1. Introduction
The Michael addition reaction is a versatile synthetic method for efficiently conjugating low-electron olefins to nucleophiles. It is mainly used for the extension of the carbon chain in organic synthesis, and for organic compounds with various functional groups. The Michael addition reaction plays an important role in polymer synthesis. In recent years, it has been widely used in emerging technologies such as biomedicine, pharmaceuticals, photonics, composites, adhesives, and coatings [1].
This paper can mainly be divided into two parts. The first part mainly introduces the mechanism of the Michael addition reaction and its main form and principle. For example, the electrophilic and nucleophile reagents in the reaction, the classification of electrophilic and nucleophile, etc. [2]. The second part introduces some specific applications of Michael addition reactions. In this paper, the rhodium-catalyzed Michael addition of alkyl aromatic benzyl C-H bonds is reviewed [3]. The catalytic mechanism of the rhodium catalyst, the reaction mechanism of the Michael addition, and the catalytic performance of the catalyst for various substrates are included in the review. This paper has the following values: it contributes to the development of the organic synthesis industry, fills the blank of the Michael addition reaction of benzyl hydrocarbon bonds of alkyl aromatics, adds a new basis for the Michael addition theory of organometallic catalysts, expands the Michael addition theory, and establishes a new view of Michael addition of benzyl hydrocarbon bonds of alkyl aromatics.
2. Michael addition
2.1. History of Michael addition
The Michael addition is a type of organic reaction discovered by Arthur Michael in 1887 [4]. The reaction involves the nucleophilic addition of a carbanion to an α,β-unsaturated carbonyl compound, resulting in the formation of a new carbon-carbon bond.
The reaction was first described in Michael's paper titled "On the Addition of Sodium Malonic Ester to Methylene Compounds: A Synthesis of Substituted Succinic Acids," which was published in the Journal of the Chemical Society [5]. In this paper, Michael reported the addition of sodium malonic ester to acrolein, resulting in the formation of β-ketobutyric acid.
Since its discovery, the Michael addition has been extensively studied and applied in organic synthesis, particularly in the formation of carbon-carbon bonds. The reaction has been used in the synthesis of natural products, pharmaceuticals, and other complex organic molecules.
One notable application of the Michael addition is in the synthesis of the anti-inflammatory drug ibuprofen. In the early 1960s, Stewart Adams and John Nicholson of Boots Pure Drug Company used the Michael reaction to synthesize the key intermediate in the synthesis of ibuprofen, which is now one of the most widely used nonsteroidal anti-inflammatory drugs [6].
2.2. Electrophiles and nucleophiles in Michael addition
The nucleophile in the Michael addition can be a wide range of compounds, including alkoxides, thiols, amines, and enolates, among others [7]. Similarly, the electrophile can also vary and include a variety of α,β-unsaturated carbonyl compounds, such as acrylates, acrylamides, and vinyl ketones. One important aspect of the Michael addition reaction is the regioselectivity of the reaction, which is determined by the nucleophile used. The nucleophile can add to the β-carbon of the α,β-unsaturated carbonyl compound or the carbonyl carbon, leading to different products.
2.3. The basic mechanism of Michael addition
The reaction mechanism typically involves two steps: the formation of an enolate intermediate, followed by the nucleophilic attack.
In the first step, the α,β-unsaturated carbonyl compound undergoes deprotonation at the α-position by a base to form an enolate intermediate. The resulting enolate intermediate is resonance-stabilized and can exist in multiple forms.
In the second step, the enolate intermediate acts as a nucleophile and attacks the electrophilic β-carbon of another molecule, resulting in the formation of a new carbon-carbon bond. The resulting product is a β-functionalized carbonyl compound, as shown in Figure 1.
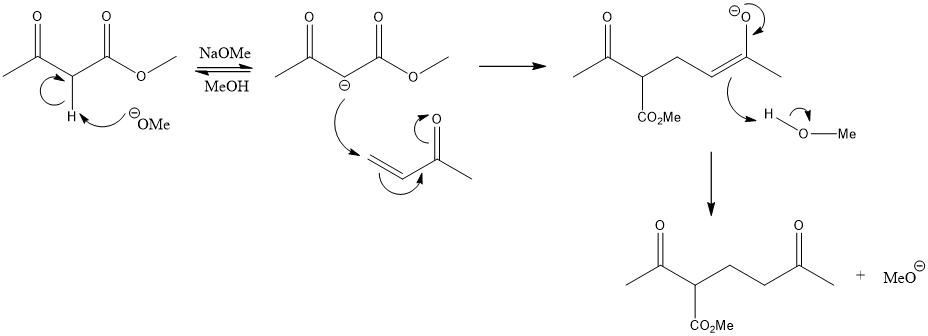
Figure 1. The mechanism of forming a 1,5-dicarbonyl though Michael addition.
