deeper insights into interface behavior. With continued innovation in materials and manufacturing, graphene may finally unlock the full potential of lithium metal batteries.
References
[1]. Cheng, X. B., Zhang, R., Zhao, C. Z., & Zhang, Q. (2017). Toward safe lithium metal anode in rechargeable batteries: a review. Chemical reviews, 117(15), 10403-10473.
[2]. Lin, D., Liu, Y., & Cui, Y. (2017). Reviving the lithium metal anode for high-energy batteries. Nature nanotechnology, 12(3), 194-206.
[3]. Liu, J., Bao, Z., Cui, Y., Dufek, E. J., Goodenough, J. B., Khalifah, P., ... & Zhang, J. G. (2019). Pathways for practical high-energy long-cycling lithium metal batteries. Nature Energy, 4(3), 180-186.
[4]. Liu, W.; Xia, Y.; Wang, W.; et al. A biomimetic graphene scaffold for stable lithium metal anodes. Adv. Energy Mater. 2021, 11, 2100205.
[5]. Yun, Q., He, Y. B., Lv, W., Zhao, Y., Li, B., Kang, F., & Yang, Q. H. (2016). Chemical dealloying derived 3D porous current collector for Li metal anodes. Advanced Materials, 28(32), 6932-6939.
[6]. Wang, H.; Li, Y.; Li, Y.; et al. Graphene‐based composites for electrochemical energy storage. Energy Storage Mater. 2019, 24, 22–31.
[7]. Yang, C., Yao, Y., He, S., Xie, H., Hitz, E., & Hu, L. (2017). Ultrafine silver nanoparticles for seeded lithium deposition toward stable lithium metal anode. Advanced materials, 29(38), 1702714.
[8]. Jin, C., Sheng, O., Luo, J., Yuan, H., Fang, C., Zhang, W., ... & Tao, X. (2017). 3D lithium metal embedded within lithiophilic porous matrix for stable lithium metal batteries. Nano Energy, 37, 177-186.
[9]. Wang, J.; Wang, H.; Zhang, J.; et al. Janus graphene films for lithium metal anodes. ACS Nano 2022, 16, 3456–3465.
[10]. Chen, K.; Pathak, R.; Gurung, A.; et al. A novel 3D graphene foam for ultrahigh-rate lithium metal anodes. Nat. Commun. 2022, 13, 1–12.
[11]. Zhang, R., Li, N. W., Cheng, X. B., Yin, Y. X., Zhang, Q., & Guo, Y. G. (2017). Advanced micro/nanostructures for lithium metal anodes. Advanced Science, 4(3), 1600445.
[12]. Liu, Y., Lin, D., Yuen, P. Y., Liu, K., Xie, J., Dauskardt, R. H., & Cui, Y. (2016). An artificial solid electrolyte interphase with high Li-ion conductivity, mechanical strength, and flexibility for stable lithium metal anodes. Advanced Materials, 29(10).
[13]. Huang, G., Han, J., Zhang, F., Wang, Z., Kashani, H., Watanabe, K., & Chen, M. (2019). Lithiophilic 3D nanoporous nitrogen‐doped graphene for dendrite‐free and ultrahigh‐rate lithium‐metal anodes. Advanced materials, 31(2), 1805334.
[14]. Zhao, H.; Deng, N.; Yan, J.; et al. Recent advances in optimizing the microstructure of Si-based anodes for lithium-ion batteries. RSC Adv. 2018, 8, 2610–2619.
[15]. Li, N. W., Shi, Y., Yin, Y. X., Zeng, X. X., Li, J. Y., Li, C. J., ... & Guo, Y. G. (2018). A flexible solid electrolyte interphase layer for long‐life lithium metal anodes. Angewandte Chemie, 130(6), 1521-1525.
[16]. Xu, R., Zhang, X. Q., Cheng, X. B., Peng, H. J., Zhao, C. Z., Yan, C., & Huang, J. Q. (2018). Artificial soft–rigid protective layer for dendrite‐free lithium metal anode. Advanced Functional Materials, 28(8), 1705838.
[17]. Zheng, G., Lee, S. W., Liang, Z., Lee, H. W., Yan, K., Yao, H., ... & Cui, Y. (2014). Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nature nanotechnology, 9(8), 618-623.
[18]. Yu, Z., Cui, Y., & Bao, Z. (2020). Design principles of artificial solid electrolyte interphases for lithium-metal anodes. Cell Reports Physical Science, 1(7).
[19]. Li, T., Zhang, X. Q., Shi, P., & Zhang, Q. (2019). Fluorinated solid-electrolyte interphase in high-voltage lithium metal batteries. Joule, 3(11), 2647-2661.
[20]. Zhou, T., Shen, J., Wang, Z., Liu, J., Hu, R., Ouyang, L., ... & Zhu, M. (2020). Regulating lithium nucleation and deposition via MOF‐derived Co@ C‐modified carbon cloth for stable Li metal anode. Advanced Functional Materials, 30(14), 1909159.