The reaction can be catalyzed by a variety of acids or bases, and the choice of catalyst can have an impact on the regioselectivity and stereoselectivity of the reaction [8].
3. Rhodium-catalyzed benzylic addition reactions of alkylarenes to Michael acceptors
3.1. Limitations and improvements in Michael addition reactions
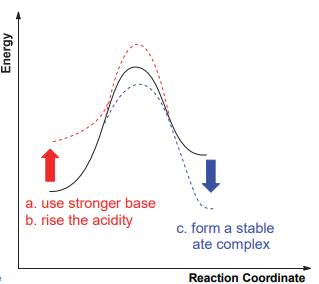
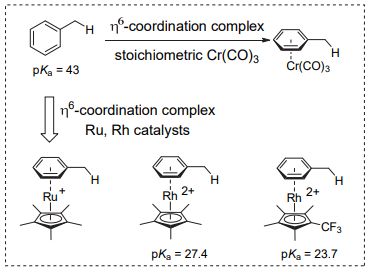
The common carbon-hydrogen bond deprotonation reaction is the process of "strong acid and base" to produce "weak acid and base", which is allowed by thermodynamics. Thermodynamic stable carboanion intermediates can be obtained. In addition to the common carbonyl α-H, which is highly acidic, most C-H bonds are relatively weak in acidity. For example, pKa of acetone in water is 30, and pKa of toluene in dimethyl sulfoxide is 43. According to the reaction process diagram, the reaction between the weak acidic C-H bond and the weak base is not supported by thermodynamics, and it is difficult for the deprotonation reaction. To reduce this influence, generally, three methods can be adopted, including a strong base reagent, an increase in the acidity of the C-H bond, and an increase in the stability of carbanion products (Figure 2.a).
Diisopropylamine lithium and even butyl lithium is difficult to deprotonate toluene due to the weak acidity of the C-H bond of benzyl toluene. The η6 coordination structures formed by toluene with metals (e.g., toluene tricarbonyl chromium) can effectively enhance the acidity of benzyl C-H bonds, which is an important way of deprotonation of toluene (Figure 2.b) [9].
|
|
(a) Deprotonation of weakly acidic C-H bonds. | (b) Enhanced acidity of benzylic C-H bonds. |
Figure 2. The way to enhance the deprotonate reaction.
In 2010, Walsh and his team [10] applied toluene with Cr tricarbonyl coordination to cross the coupling reaction catalyzed by transition metal, and realized benzyl arylation and allylation of a series of toluene complexes under alkali conditions. However, the metal π complex can enhance the acidity of the benzyl C-H bond, thus achieving toluene deprotonation. However, the limited range of substrates and complex synthesis and dementalization of products limit the application of this method.
3.2. Rhodium-catalyzed addition of benzyl C-H bonds to Michael receptors
Carbanions produced by the C(SP)-H bond key nuclear test agent are used for a series of classic \( C(SP)-C-({SP^{3}}) \) keys, including aldol reactions [11], Michael adding reactions [12], and Mannich reactions [13]. Because the electrical negativeness between carbon and hydrogen atoms is very small, it is difficult to be elapsed by the C-H key activated by strong electron attractive groups (such as cymbal). Based on the basic knowledge of deuteration reactions catalyzed by methanol benzyl hydrocarbon binding to olefin, the Shi hang group recently performed rhodium-catalyzed benzyl carbon intermolecular REDOX reactions in a Michael receptor [14]. Compared with hydrogen residents, the neutron nucleus and oxidation reactions of the hydrocarbon-based hydrocarbon keys are affected by the electronic philosophy compounds, space blockers, and other factors, and have considerable richness challenges. Through the restoration system, the catalytic quality and performance of different alternative cyclopenne transition metals were studied, and various catalysts of various cyclopanane catalysts have certain electrochemical properties. The oxidation reaction of carbonide and 1,1-dilate sulfonal polyvinyl chloride and the catalytic performance of the catalyst for this reaction is shown in Figure 3.
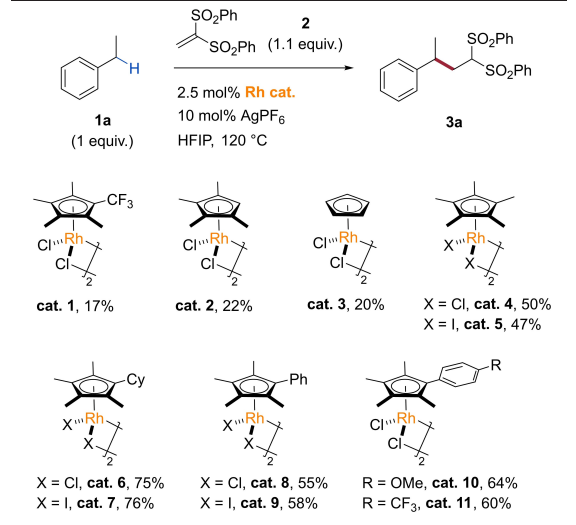
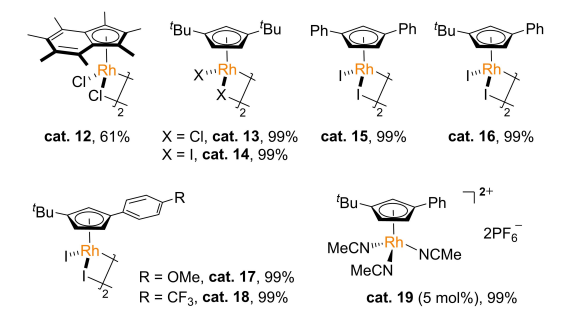
Figure 3. Catalysis selected in the 1,1-dilate sulfonal polyvinyl chloride reaction.
With excellent electrochemical properties and selectivity of the third, primary, and secondary, and the effective connection of primary and dilate hydrocarbons, this reaction is compatible with halogen bulbs, ester base, and active species, showing an excellent nuclear range. The author tries to use cyclomerized metharium hydrochloride as a Michael receptor, and it is necessary to react under which conditions to get high revenue in the final adding products. Due to its relatively poor infancy, the most common Michael receptor, such as acrylic hydrochloric acid resin, cannot participate in the reaction. The current scope of the reaction is also limited to Michael strong receptor ionic compounds.
The anions and cations of cyclopentadienyl rhodium were added to the solid material of carbon disulfide and the final product of the reaction. During the reaction, carbon disulfide and rhodium π transition metal were selectively obtained. Figure 4 finds out under what conditions can this transition metal heat 1,1-diphenyl sulfonyl polyvinyl chloride to undergo an efficient and stable addition reaction.
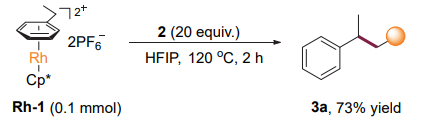
Figure 4. Addition of Rh-complex.
These results indicate that carbon disulfide is the key to the reaction of fine chemical products with rhodium π transition metal. The carbon disulfide is not strongly relative to the end product and coordination potential is the most important factor in the process of selective single addition of the end product. Benzyl cyclopropane was able to perform a smooth REDOX reaction if no organic contamination by cyclopropane was observed. The results of this experiment exclude the possibility of benzyl radical and benzyl nonmetallic fine chemical products.
As a result, members of Shi's research group developed a Michael REDOX reaction of methanol to olefin benzyl hydrocarbon bonds and the strong ionic compound adiponic acid. This reaction has excellent anionic compatibility and a wide range of substrates in all aspects of methanol to olefin nucleophile. From the point of view of the required reaction conditions, it can be seen that the author has undoubtedly achieved the rhodium-catalyzed Michael REDOX reaction of benzyl hydrocarbon bond under the condition of "no base". Different from the expected reaction equation, the rhodium catalyst gradually formed π-transition metal into olefin with methanol, which continuously enhanced the basic material of benzyl hydrocarbon bond, but may not participate in the specific process of the complete cracking of the intermolecular forces of carbon.