[21]. Huang, G.; Han, J.; Zhang, F.; et al. Lithiophilic 3D porous CuZn current collector for stable lithium metal batteries. ACS Energy Lett. 2019, 4, 109–115.
[22]. Zhang, Y., Wang, C., Pastel, G., Kuang, Y., Xie, H., Li, Y., ... & Hu, L. (2018). 3D wettable framework for dendrite‐free alkali metal anodes. Advanced Energy Materials, 8(18), 1800635.
[23]. Chang, J., Shang, J., Sun, Y., Ono, L. K., Wang, D., Ma, Z., Huang, Q., Chen, D., Liu, G., Cui, Y., Qi, Y., & Zheng, Z. (2018). Flexible and stable high-energy lithium-sulfur full batteries with only 100% oversized lithium. Nature communications, 9(1), 4480.
[24]. Liu, F., Xu, R., Hu, Z., Ye, S., Zeng, S., Yao, Y., ... & Yu, Y. (2019). Regulating lithium nucleation via CNTs modifying carbon cloth film for stable Li metal anode. Small, 15(5), 1803734.
[25]. Xu, Y., Li, T., Wang, L., & Kang, Y. (2019). Interlayered dendrite‐free lithium plating for high‐performance lithium‐metal batteries. Advanced Materials, 31(29), 1901662.
[26]. Li, S., Liu, Q., Zhou, J., Pan, T., Gao, L., Zhang, W., ... & Lu, Y. (2019). Hierarchical Co3O4 nanofiber–carbon sheet skeleton with superior Na/Li‐philic property enabling highly stable alkali metal batteries. Advanced Functional Materials, 29(19), 1808847.
[27]. Xiang, J., Yuan, L., Shen, Y., Cheng, Z., Yuan, K., Guo, Z., ... & Huang, Y. (2018). Improved rechargeability of lithium metal anode via controlling lithium‐ion flux. Advanced Energy Materials, 8(36), 1802352.
[28]. Qiu, H., Tang, T., Asif, M., Huang, X., & Hou, Y. (2019). 3D porous Cu current collectors derived by hydrogen bubble dynamic template for enhanced Li metal anode performance. Advanced Functional Materials, 29(19), 1808468.
Cite this article
Yang,H. (2025). Recent Process of 2D Graphene for High-Energy-Density Lithium Anode. Applied and Computational Engineering,209,1-6.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of CONF-MCEE 2026 Symposium: Advances in Sustainable Aviation and Aerospace Vehicle Automation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Cheng, X. B., Zhang, R., Zhao, C. Z., & Zhang, Q. (2017). Toward safe lithium metal anode in rechargeable batteries: a review. Chemical reviews, 117(15), 10403-10473.
[2]. Lin, D., Liu, Y., & Cui, Y. (2017). Reviving the lithium metal anode for high-energy batteries. Nature nanotechnology, 12(3), 194-206.
[3]. Liu, J., Bao, Z., Cui, Y., Dufek, E. J., Goodenough, J. B., Khalifah, P., ... & Zhang, J. G. (2019). Pathways for practical high-energy long-cycling lithium metal batteries. Nature Energy, 4(3), 180-186.
[4]. Liu, W.; Xia, Y.; Wang, W.; et al. A biomimetic graphene scaffold for stable lithium metal anodes. Adv. Energy Mater. 2021, 11, 2100205.
[5]. Yun, Q., He, Y. B., Lv, W., Zhao, Y., Li, B., Kang, F., & Yang, Q. H. (2016). Chemical dealloying derived 3D porous current collector for Li metal anodes. Advanced Materials, 28(32), 6932-6939.
[6]. Wang, H.; Li, Y.; Li, Y.; et al. Graphene‐based composites for electrochemical energy storage. Energy Storage Mater. 2019, 24, 22–31.
[7]. Yang, C., Yao, Y., He, S., Xie, H., Hitz, E., & Hu, L. (2017). Ultrafine silver nanoparticles for seeded lithium deposition toward stable lithium metal anode. Advanced materials, 29(38), 1702714.
[8]. Jin, C., Sheng, O., Luo, J., Yuan, H., Fang, C., Zhang, W., ... & Tao, X. (2017). 3D lithium metal embedded within lithiophilic porous matrix for stable lithium metal batteries. Nano Energy, 37, 177-186.
[9]. Wang, J.; Wang, H.; Zhang, J.; et al. Janus graphene films for lithium metal anodes. ACS Nano 2022, 16, 3456–3465.
[10]. Chen, K.; Pathak, R.; Gurung, A.; et al. A novel 3D graphene foam for ultrahigh-rate lithium metal anodes. Nat. Commun. 2022, 13, 1–12.