Benzyl C-H bonds are activated and functionalized by deprotonation. Therefore, although there is no added "base" in the reaction conditions, the role and function of "base" in the reaction process are still worth further discussion. It is believed that the combination of aromatics activation by rhodium catalyst and alkali deprotonation will enable more interesting reactions.
4. Conclusion
This paper provides a comprehensive overview of the Michael addition reaction, including the drugs and conditions necessary for the reaction, as well as an illustration of the reaction mechanism to aid in the understanding of the process.
The first part of the introduction delves into the history and evolution of the Michael addition reaction, providing a detailed understanding of its development. A thorough understanding of the Michael addition reaction and its history is crucial to understanding the modern significance of this important reaction. This information will be covered in the article. The second part of the paper provides a detailed analysis of the various factors related to the Michael addition reaction process. These factors include those affecting the deprotonation step, which is critical to the successful execution of the reaction. The paper summarizes the causes of these factors and presents an improvement plan to ensure that the reaction proceeds smoothly. Additionally, the paper introduces the rhodium-catalyzed Michael addition of the alkyl aromatics benzyl C-H bond, which represents an important advancement in the field. It also provides examples of previous studies in this area before presenting the current study's findings on how the catalyst works and which substrates it is most suitable for. This thorough analysis of the rhodium-catalyzed Michael addition of the alkyl aromatics benzyl C-H bond highlights the potential of this reaction in organic synthesis and underscores the importance of continued research in this area.
The main shortcomings of this paper are as follows: the introduction to the mechanism of the Michael addition reaction gives only one example, which may be difficult to understand. There are few data on the rhodium-catalyzed Michael addition of the alkyl arene benzyl C-H bond, which makes it impossible to evaluate the results objectively.
References
[1]. Mather, B. D., Viswanathan, K., Miller, K. M., Long, T. E., Long, D. Q. and Huang, M. Q. (2003). Michael addition reaction and its application. Journal of Advanced Correspondence Education (Natural Science Edition) (02), 39-42.
[2]. Smith, M. B. and March, J. (2013). March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure. 7th ed. Hoboken, New Jersey: Wiley.
[3]. Mather, B. D., Viswanathan, K., Miller, K. M., Long, T. E. (2006). Michael addition reactions in macromolecular design for emerging technologies. Progress in Polymer Science 31(5), 487-531, ISSN 0079-6700. https://doi.org/10.1016/j.progpolymsci.2006.03.001.
[4]. Michael, A. (1887). Über die Addition von Natriumacetessig-und Natriummalonsäureäthern zu den Methylene der aromatischen α- und β-ungesättigten Säuren. J. Prakt. Chem. 35, 349-356.
[5]. Clayden, J., Greeves, N., Warren, S. and Wothers, P. (2012). Organic chemistry. Oxford University Press.
[6]. Adams, S. S. and Nicholson, J. S. (1966). The synthesis of ibuprofen. Journal of chemical education 43(10), 581-584.
[7]. Mahrwald, R. (Ed.). (2012). Modern Michael addition reactions. John Wiley & Sons.
[8]. Wiberg, K. B., Abood, S. D. and Maitra, A. M. (1962). Mechanism of the Michael Reaction. J. Am. Chem. Soc. 84 (16), 3148-3152. DOI: 10.1021/ja00876a011.
[9]. Michon, C., Liu, S. Y., Hiragushi, S. Y., Uenishi, J’ichi and Uemura, M. (2008). Gold(I)-Catalyzed Cycloisomerization of (Arene)chromium Complexes with Enyne Bonds Directed Towards Axially Chiral Biaryls. Organomet, 1321-1324.
[10]. McGrew, G. I., Temaismithi, J., Carroll, P. J. and Walsh, P. J. (2010). Angew. Chem., Int. Ed., 49, 5541.
[11]. Mukaiya, T. (1982). Organic Reaction 28, 203-331; b) Heathcock, C. H. (1991). Selectivity, strategy and efficiency in modern organic chemistry. Comprehensive organic synthesis 2, 133-179. Pergamon Press, Oxford.
[12]. Bergmann, E. D., Ginsburg, D. and Pappo, R. (1959). Organic Reaction 10, 179-556; b) Kanai, M. and Catal. M. S. (2000). Asymmetric Synth. (2nd Edition), 569-592; c) Narasaka, K., Soai, K. and Mukaiyama, T. (1974). Chem. Lett. 3, 1223-1224.
[13]. Blicke, F. F. (1942). Organic Reaction 1, 303-341; b) Allochio Filho, J. F., Lemos, B. C., de Souza, A. S., Pinheiro, S. and Greco, S. J. (2017). Tetrahedron 73, 6977-7004; c) Farhid, H. Khodkari, V., Nazeri, M. T., Javanbakht, S., Shaabani, A. (2021). Org. Biomol. Chem. 19, 3318-3358.
[14]. Li, Y., Wu, W. Q., Zhu, H., et al. (2022). Rhodium-catalyzed benzylic addition reactions of alkylarenes to Michael acceptors. Angew. Chem. Int. Ed. 61, 134.
Cite this article
Liu,Z. (2023). Michael addition reaction and its examples. Applied and Computational Engineering,24,1-6.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2023 International Conference on Functional Materials and Civil Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Mather, B. D., Viswanathan, K., Miller, K. M., Long, T. E., Long, D. Q. and Huang, M. Q. (2003). Michael addition reaction and its application. Journal of Advanced Correspondence Education (Natural Science Edition) (02), 39-42.
[2]. Smith, M. B. and March, J. (2013). March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure. 7th ed. Hoboken, New Jersey: Wiley.
[3]. Mather, B. D., Viswanathan, K., Miller, K. M., Long, T. E. (2006). Michael addition reactions in macromolecular design for emerging technologies. Progress in Polymer Science 31(5), 487-531, ISSN 0079-6700. https://doi.org/10.1016/j.progpolymsci.2006.03.001.
[4]. Michael, A. (1887). Über die Addition von Natriumacetessig-und Natriummalonsäureäthern zu den Methylene der aromatischen α- und β-ungesättigten Säuren. J. Prakt. Chem. 35, 349-356.
[5]. Clayden, J., Greeves, N., Warren, S. and Wothers, P. (2012). Organic chemistry. Oxford University Press.
[6]. Adams, S. S. and Nicholson, J. S. (1966). The synthesis of ibuprofen. Journal of chemical education 43(10), 581-584.
[7]. Mahrwald, R. (Ed.). (2012). Modern Michael addition reactions. John Wiley & Sons.
[8]. Wiberg, K. B., Abood, S. D. and Maitra, A. M. (1962). Mechanism of the Michael Reaction. J. Am. Chem. Soc. 84 (16), 3148-3152. DOI: 10.1021/ja00876a011.
[9]. Michon, C., Liu, S. Y., Hiragushi, S. Y., Uenishi, J’ichi and Uemura, M. (2008). Gold(I)-Catalyzed Cycloisomerization of (Arene)chromium Complexes with Enyne Bonds Directed Towards Axially Chiral Biaryls. Organomet, 1321-1324.
[10]. McGrew, G. I., Temaismithi, J., Carroll, P. J. and Walsh, P. J. (2010). Angew. Chem., Int. Ed., 49, 5541.
[11]. Mukaiya, T. (1982). Organic Reaction 28, 203-331; b) Heathcock, C. H. (1991). Selectivity, strategy and efficiency in modern organic chemistry. Comprehensive organic synthesis 2, 133-179. Pergamon Press, Oxford.
[12]. Bergmann, E. D., Ginsburg, D. and Pappo, R. (1959). Organic Reaction 10, 179-556; b) Kanai, M. and Catal. M. S. (2000). Asymmetric Synth. (2nd Edition), 569-592; c) Narasaka, K., Soai, K. and Mukaiyama, T. (1974). Chem. Lett. 3, 1223-1224.
[13]. Blicke, F. F. (1942). Organic Reaction 1, 303-341; b) Allochio Filho, J. F., Lemos, B. C., de Souza, A. S., Pinheiro, S. and Greco, S. J. (2017). Tetrahedron 73, 6977-7004; c) Farhid, H. Khodkari, V., Nazeri, M. T., Javanbakht, S., Shaabani, A. (2021). Org. Biomol. Chem. 19, 3318-3358.
[14]. Li, Y., Wu, W. Q., Zhu, H., et al. (2022). Rhodium-catalyzed benzylic addition reactions of alkylarenes to Michael acceptors. Angew. Chem. Int. Ed. 61, 134.