[11]. Zhang, R., Li, N. W., Cheng, X. B., Yin, Y. X., Zhang, Q., & Guo, Y. G. (2017). Advanced micro/nanostructures for lithium metal anodes. Advanced Science, 4(3), 1600445.
[12]. Liu, Y., Lin, D., Yuen, P. Y., Liu, K., Xie, J., Dauskardt, R. H., & Cui, Y. (2016). An artificial solid electrolyte interphase with high Li-ion conductivity, mechanical strength, and flexibility for stable lithium metal anodes. Advanced Materials, 29(10).
[13]. Huang, G., Han, J., Zhang, F., Wang, Z., Kashani, H., Watanabe, K., & Chen, M. (2019). Lithiophilic 3D nanoporous nitrogen‐doped graphene for dendrite‐free and ultrahigh‐rate lithium‐metal anodes. Advanced materials, 31(2), 1805334.
[14]. Zhao, H.; Deng, N.; Yan, J.; et al. Recent advances in optimizing the microstructure of Si-based anodes for lithium-ion batteries. RSC Adv. 2018, 8, 2610–2619.
[15]. Li, N. W., Shi, Y., Yin, Y. X., Zeng, X. X., Li, J. Y., Li, C. J., ... & Guo, Y. G. (2018). A flexible solid electrolyte interphase layer for long‐life lithium metal anodes. Angewandte Chemie, 130(6), 1521-1525.
[16]. Xu, R., Zhang, X. Q., Cheng, X. B., Peng, H. J., Zhao, C. Z., Yan, C., & Huang, J. Q. (2018). Artificial soft–rigid protective layer for dendrite‐free lithium metal anode. Advanced Functional Materials, 28(8), 1705838.
[17]. Zheng, G., Lee, S. W., Liang, Z., Lee, H. W., Yan, K., Yao, H., ... & Cui, Y. (2014). Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nature nanotechnology, 9(8), 618-623.
[18]. Yu, Z., Cui, Y., & Bao, Z. (2020). Design principles of artificial solid electrolyte interphases for lithium-metal anodes. Cell Reports Physical Science, 1(7).
[19]. Li, T., Zhang, X. Q., Shi, P., & Zhang, Q. (2019). Fluorinated solid-electrolyte interphase in high-voltage lithium metal batteries. Joule, 3(11), 2647-2661.
[20]. Zhou, T., Shen, J., Wang, Z., Liu, J., Hu, R., Ouyang, L., ... & Zhu, M. (2020). Regulating lithium nucleation and deposition via MOF‐derived Co@ C‐modified carbon cloth for stable Li metal anode. Advanced Functional Materials, 30(14), 1909159.
[21]. Huang, G.; Han, J.; Zhang, F.; et al. Lithiophilic 3D porous CuZn current collector for stable lithium metal batteries. ACS Energy Lett. 2019, 4, 109–115.
[22]. Zhang, Y., Wang, C., Pastel, G., Kuang, Y., Xie, H., Li, Y., ... & Hu, L. (2018). 3D wettable framework for dendrite‐free alkali metal anodes. Advanced Energy Materials, 8(18), 1800635.
[23]. Chang, J., Shang, J., Sun, Y., Ono, L. K., Wang, D., Ma, Z., Huang, Q., Chen, D., Liu, G., Cui, Y., Qi, Y., & Zheng, Z. (2018). Flexible and stable high-energy lithium-sulfur full batteries with only 100% oversized lithium. Nature communications, 9(1), 4480.
[24]. Liu, F., Xu, R., Hu, Z., Ye, S., Zeng, S., Yao, Y., ... & Yu, Y. (2019). Regulating lithium nucleation via CNTs modifying carbon cloth film for stable Li metal anode. Small, 15(5), 1803734.
[25]. Xu, Y., Li, T., Wang, L., & Kang, Y. (2019). Interlayered dendrite‐free lithium plating for high‐performance lithium‐metal batteries. Advanced Materials, 31(29), 1901662.
[26]. Li, S., Liu, Q., Zhou, J., Pan, T., Gao, L., Zhang, W., ... & Lu, Y. (2019). Hierarchical Co3O4 nanofiber–carbon sheet skeleton with superior Na/Li‐philic property enabling highly stable alkali metal batteries. Advanced Functional Materials, 29(19), 1808847.
[27]. Xiang, J., Yuan, L., Shen, Y., Cheng, Z., Yuan, K., Guo, Z., ... & Huang, Y. (2018). Improved rechargeability of lithium metal anode via controlling lithium‐ion flux. Advanced Energy Materials, 8(36), 1802352.
[28]. Qiu, H., Tang, T., Asif, M., Huang, X., & Hou, Y. (2019). 3D porous Cu current collectors derived by hydrogen bubble dynamic template for enhanced Li metal anode performance. Advanced Functional Materials, 29(19), 1808468.









